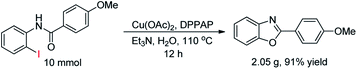Open Access Article
Open Access ArticleEfficient Cu-catalyzed intramolecular O-arylation for synthesis of benzoxazoles in water†
Yanling Tang‡
,
Minxin Li‡,
Hui Gao*,
Gaoxiong Rao and
Zewei Mao *
*
College of Pharmaceutical Science, Yunnan University of Chinese Medicine, Kunming 650500, P.R. China. E-mail: gaohui_em@126.com; ydmason@163.com; maozw@ynutcm.edu.cn; Fax: +86 871 65918232; Tel: +86 871 65918232
First published on 7th April 2020
Abstract
An efficient method was developed for synthesis of benzoxazoles by Cu-catalyzed intramolecular O-arylation of o-halobenzanilides in water. This strategy provides several advantages, such as high yields, water as a green solvent and functional groups tolerance.
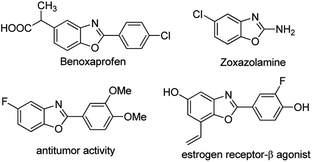
Benzoxazole derivatives not only exist in several important natural products, but are also present in many important classes of organic compounds due to their biological, pharmaceutical and catalytic properties, which have attracted more and more attention from pharmaceutical and synthetic chemists (Fig. 1).1,2 Commonly, the preparation of benzoxazoles has attracted considerable attention. Traditional methods to construction of 2-aryl benzoxazoles involve the condensation of 2-aminophenol with carboxylic acids or aldehydes under oxidative conditions, which unfortunately often suffer from the use of harsh reaction conditions involving strong acids or highly toxic reagents in combination with high temperatures.3
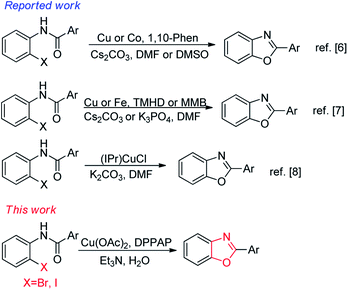
In the last decades, some of these drawbacks have been overcome by the development of transition-metal-catalyzed processes for C–O bond formations under comparatively milder reaction conditions.4 Until now, there are various reactions reported for the construction of benzoxazoles by intramolecular O-arylation of orthohaloanilides under milder reaction conditions.5 Evindar et al. have improved the formation of benzoxazoles via a CuI/1,10-phenanthroline-catalyzed cyclization of o-haloanilides in the presence of Cs2CO3 in DMF.6a Saha et al. have reported cobalt(II)/1,10-phenanthroline-catalyzed the intramolecular C–O cross-couplings of o-halobenzanilides to give benzoxazoles in DMSO.6b Dai et al. have prepared 2-substituted benzoxazoles via Cu-catalysed intramolecular coupling cyclization reactions using methyl 2-methoxybenzoate (MMB) as the ligand in DMF.7a Bonnamour et al. have obtained benzoxazole derivatives by iron-catalyzed intramolecular O-arylation reaction and 2,2,6,6-tetramethyl-3,5 heptanedione (TMHD) as the cocatalyst in DMF.7b Tapia et al. described an efficient synthesis of 2-arylbenzoxazoles using (IPr)CuCl via intramolecular coupling cyclization of 2-haloanilides in DMF.8 Although many improvements have been achieved for the synthesis of 2-substituted benzoxazoles from o-halobenzanilides through intramolecular O-arylation by transition-metal-catalyzed processes, many approaches reported so far were harsh conditions, such as long reaction time, costly and toxic solvents.3,9 Therefore, there is still worthwhile to develop an economic, efficient and eco-friendly catalytic system (Scheme 1).
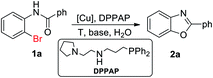
To the best of our knowledge, no report on the synthesis of benzoxazoles from o-halobenzanilides in water has been reported so far. In initiatory work, we have found that 1-[2-(N-(3-diphenylphosphinopropyl))aminoethyl]pyrrolidine (DPPAP) could promoted transition metal-catalyzed reactions as NNP-type pincer ligand, which accelerated Cu-catalyzed intramolecular O-arylation of o-halobenzanilides to form benzoxazoles in water. In present work, we optimum reaction conditions based on initiatory study, and report a new, efficient and green Cu-catalyzed synthesis of benzimidazoles in water.
