Open Access Article
Open Access ArticleLiquid photonic crystal detection reagent for reliable sensing of Cu2+ in water†
Yixin Zhang and
Jianping Ge *
*
School of Chemistry and Molecular Engineering, Shanghai Key Laboratory of Green Chemistry and Chemical Processes, East China Normal University, Shanghai 200062, China. E-mail: jpge@chem.ecnu.edu.cn; Tel: +86-21-62224356
First published on 17th March 2020
Abstract
A traditional hydrogel photonic crystal sensor is prepared by multiple steps, including colloidal assembly, polymerization, and recognition group modification, and its measurement repeatability is a challenge due to the inevitable deviations in sensor fabrication and application. In this work, a salicylic acid-containing “SiO2/propylene carbonate” liquid photonic crystal (Sal-LPC), as a new photonic sensing material, was developed to demonstrate reliable measurement of Cu2+ in water. When the Sal-LPC reagent was mixed with the test sample, the Cu2+ promoted the release of H+ from Sal and shrank the photonic crystal lattice, so that the Cu2+ concentration could be determined by the reflection blueshift of liquid PC. The Sal-LPC reagent showed a stronger response to Cu2+ than to other cations, and its sensitivity and measurement range could be optimized by the particle fraction and Sal dosage. Compared to traditional PC hydrogel sensors, the liquid PC reagent was composed of colloidal particles and responsive species, which required no strict control in synthesis. More importantly, the optical response of the liquid PC reagent was scarcely affected by changes in synthesis, storage, or application, and it could interact with the analyte quickly and quantitatively, which ensured accurate and repeatable measurement in either chemical analysis or environmental monitoring.
Introduction
Photonic crystals (PCs) are periodic dielectric structures which forbid the propagation of electromagnetic waves of certain frequency ranges. Once an external light source is projected onto the PC, it shows characteristic reflection, transmission, and structural colors due to the modulation of light by the photonic bandgap. A responsive photonic crystal (RPC) is one kind of smart PC material, which changes periodic dielectric structures and thereby optical properties along with a change in external stimuli.1–5 Due to the intrinsic relationship between external stimuli, PC structure, and optical signals, which form a basic framework for a sensing mechanism, these responsive photonic crystals have been broadly investigated as PC sensors. Many chemical or physical parameters in which people are interested have been measured, either qualitatively through color changes or quantitatively by shifts in reflection peaks. In the past decade, researchers have developed various PC sensors to detect temperature,6–8 mechanical force,9–11 viscosity,12 porosity,13 magnetic fields,14–16 humidity,17–20 pH value,21,22 ions,23–26 vapor,27–29 microbes,30 and biomolecules.31–34 Such enthusiasm should continue in the coming years, considering the practical applications of these materials.Among all PC sensors, a chemical sensor for the detection of ions and molecules has gained a lot of attention, not only because it was developed very early35–37 and studied extensively in history, but also because it was considered to be a prototype for other PC sensors. Generally, a PC-based ion sensor was composed of colloidal crystals embedded in hydrogels or inverse opaline hydrogels, whose polymer networks were covalently anchored with the recognition groups. Once the target ions entered the PC gels, they would react with the recognition group, shrink/expand the gel, and eventually lead to a change in reflection signals. For example, Asher et al. developed an 8-hydroxyquinoline modified PC hydrogel for the detection of Cu2+ in drinking water.38 As the Cu2+ concentration increased, the binary coordination complex between Cu2+ and 8-hydroxyquinoline changed to a univalent complex and the hydrogel swelled so that the Cu2+ concentration could be measured by the redshift of the reflection peak. In another instance, a crown ether grafted PC hydrogel was reported to detect the smallest metal ion Be2+ based on the selective chelation of crown ether and cations.39 The Be2+ concentration could be measured according to the reflection redshift of the PC hydrogel, as the chelation would promote the swelling of the hydrogel and thereby the expansion of the PC lattice. Although polymeric PC based chemical sensors had already been demonstrated through the detection of different ions and molecules, there were still disadvantages for the traditional PC sensor. The fabrication of PC hydrogel sensors usually required multiple steps, including colloidal assembly, hydrogel polymerization, and recognition group modification. The change in optical signals was affected by the loading amount of recognition group, the fixation of PC film on the substrate, and even the testing position. Therefore, precise and repeatable measurement results could be a challenge for the commercialization of these traditional sensors. In previous work, most studies focused on the sensitivity and measurement range, but very little attention was paid to repeatability among individual sensors.
In this work, we would like to demonstrate a new kind of liquid PC based chemical sensor through its detection of Cu2+ in water. A liquid PC was prepared by the thermal evaporation induced supersaturation of colloidal particles followed by their self-assembly in solution.40 Compared to traditional methods, this was an efficient way to obtain highly crystalline colloidal PCs at a large scale, which guarantees their application in related devices. Liquid PCs have a vast potential to serve as detection reagents since their optical signals are sensitive to many physicochemical parameters in solution, including particle volume fraction, pH value, ionic strength, solvent polarity, and viscosity. Here, a salicylic acid-containing “SiO2/propylene carbonate” liquid photonic crystal (Sal-LPC) is an accurately sensitive reagent to Cu2+ concentration, because the Cu2+ promotes the release of H+ from Sal and shrinks the photonic crystal lattice so that the Cu2+ concentration can be determined by the reflection blueshift of the liquid PC. The Sal-LPC reagent had a stronger response to Cu2+ than to other cations, and its measurement range could be optimized by the Sal dosage. Compared to traditional PC hydrogel sensors, the liquid PC reagent was composed of a mixed solution of colloidal particles and responsive species, which required no strict control over synthesis and modification of photonic crystals. More importantly, the optical response of the liquid PC reagent was scarcely affected by changes in synthesis, storage, or application, and it could interact with the analyte quickly and quantitatively, all of which ensured an accurate and repeatable response for this new sensing material.
Experimental
Materials
Tetraethylorthosilicate (TEOS, 98%), aqueous ammonia (NH3·H2O, 28%), salicylic acid (Sal), and zinc nitrate hexahydrate (Zn(NO3)2·6H2O) were purchased from Sinopharm Chemical Reagent Co. Ltd. Ethanol (EtOH, 99.9%) and copper nitrate trihydrate (Cu(NO3)2·3H2O) were purchased from J&K Co. Ltd. Propylene carbonate (PC, 99%), aluminum nitrate nonahydrate (Al(NO3)3·9H2O), cadmium nitrate tetrahydrate (Cd(NO3)2·4H2O), cobalt nitrate hexahydrate (Co(NO3)2·6H2O), magnesium nitrate hexahydrate (Mg(NO3)2·6H2O), lead nitrate (Pb(NO3)2) and nickel nitrate hexahydrate (Ni(NO3)2·6H2O) were all purchased from Aladdin Co. Ltd. Chromic nitrate nonahydrate (Cr(NO3)3·9H2O) and iron nitrate nonahydrate (Fe(NO3)3·9H2O) were all purchased from Sigma-Aldrich Co. Ltd. All chemicals were used as received without further treatment.Preparation of Sal-LPC detection reagent
Firstly, monodispersed SiO2 colloidal particles were prepared by a modified Stöber method. After washing with ethanol three times, SiO2 colloidal particles (0.025 cm3) were dispersed in ethanol (1 mL) to form a stable colloidal dispersion. Propylene carbonate (75 μL) and the ethanol solution of salicylic acid (0.5 mM, 10 μL) were then added to the above solution and mixed with it by a vortex mixer. Subsequently, the mixture was transferred to an oven at 90 °C for 2 h, where ethanol was evaporated to produce a salicylic acid-containing SiO2/PC liquid photonic crystal (Sal-LPC) detection reagent with a total volume of 100 μL. The preparations of Sal-LPC reagents with different Sal concentrations, particle volume fractions, and particle diameters were the same as in the above procedures, except for the indicated changes made in preparation.Working curves for measurement of Cu2+ concentration
The working curve for the measurement of Cu2+ concentration (CCu2+) was established by measuring the reflection wavelength change of the liquid PC reagent when it was mixed with an aqueous solution of Cu2+ at different concentrations. In a typical test, the Sal-LPC reagent (100 μL) was mixed with deionized water (10 μL) to form a homogenous suspension. The Sal-LPC reagent with deionized water was kept for 5 min to ensure complete interaction. Some of the above mixed solution (5 μL) was taken out and sandwiched between two glass slides to form a liquid film with a thickness of 100 μm. After being kept for 5 min, liquid colloidal PC spontaneously precipitated from the liquid film and its reflection wavelength was measured and recorded as λ0. On the other hand, the Sal-LPC reagent (100 μL) was mixed with an aqueous solution of Cu2+ (10 μL) to form another homogenous suspension and a new liquid PC, whose reflection wavelength was measured to be λ′. Then, the reflection wavelength change (Δλ = λ′ − λ0) caused by the introduction of Cu2+ could be determined according to the two experiments. Along with the change in CCu2+ in standard solution, Δλ was measured accordingly, which were used to plot a working curve (Δλ–c) for the measurement of Cu2+ concentration.Detection of Cu2+ concentration in aqueous solution
The Sal-LPC reagents (100 μL) were mixed with deionized water (10 μL) or an aqueous solution of Cu2+ (10 μL) to form two liquid PCs, respectively. The reflection wavelength change (Δλ) could be obtained by the same procedures mentioned above. Finally, the Cu2+ concentration could be determined from the working curve and Δλ.Characterizations
Digital photos of liquid PCs were taken with a Sony NEX-3N digital camera. The optical microscope images of liquid PC were taken with an Olympus BXFM reflection-type microscope operated in dark-field mode. The reflection spectra were measured with an Ocean Optics Maya 2000 Pro spectrometer with incident angle and detection angle fixed at 0°. The arrangement of particles in liquid photonic crystals (after drying) was observed with a Phenom G2 Pro scanning electron microscope. The pH was measured with a Mettler Toledo FiveEasy pH meter.Results and discussion
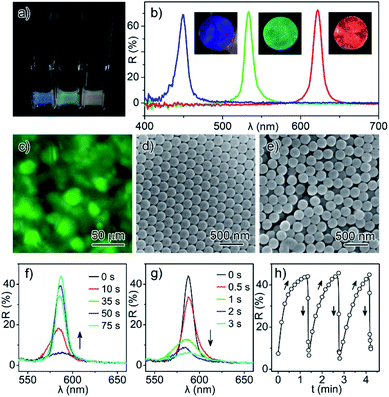