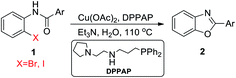
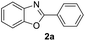
Reaction between N-(2-bromophenyl)benzamide (1a) was selected as a model substrate and various copper sources were tested including CuI, CuSO4, Cu(OAc)2 and Cu(OTf)2 to check and identify optimization of reaction conditions. As shown in Table 1, the reaction results were distinguishable catalyzed by various copper sources. In general, CuI could lead to product in low yield, and CuSO4 was not active to this action and no product was obtained (Table 1, entries 1–6). To our surprise, Cu(OTf)2 and Cu(OAc)2 enhanced the reaction yields obviously (Table 1, entries 7–15). In order to study the effects of different types of bases on the reaction, 4 bases were employed including inorganic and organic base such as K3PO4, K2CO3, TMEDA and Et3N. We could see that compound 2a was formed in the presence of TMEDA and Et3N in better yields than that of K3PO4 and K2CO3, and there was nearly no difference.
| Entry | [Cu] | Base | T (°C) | Time (h) | Yield (%) |
|---|---|---|---|---|---|
| a All reactions were performed using 1a (1 mmol), [Cu] (5 mol%), DPPAP (10 mol%), base (2 mmol) in water (3 mL).b Isolated yield after chromatographic purification. | |||||
| 1 | CuI | K2CO3 | 100 | 12 | 22 |
| 2 | CuI | K3PO4 | 100 | 12 | 20 |
| 3 | CuI | TMEDA | 100 | 12 | 38 |
| 4 | CuI | Et3N | 100 | 12 | 36 |
| 5 | CuSO4 | TMEDA | 100 | 12 | Trace |
| 6 | CuSO4 | Et3N | 100 | 12 | Trace |
| 7 | Cu(OTf)2 | TMEDA | 100 | 12 | 64 |
| 8 | Cu(OTf)2 | Et3N | 100 | 12 | 62 |
| 9 | Cu(OAc)2 | TMEDA | 100 | 12 | 84 |
| 10 | Cu(OAc)2 | Et3N | 100 | 12 | 83 |
| 11 | Cu(OAc)2 | Et3N | 90 | 12 | 70 |
| 12 | Cu(OAc)2 | Et3N | 110 | 12 | 89 |
| 13 | Cu(OAc)2 | Et3N | 110 | 6 | 62 |
| 14 | Cu(OAc)2 | Et3N | 110 | 24 | 81 |
| 15 | Cu(OAc)2 | Et3N | 120 | 12 | 72 |
Furthermore, we examined the effects of temperature and time on the reaction. With the increasing of temperature from 90 °C to 110 °C, the yield had increased to 89% (Table 1, entry 12). However, further increasing temperature led to decline of yield(Table 1, entry 15). In addition, reaction time from 6 h to 12 h was favorable to reaction obviously. However, further increase of reaction time to 24 h had negative effect on the yield of benzoxazole (Table 1, entry 14). Therefore, it was clear that the best reaction was performed taking Cu(OAc)2 (5 mol%) and DPPAP (10 mol%) as the catalyst in water in present of Et3N (2 eq.) for 12 h at 110 °C.
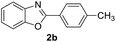
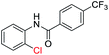
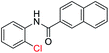
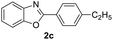
With the optimal reaction conditions in hand, a series of o-halobenzanilides were investigated to study the scope of Cu-catalyzed intramolecular O-arylation. As shown in Table 2, to our delight, the O-arylation of various substituted 2-bromobenzanilides or 2-iodobenzanilides under standard reaction condition gave the corresponding title products in good to high yields, and 2-iodobenzanilides were more active than 2-bromobenzanilides (Table 2). In order to study the influence of substituted groups, various substituted o-halobenzanilides were performed including electron donating groups (CH3, C2H5, CH3O, F, Cl, Br) and withdrawing group (CF3). From the results, we could find that this reaction condition was beneficial to prepare 2-substituted benzoxazoles, and substitutions on amide were tolerated. In general, electron donating groups afforded the corresponding benzoxazoles in higher yields than that of CF3. Especially, N-(2-iodophenyl)-4-methoxybenzamide formed 2d in the best yield (93%), and N-(2-bromophenyl)-2,3,4,5-tetrafluorobenzamide formed 2g in the lowest yield (70%).
| Entry | 1 | 2 | Yieldc (%) | |
|---|---|---|---|---|
| present | reported | |||
| a All reactions were carried out using o-halobenzanilides (1 mmol), Cu(OAc)2 (0.05 mmol), DPPAP (0.1 mmol), Et3N (2 mmol) in water (3 mL) for 12 h at 110 °C.b Isolated yield.c ‘—’ presented no report yield from o-halobenzanilides. | ||||
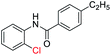
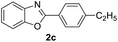
| 1 |  |
 |
89 | 78 (ref. 7a) |
| 2 | 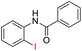 |
92 | 87 (ref. 7a) | |
| 3 | 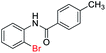 |
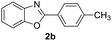 |
90 | 79 (ref. 8) |
| 4 | 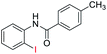 |
92 | 87 (ref. 8) | |
| 5 | 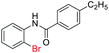 |
 |
81 | — |
| 6 | 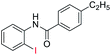 |
87 | — | |
| 7 | 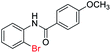 |
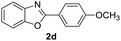 |
88 | 75 (ref. 8) |
| 8 | 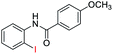 |
93 | 82 (ref. 8) | |
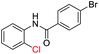
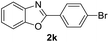
| 9 | 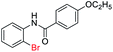 |
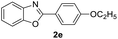 |
82 | — |
| 10 | 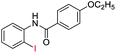 |
85 | — | |
| 11 | 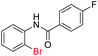 |
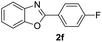 |
74 | 80 (ref. 7a) |
| 12 | 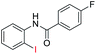 |
81 | 91 (ref. 7a) | |
| 13 | 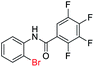 |
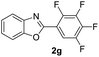 |
70 | — |
| 14 | 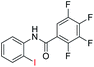 |
77 | — | |
| 15 | 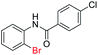 |
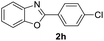 |
79 | 77 (ref. 8) |
| 16 | 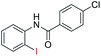 |
85 | 86 (ref. 8) | |
| 17 | 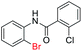 |
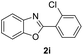 |
74 | — |
| 18 | 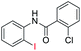 |
83 | — | |
| 19 | 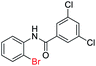 |
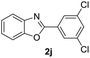 |
86 | — |
| 20 | 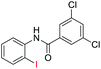 |
89 | — | |
| 21 | 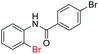 |
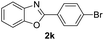 |
83 | 92 (ref. 6a) |
| 22 | 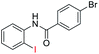 |
90 | — | |
| 23 | 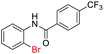 |
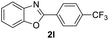 |
76 | — |
| 24 | 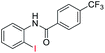 |
88 | — | |
| 25 | 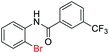 |
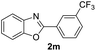 |
76 | — |
| 26 | 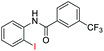 |
85 | — | |
| 27 | 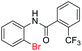 |
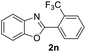 |
73 | — |
| 28 | 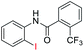 |
78 | — | |
| 29 | 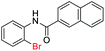 |
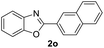 |
89 | — |
| 30 | 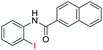 |
92 | — | |
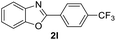
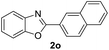
In addition, the intramolecular O-arylation of various ortho, meta and para substituted o-halobenzanilides were carried out using the Cu(OAc)2/DPPAP catalyst system. As shown in Table 2, all desired products were obtained in good yields, and position of both electron donating and withdrawing groups did not have obvious influence on applicability. It was gratified that N-(2-bromophenyl)-2,3,4,5-tetrafluorobenzamide, N-(2-iodophenyl)-2,3,4,5-tetrafluoro benzamide, N-(2-bromophenyl)-3,5-dichlorobenzamide and N-(2-iodophenyl)-3,5-dichlorobenzamide could afford the corresponding products 2g and 2j in good yields (70%, 77%, 86% and 89%, respectively) under optimal reaction conditions.
From above results, our improved protocol for synthesis of benzoxazoles by intramolecular O-arylation of o-halobenzanilides (Br, I) in water is significantly applicative under Cu/DPPAP catalyst system. Delighted with this results, we were eager to study O-arylation of 2-chlorobenzanilides. Therefore, six 2-chlorobenzanilides bearing different substituted groups were selected as the model reaction under standard conditions. As shown in Table 3, 2-chlorobenzanilides displayed poor reactivity under this conditions, and benzoxazoles were obtained in low yields (<30%), respectively.