A liquid-form colloidal photonic crystal (PC), as the major sensing material, was first prepared by thermal evaporation induced supersaturation of colloidal particles followed by their self-assembly in solution.40 In a typical synthesis, ethanol was removed from the ethanol/propylene carbonate solution of the particles by heating, which drove the supersaturation of SiO2 particles in propylene carbonate and thereby the simultaneous precipitation of liquid PCs in a few minutes (Fig. 1a and b). With a change in particle size and the kind of high boiling point solvent, SiO2 based liquid photonic crystals with blue, green, or red structural colors could be successfully prepared (Fig. S1†). In this colloidal system, some of the particles were assembled into solvent-wrapped photonic crystals, and the rest of them underwent Brownian motion in solution, forming a microscale separation of crystal phase and amorphous phase, as indicated by the green and black domains in the optical microscope image (Fig. 1c). When the dried liquid PCs were observed by SEM, one could also find the ordered and disordered particle arrangements corresponding to these two phases (Fig. 1d and e). The coexistence of crystal and amorphous phases, as well as the metastable structure, gave the liquid PCs reversible assembly and disassembly characteristics, which could be proved by the periodic change in reflection intensities (Fig. 1f–h). Under weak external interference, the liquid PCs would disassemble in 3 seconds along with the disappearance of the reflection signal, because the SiO2 particles in the colloidal crystals were wrapped in solvent but not fixed as the solid PC. However, once the supersaturated colloidal solution was left undisturbed for a few minutes, the liquid PCs would assemble again and recover the reflection signal to the maximum value.The liquid PC was suitable to serve as a detection reagent due to its sensitive response to multiple physicochemical parameters, flexible introduction of various detection functions, and integration of sensing and expression. First of all, the liquid PCs were sensitive to the physical properties and the chemical environment of their precursor solution. Any change in particle concentration, pH value, ionic strength, solvent polarity, and viscosity etc. would change the structure of colloidal crystals and the reflection signals, which meant the liquid PCs were potentially usable as a liquid-form sensing material. Secondly, various detection functions could be introduced to the liquid PC system via the addition of responsive components, which would be more convenient and controllable than the chemical grafting of recognition groups in traditional hydrogel PC sensors. Thirdly, the reversible assembly and disassembly of the liquid PC system realized an excellent integration of efficient sensing and accurate expression. In the state of the supersaturated colloid solution, the detection reagent could fully interact with the analyte, which guaranteed a quick arrival at the equilibrium state and repeatable results from parallel tests. While, in the state of colloidal crystals, the detection reagent showed the characteristic reflection signals for the physical/chemical stimuli, so that one could acquire the related information of reflection wavelength shift or intensity changes. In this work, a salicylic acid-containing “SiO2/propylene carbonate” liquid photonic crystal (Sal-LPC) for the detection of Cu2+ cations was developed to demonstrate the feasibility of this new sensing material. Although the detection of various ions and molecules by PC sensors had been reported in the literature, this was indeed the first time it was proved in a liquid PC system.
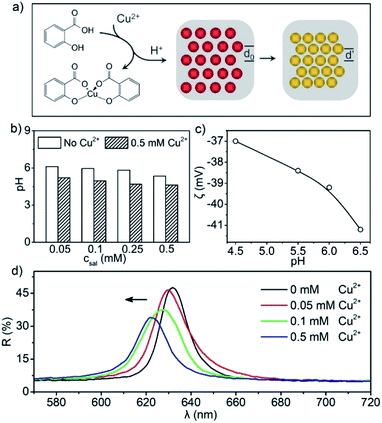
Through experimental investigations, the sensing of Cu2+ by a Sal-LPC detection reagent was found to be realized based on the response delivery among the Cu2+ concentration, pH value, particle surface charge, lattice constant, and reflection signals (Fig. 2a). It should be mentioned that the introduction of Sal to liquid PC would not destroy the photonic structure, which made it usable for Cu2+ sensing (Fig. S2†). When the aqueous solution of Cu2+ was mixed with the Sal-LPC reagent, a Cu(Sal)2 complex formed along with the release of H+ into the solution. This could be verified by the decrease in pH value in parallel experiments where an aqueous solution of Cu2+ was added to the ethanol solution of Sal (Fig. 2b). The released H+ then inhibited the deprotonation of the silanol group (Si–OH) on the surface of SiO2 particles and weakened its surface charge. Along with the decrease in pH value from 6.5 to 4.5, the zeta potential of the SiO2 particles changed from −41 to −37 mV (Fig. 2c). Since the colloidal assembly was closely related to the electrostatic repulsion between SiO2 particles, the decrease in surface charge would eventually lead to a shrinkage in the photonic crystal lattice and a blueshift of the reflection peak. Therefore, as the Cu2+ concentration increased from 0 to 0.5 mM, the reflection peak of Sal-LPC did blueshift as expected (Fig. 2d). It should be noted that detailed information about the Sal-LPC reagents and the Cu2+ solution for every test in this manuscript was summarized in Table S1.† The increase in Cu2+ concentration would increase the ionic strength of the solution, which might also cause a decrease in particle surface charge and reflection blueshift. However, for relatively low concentrations of Cu2+, the blueshift of the liquid PC (without Sal) caused by the addition of Cu2+ (Fig. S3†) was much smaller than the blueshift in the above working mechanism, which excluded the interference of Cu2+ itself and proved that the reflection blueshift was caused by the reaction between Sal and Cu2+.
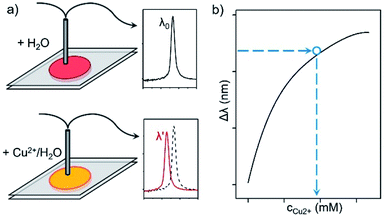
For a typical detection, the concentration of Cu2+ was determined by the measurement of reflection blueshift of the liquid PC, followed by a transformation according to the “c–Δλ” working curve (Fig. 3). In order to avoid the dilution-induced reflection wavelength change, deionized water with the same volume as the Cu2+ solution was first mixed with the Sal-LPC reagent, whose reflection wavelength was measured and recorded as λ0. Similarly, a Cu2+ solution of unknown concentration was mixed with the Sal-LPC reagent, which gave a new reflection wavelength (λ′). According to the reflection blueshift, the Cu2+ concentration could finally be determined from a pre-plotted “c–Δλ” curve. The working curve for the LPC reagent could be achieved by the same experiments as above except for the use of standard Cu2+ solutions of known concentrations. Since the working curve could indicate the sensitivity and the measurement range of the LPC reagent, our investigation started from these curves in order to optimize the optical response to Cu2+.
 | ||
| Fig. 3 Measuring the Cu2+ concentration according to a calibrated “Δλ–c” working curve and the measurement of “Δλ” between tested and blank samples. | ||
The influence of Sal dosage was studied first as Sal molecules were the responsive component in the detection reagent. In the practical experiments, we had prepared Sal-LPC reagents with Sal concentrations of 0.05, 0.1, 0.25, and 0.5 mmol L−1 and studied their response to Cu2+ ions with concentrations from 0 to 0.5 mM (Fig. 4). The detection range was selected as 0–0.5 mM because industrial wastewater usually contains Cu2+ with concentrations of 15–30 mg mL−1 (0.24–0.47 mM), and it is not allowed to be poured into rivers and lakes unless the concentration had been decreased to 2 mg mL−1 (0.03 mM). All experiments showed the same result that the reflection peak blueshifted along with an increase in Cu2+ concentration. Taking the Sal-LPC reagent containing 0.25 mmol L−1 Sal as a typical example, its reflection peak changed from 652 nm to 632 nm as the Cu2+ concentration increased from 0 to 0.5 mmol L−1. After organizing all the data into “Δλ–c” curves, one could see that the reflection blueshift (−Δλ) was always fast at the beginning and became slower afterward (Fig. 5). The inflection points were all around the stoichiometry point where the molar ratio of Cu2+ and Sal was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 except for the last case. Before the inflection point, H+ cations were stoichiometrically produced along with the addition of Cu2+ and the gradual formation of Cu(Sal)22− and CuSal, so that the reflection blueshift was sensitive to concentration of Cu2+. After the inflection point, H+ cations were produced due to the addition of excessive Cu2+ and the movement of chemical equilibrium towards the formation of Cu–Sal, so that the reflection blueshift became slower. According to our experiments, the reflection blueshift could respond monotonously to Cu2+ concentration until the Cu2+/Sal ratio reached 2
1 except for the last case. Before the inflection point, H+ cations were stoichiometrically produced along with the addition of Cu2+ and the gradual formation of Cu(Sal)22− and CuSal, so that the reflection blueshift was sensitive to concentration of Cu2+. After the inflection point, H+ cations were produced due to the addition of excessive Cu2+ and the movement of chemical equilibrium towards the formation of Cu–Sal, so that the reflection blueshift became slower. According to our experiments, the reflection blueshift could respond monotonously to Cu2+ concentration until the Cu2+/Sal ratio reached 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
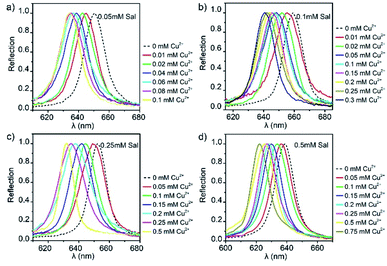 | ||