In addition, we performed the reaction on a gram-scale by taking 10 mmol of N-(2-iodophenyl)-4-methoxybenzamide in 30 mL water at 110 °C for 12 h (Scheme 2). The reaction proceeded smoothly to form desired product 2d (2.05 g, 91% yield).
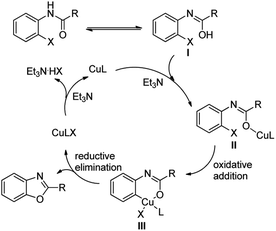
Although the mechanism of the present reaction was not yet clear, based on experimental results mentioned and copper-catalyzed C–O bond formations in the literature,6a,10 the most probable mechanism was proposed and depicted for the Cu-catalytic intramolecular O-arylation of o-halobenzanilides as shown in Scheme 3. Firstly, o-halobenzanilide converted to enol I, followed by coordination of the CuL catalyst and Et3N to give II. Subsequently, intermediate III was formed by oxidative insertion. After that, III underwent reductive elimination to give benzoxazole as well as CuLX. Finally, CuLX was transformed into CuL catalyst for reuse in presence of Et3N.
Conclusions
In conclusion, we have developed an efficient method for synthesis of benzoxazoles by Cu-catalyzed intramolecular O-arylation of o-halobenzanilides in water. This protocol is applicable to a wide variety of o-halobenzanilides bearing electron donating as well as electron withdrawing groups, which features high yields, water as green solvent and functional groups tolerable. The simple and green catalytic system provides a valuable synthetic approach for industrial applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81560620), the Yunnan Provincial Science and Technology Department-Applied Basic Research Joint Special Funds of Yunnan University of Chinese Medicine (2017FF117(-023)).Notes and references
- (a) S. M. Johnson, S. Connelly, I. A. Wilson and J. W. Kelly, J. Med. Chem., 2008, 51, 260 CrossRef CAS PubMed; (b) N. Mishra, A. S. Singh, A. K. Agrahari, S. K. Singh, M. Singh and V. K. Tiwari, ACS Comb. Sci., 2019, 21, 389 CrossRef CAS PubMed; (c) G. Daletos, N. J. de Voogd, W. E. G. Muller, V. Wray, W. Lin, D. Feger, M. Kubbutat, A. H. Aly and P. Proksch, J. Nat. Prod., 2014, 77, 218 CrossRef CAS PubMed; (d) S. Aiello, G. Wells, E. L. Stone, H. Kadri, R. Bazzi, D. R. Bell, M. F. G. Stevens, C. S. Matthews, T. D. Bradshaw and A. D. Westwell, J. Med. Chem., 2008, 51, 5135 CrossRef CAS PubMed.
- (a) L. Leventhal, M. R. Brandt, T. A. Cummons, M. J. Piesla, K. E. Rogers and H. A. Harris, Eur. J. Pharmacol., 2006, 553, 146 CrossRef CAS PubMed; (b) L. Q. Sun, J. Chen, M. Cruce, J. A. Deskus, J. R. Epperson, K. Takaki, G. Johnson, L. Iben, C. D. Malhe, E. Ryan and C. Xu, Bioorg. Med. Chem. Lett., 2004, 14, 3799 CrossRef CAS PubMed; (c) E. Oksuzoglu, O. Temiz-Arpaci, B. Tekiner-Gulbas, H. Eroglu, G. Sen, S. Alper, I. Yildiz, N. Diril, E. Aki-Sener and I. Yalcin, Med. Chem. Res., 2007, 16, 1 CAS; (d) J. Boyer, E. Arnoult, M. Médebielle, J. Guillemont and J. Unge, J. Med. Chem., 2011, 54, 7974 CrossRef CAS PubMed.
- (a) K. Bougrin, A. Loupy and M. Soufiaoui, Tetrahedron Lett., 1998, 54, 8055 CrossRef CAS; (b) J. Chang, K. Zhao and S. Pan, Tetrahedron Lett., 2002, 43, 951 CrossRef CAS; (c) K. Bougrin, A. Loupy and M. Soufiaoui, Tetrahedron, 1998, 54, 8055 CrossRef CAS; (d) S. M. Inamdar, V. K. More and S. K. Mandal, Tetrahedron Lett., 2013, 54, 579 CrossRef CAS; (e) Y. Kawashita, N. Nakamichi, H. Kawabata and M. Hayashi, Org. Lett., 2003, 5, 3713 CrossRef CAS PubMed.