| Fig. 4 Optical response of liquid PCs containing (a) 0.05 mM, (b) 0.1 mM, (c) 0.25 mM, and (d) 0.5 mM Sal to the change in Cu2+ concentration. | ||
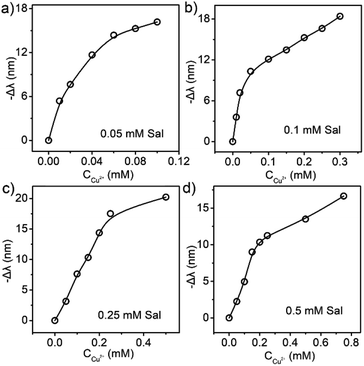 | ||
| Fig. 5 The relationship between the Cu2+ concentration and the reflection blueshift of liquid PCs containing (a) 0.05 mM, (b) 0.1 mM, (c) 0.25 mM, and (d) 0.5 mM Sal. | ||
Through the control of Sal concentration, the Sal-LPC reagent was able to measure the concentration of Cu2+ aqueous solution in different ranges. Four groups of parallel experiments showed that the measurement ranges of Cu2+ concentration were 0–0.1 mM, 0–0.3 mM, 0–0.5 mM, and 0–0.75 mM for Sal-LPC reagents with Sal dosages of 0.05 mM, 0.1 mM, 0.25 mM, and 0.5 mM. The maximum reflection blueshifts were measured to be 16 nm, 18.4 nm, 20.2 nm, and 16.6 nm, respectively (Fig. 5). Higher Sal dosage favored the detection of Cu2+ of higher concentrations because the Cu2+-induced release of H+ and reflection blueshift required a matched supply of Sal. However, a very high dosage of Sal might lead to a threshold of maximum sensible Cu2+ concentration, as the large amount of released H+ would weaken the response of Sal-LPC to a further supply of H+.
In addition to the Sal dosage, the volume fraction of SiO2 particles, which determined the initial lattice constant of the photonic crystals, was found to be critical to the sensitivity of the Sal-LPC reagent. Here, SiO2 particles with volume fractions of 20%, 25%, 28%, and 30% and Sal with a concentration of 0.25 mM were mixed with propylene carbonate to form 4 kinds of Sal-LPC reagents, which were used to detect Cu2+ with concentrations ranging from 0 mM to 0.5 mM (Fig. S4†). Similar to the Sal control experiments, all the reflection blueshifts increased monotonously with the increasing Cu2+ concentration, and the increase became slower when the Cu2+/Sal ratio reached 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 6). However, the overall reflection blueshift, which determined the sensitivity, showed a volcano trend with the particle volume fraction. The reflection blueshifts of Sal-LPC reagents with particle volume fractions of 20%, 25%, 28%, and 30% in response to 0.5 mM Cu2+ solution were measured to be 14 nm, 20 nm, 13 nm, and 8 nm, respectively. Generally, a relatively lower volume fraction of particles would provide a larger particle interspacing in the liquid PCs at the beginning, which left more space for lattice shrinkage and a larger reflection blueshift in response to the same Cu2+ solution. However, a very low particle fraction of Sal-LPC, such as 20%, would decrease its response to Cu2+, possibly due to the short supply of Si–OH in the system. Our experiments revealed that the Sal-LPC reagent with a particle volume fraction of 25% showed the best sensitivity in response to the Cu2+ solution.
1 (Fig. 6). However, the overall reflection blueshift, which determined the sensitivity, showed a volcano trend with the particle volume fraction. The reflection blueshifts of Sal-LPC reagents with particle volume fractions of 20%, 25%, 28%, and 30% in response to 0.5 mM Cu2+ solution were measured to be 14 nm, 20 nm, 13 nm, and 8 nm, respectively. Generally, a relatively lower volume fraction of particles would provide a larger particle interspacing in the liquid PCs at the beginning, which left more space for lattice shrinkage and a larger reflection blueshift in response to the same Cu2+ solution. However, a very low particle fraction of Sal-LPC, such as 20%, would decrease its response to Cu2+, possibly due to the short supply of Si–OH in the system. Our experiments revealed that the Sal-LPC reagent with a particle volume fraction of 25% showed the best sensitivity in response to the Cu2+ solution.
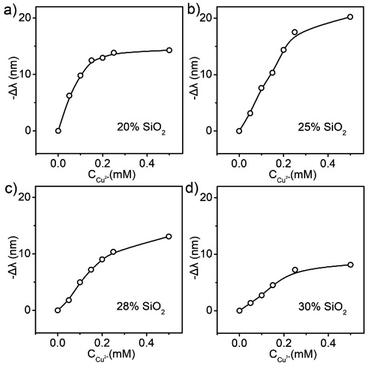 | ||
| Fig. 6 The relationship between the Cu2+ concentration and the reflection blueshift of liquid PCs with particle volume fractions of (a) 20%, (b) 25%, (c) 28%, and (d) 30%. | ||
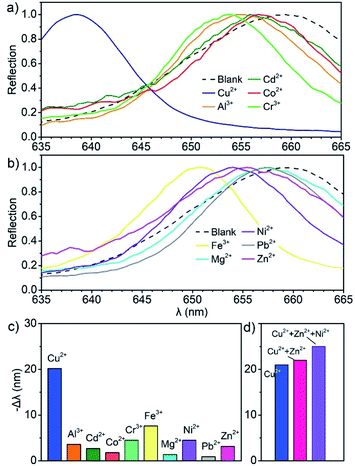
The Sal-LPC reagent showed a sensitive response to Cu2+ due to the great tendency to form a Cu–Sal complex in the liquid PC environment. In a comparative experiment, the Sal-LPC reagent with an SiO2 particle fraction of 25% and Sal concentration of 0.25 mM was used to detect aqueous solutions of Cu2+, Al3+, Cd2+, Co2+, Cr3+, Fe3+, Mg2+, Ni2+, Pb2+, and Zn2+ with concentrations of 0.5 mM (Fig. 7a–c). The reflection blueshift of the Sal-LPC reagent was 20 nm in response to Cu2+, while those in response to the other metal cations were all below 4.5 nm except for the response to Fe3+ at 7.6 nm. The stronger response of the Sal-LPC reagent to Cu2+ could be explained by the large formation constant of the Cu–Sal complex (Table S2†), which promoted the disassociation of protons from Sal and the lattice shrinkage of PC structures, and thereby the blueshift of the reflection signals. It was unusual that the reflection blueshift of Sal-LPC in response to Fe3+ and Al3+ were not large, considering that the 1st formation constants of Fe–Sal and Al–Sal complexes are even larger than that of Cu2+. Further studies indicated that the introduction of Fe3+ and Al3+ in a water-containing solution did lead to the generation of more H+ ions. However, in a less aqueous solution, Cu2+ promoted the release of more H+ (Table S3†). Thanks to the strong response to Cu2+, the Sal-LPC reagent was able to detect Cu2+ with disturbance from other metal ions (Fig. 7d). In the presence of 0.5 mM Zn2+, the reflection blueshift of Sal-LPC in response to 0.5 mM Cu2+ was 22 nm, which showed a 4.7% deviation from the response to pure Cu2+ solution. While, with the coexistence of 0.5 mM Zn2+ and 0.5 mM Ni2+, the reflection blueshift was measured to be 25 nm, and the deviation reached 19%.
The liquid PC based detection reagent showed a highly repeatable response and good reliability in the practical tests. Traditional PC sensors for ions and molecules are usually fabricated through the modification of photonic crystal hydrogels with functional groups, which is effective in changing the photonic structures under different chemical stimuli. However, a repeatable response could be a challenge, considering the difficulty in controlling the loading amount of responsive groups, and the inevitable differences due to storage, fixation of hydrogel on the substrate, and the spectra recording positions. Unlike traditional PC sensors, the liquid PC detection reagent has intrinsic advantages in producing repeatable responses, because the analyte and the recognition species are mixed thoroughly and interact quantitatively, bypassing the complicated synthesis procedures and directly reporting precise results.
Here, the highly repeatable response was demonstrated by four groups of comparative measurements, which utilized the Sal-LPC reagents from different synthetic batches or composed of SiO2 particles with different sizes, as well as the same reagent after storage for several days or at different humidities (Fig. S5–S8†). In these experiments, all the Sal-LPC reagents contained 25% of SiO2 particles and 0.25 mM of Sal, and their responses to 0–0.5 mM of Cu2+ aqueous solution were compared to study the repeatability (Table S1†). Similar to the investigation of the influence of Sal, the original reflection spectra were first converted to reflection wavelength blueshifts, whose deviations from the average results of all the tests were then plotted as a function of Cu2+ concentration (Fig. 8). The scatter plots indicated that the errors were well controlled below 5% over most measurement ranges, except for the case of low Cu2+ concentration. For a specific Sal-LPC reagent with a Sal concentration of c, its measurement ranges of Cu2+ concentration usually started from 0 mM to 2c mM, where the reflection blueshift accordingly changed from 0 nm to about 20 nm. When the Cu2+ concentration to be analysed was low, the absolute value of Δλ was very small and close to the resolution of the spectrometer, so that the measurement error reached 10% to 20%. Although such inaccuracy was an intrinsic characteristic of the LPC reagent, the detection of low Cu2+ concentration could be realized by using a Sal-LPC reagent with a lower Sal dosage, which improved the accuracy in an alternative way. The comparative experiments showed that the Sal-LPC reagents prepared in different batches from the same source materials gave very close results. Replacing the SiO2 particles with larger/smaller particles changed the original reflection peak of Sal-LPC, but it did not influence the optical response determined by the reflection blueshift. Furthermore, such LPC reagents were less affected by a change in humidity, and the measurements on different days after storage were also identical. Therefore, the Sal-LPC reagent had a long shelf life and good stability, which ensured the precise measurements.
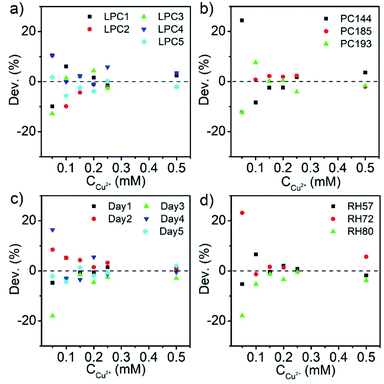 | ||
| Fig. 8 Deviation of “−Δλ” measured by (a) Sal-LPC prepared in different batches, (b) Sal-LPC prepared by different-sized particles, and the same Sal-LPC (c) in 5 days or (d) at different humidities. | ||
Conclusion
In summary, a salicylic acid-containing “SiO2/propylene carbonate” liquid photonic crystal (Sal-LPC) reagent for the detection of Cu2+ was developed to demonstrate a new PC based sensing material. When the Sal-LPC reagent was mixed with the Cu2+ solution, the Cu2+ would promote the release of H+ from Sal and shrink the lattice constant of liquid PC, so that the Cu2+ concentration could be determined by the reflection blueshift of liquid PC. The measurement range and the sensitivity of the Sal-LPC reagent could be adjusted through control of the Sal dosage and SiO2 particle volume fraction. The Sal-LPC reagent showed a stronger response to Cu2+ rather than other cations due to the formation of a more stable Cu–Sal complex in the liquid PC environment. More importantly, the Sal-LPC reagents possessed a highly repeatable response, no matter whether the reagent was prepared in different batches with different-sized particles or used under moist conditions after storage. Compared to the traditional PC hydrogel sensors, the liquid PC system studied in this work proposes a new strategy to design a photonic crystal based sensing material. It could be prepared without complicated procedures for PC assembly or modification of functional groups, and it was able to interact thoroughly with the analyte through mixing to produce reproducible and reliable results, both of which proved its potential in chemical analysis and environmental monitoring.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. P. Ge thanks the funds from the National Natural Science Foundation of China (21671067, 21972046).References
- C. Fenzl, T. Hirsch and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2014, 53, 3318–3335 CrossRef CAS PubMed
.
- J. Hou, M. Li and Y. Song, Nano Today, 2018, 22, 132–144 CrossRef CAS
.
- M. Wang and Y. Yin, J. Am. Chem. Soc., 2016, 138, 6315–6323 CrossRef CAS PubMed
.
- Y. Zhao, L. Shang, Y. Cheng and Z. Gu, Acc. Chem. Res., 2014, 47, 3632–3642 CrossRef CAS PubMed
.
- F. A. Harraz, Sens. Actuators, B, 2014, 202, 897–912 CrossRef CAS
.
- F. Liu, S. Zhang, X. Jin, W. Wang and B. Tang, ACS Appl. Mater. Interfaces, 2019, 11, 39125–39131 CrossRef CAS PubMed
.
- S.-J. Jeon, M. C. Chiappelli and R. C. Hayward, Adv. Funct. Mater., 2016, 26, 722–728 CrossRef CAS
.
- M. Chen, L. Zhou, Y. Guan and Y. Zhang, Angew. Chem., Int. Ed., 2013, 52, 9961–9965 CrossRef CAS PubMed
.
- Y. Cho, S. Y. Lee, L. Ellerthorpe, G. Feng, G. Lin, G. Wu, J. Yin and S. Yang, Adv. Funct. Mater., 2015, 25, 6041–6049 CrossRef CAS
.
- D. Yang, S. Ye and J. Ge, Adv. Funct. Mater., 2014, 24, 3197–3205 CrossRef CAS
.
- W. Lu, H. Li, B. Huo, Z. Meng, M. Xue, L. Qiu, S. Ma, Z. Yan, C. Piao and X. Ma, Sens. Actuators, B, 2016, 234, 527–533 CrossRef CAS
.
- Y. Zhang, Q. Fu and J. Ge, Small, 2017, 13, 1603351 CrossRef PubMed
.
- B. Zhu, Q. Fu, K. Chen and J. Ge, Angew. Chem., Int. Ed., 2018, 57, 252–256 CrossRef CAS PubMed
.
- Y. Zhang, Y. Jiang, X. Wu and J. Ge, J. Mater. Chem. C, 2017, 5, 9288–9295 RSC
.
- S. Zhang, C. Li, Y. Yu, Z. Zhu, W. Zhang, R. Tang, W. Sun, W. Xie, Y. Li, J. Yu, L. He and X. Zhang, J. Mater. Chem. C, 2018, 6, 5528–5535 RSC
.
- H. Wang, S. Yang, S.-N. Yin, L. Chen and S. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 8827–8833 CrossRef CAS PubMed
.
- D. Kou, W. Ma, S. Zhang, J. L. Lutkenhaus and B. Tang, ACS Appl. Mater. Interfaces, 2018, 10, 41645–41654 CrossRef CAS PubMed
.
- S. Kim, S. G. Han, Y. G. Koh, H. Lee and W. Lee, Sensors, 2018, 18, 1357 CrossRef PubMed
.
- T. Lu, S. Zhu, Z. Chen, W. Wang, W. Zhang and D. Zhang, Nanoscale, 2016, 8, 10316–10322 RSC
.
- Y. Y. Diao, X. Y. Liu, G. W. Toh, L. Shi and J. Zi, Adv. Funct. Mater., 2013, 23, 5373–5380 CrossRef CAS
.
- Q. Yang, S. Zhu, W. Peng, C. Yin, W. Wang, J. Gu, W. Zhang, J. Ma, T. Deng, C. Feng and D. Zhang, ACS Nano, 2013, 7, 4911–4918 CrossRef CAS PubMed
.
- C. P. Tsangarides, A. K. Yetisen, F. d. C. Vasconcellos, Y. Montelongo, M. M. Qasim, T. D. Wilkinson, C. R. Lowe and H. Butt, RSC Adv., 2014, 4, 10454–10461 RSC
.
- W. Liu, L. Li, S. Liu, B. Liu, Z. Wu and J. Deng, J. Mater. Chem. C, 2019, 7, 8946–8953 RSC
.
- J. Qin, B. Dong, X. Li, J. Han, R. Gao, G. Su, L. Cao and W. Wang, J. Mater. Chem. C, 2017, 5, 8482–8488 RSC
.
- S. Liu, L. Qin, Z. Ni and M. Chen, Anal. Methods, 2017, 9, 5791–5796 RSC
.
- M. Moirangthem, R. Arts, M. Merkx and A. P. H. J. Schenning, Adv. Funct. Mater., 2016, 26, 1154–1160 CrossRef CAS
.
- C. Liu, C. Yao, Y. Zhu, J. Ren, K. Lan, H. Peng and L. Ge, RSC Adv., 2014, 4, 27281–27285 RSC
.
- Y. Zhang, Y. Sun, J. Lid, P. Guo, Z. Cai and J.-J. Wang, Sens. Actuators, B, 2019, 291, 67–73 CrossRef CAS
.
- C.-S. Wu, P.-Y. Tsai, T.-Y. Wang, E.-L. Lin, Y.-C. Huang and Y.-W. Chiang, Anal. Chem., 2018, 90, 4847–4855 CrossRef CAS PubMed
.
- Z. Cai, D. H. Kwak, D. Punihaole, Z. Hong, S. S. Velankar, X. Liu and S. A. Asher, Angew. Chem., Int. Ed., 2015, 54, 13036–13040 CrossRef CAS PubMed
.
- X. Hu, Y. Wang, H. Liu, J. Wang, Y. Tan, F. Wang, Q. Yuan and W. Tan, Chem. Sci., 2017, 8, 466–472 RSC
.
- G. Li, F. Xiao, S. Liao, Q. Chen, J. Zhou, Z. Wu and R. Yu, Sens. Actuators, B, 2018, 277, 591–597 CrossRef CAS
.
- M. Qin, Y. Huang, Y. Li, M. Su, B. Chen, H. Sun, P. Yong, C. Ye, F. Li and Y. Song, Angew. Chem., Int. Ed., 2016, 55, 6911–6914 CrossRef CAS PubMed
.
- W. Liu, L. Shang, F. Zheng, J. Lu, J. Qian, Y. Zhao and Z. Gu, Small, 2014, 10, 88–93 CrossRef CAS PubMed
.
- J. H. Holtz and S. A. Asher, Nature, 1997, 389, 829–832 CrossRef CAS PubMed
.
- S. A. Asher, J. Holtz, J. Weissman and G. S. Pan, MRS Bull., 1998, 23, 44–50 CrossRef CAS
.
- Y. N. Xia, B. Gates, Y. D. Yin and Y. Lu, Adv. Mater., 2000, 12, 693–713 CrossRef CAS
.
- S. A. Asher, A. C. Sharma, A. V. Goponenko and M. M. Ward, Anal. Chem., 2003, 75, 1676–1683 CrossRef CAS PubMed
.
- J. Qin, B. Dong, L. Cao and W. Wang, J. Mater. Chem. C, 2018, 6, 4234–4242 RSC
.
- D. Yang, S. Ye and J. Ge, J. Am. Chem. Soc., 2013, 135, 18370–18376 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: OM and SEM images of liquid PCs, influence of Cu2+ or Sal upon reflection signals, reflection blueshift of liquid PCs with different particle volume fraction, reproducibility in measurements of reflection blueshift, the composition of all liquid PCs in this work, the formation constant of metal–salicylic acid complex, and the disassociation of Sal promoted by metal cation. See DOI: 10.1039/d0ra01014f |
| This journal is © The Royal Society of Chemistry 2020 |