- (a) N. Barbero, M. Carril, R. SanMartin and E. Domínguez, Tetrahedron, 2007, 63, 10425 CrossRef CAS; (b) S. Ueda and H. Nagasawa, Angew. Chem., Int. Ed., 2008, 47, 6411 CrossRef CAS PubMed; (c) N. Khatun, S. Guin, S. K. Rout and B. K. Patel, RSC Adv., 2014, 4, 10770 RSC; (d) J. Peng, C. Zong, M. Ye, T. Chen, D. Gao, Y. Wang and C. Chen, Org. Biomol. Chem., 2011, 9, 1225 RSC; (e) A. D. G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef PubMed; (f) A. Ahmed, R. Singha and J. K. Ray, Tetrahedron Lett., 2015, 56, 2167 CrossRef CAS; (g) X. Zhang, W. Zeng, Y. Yang, H. Huang and Y. Liang, Org. Lett., 2014, 16, 876 CrossRef CAS PubMed; (h) L. Xiao, H. Gao, J. Kong, G. Liu, X. Peng and S. Wang, Chin. J. Org. Chem., 2014, 34, 1048 CrossRef CAS.
- (a) S. Sasmal, I. Sen, R. G. Hall and S. Pal, Tetrahedron Lett., 2015, 56, 1374 CrossRef CAS; (b) Y. Yuan, I. Thomé, S. H. Kim, D. Chen, A. Beyer, J. Bonnamour, E. Zuidema, S. Chang and C. Bolm, Adv. Synth. Catal., 2010, 352, 2892 CrossRef CAS; (c) N. Barbero, M. Carril, R. SanMartin and E. Domínguez, Tetrahedron, 2007, 63, 10425 CrossRef CAS; (d) R. D. Viirre, G. Evindar and R. A. Batey, J. Org. Chem., 2008, 73, 3452 CrossRef CAS PubMed; (e) N. Barbero, M. Carril, R. SanMartin and E. Domínguez, Tetrahedron, 2007, 63, 10425 CrossRef CAS; (f) J. Sedelmeier, F. Lima, A. Litzler, B. Martin and F. Venturoni, Org. Lett., 2013, 15, 5546 CrossRef CAS PubMed; (g) D. Suresh, A. Dhakshinamoorthy and K. Pitchumani, Tetrahedron Lett., 2013, 54, 6415 CrossRef CAS.
- (a) G. Evindar and R. A. Batey, J. Org. Chem., 2006, 71, 1802 CrossRef CAS PubMed; (b) P. Saha, M. A. Ali, P. Ghosh and T. Punniyamurty, Org. Biomol.Chem., 2010, 8, 5692 RSC.
- (a) F. Wu, J. Zhang, Q. Wei, P. Liu, J. Xie, H. Jiang and B. Dai, Org. Biomol. Chem., 2014, 12, 9696 RSC; (b) J. Bonnamour and C. Bolm, Org. Lett., 2008, 10, 2665 CrossRef CAS PubMed.
- J. I. Urzúa, R. Contreras, C. O. Salas and R. A. Tapia, RSC Adv., 2016, 6, 82401 RSC.
- (a) K. Bougrin, A. Loupy and M. Soufiaoui, Tetrahedron, 1998, 54, 8055 CrossRef CAS; (b) G. Alenhoff and F. Glorius, Adv. Synth. Catal., 2004, 346, 1661 CrossRef; (c) J. Chang, K. Zhao and S. Pan, Tetrahedron Lett., 2002, 43, 951 CrossRef CAS; (d) M. Carril, R. SanMartin, I. Tellitu and E. Domínguez, Org. Lett., 2006, 8, 1467 CrossRef CAS PubMed.
- (a) G. D. Allred and L. S. Liebskind, J. Am. Chem. Soc., 1996, 118, 2748 CrossRef CAS; (b) E. R. Strieter, D. G. Blackmond and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4120 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and spectra data. See DOI: 10.1039/d0ra00570c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |