Open Access Article
Open Access ArticleSynthesis of plasmonic Fe/Al nanoparticles in ionic liquids†
Alexa Schmitz a,
Hajo Meyerb,
Michael Meischeinb,
Alba Garzón Manjón
a,
Hajo Meyerb,
Michael Meischeinb,
Alba Garzón Manjón c,
Laura Schmolkea,
Beatriz Giesen
c,
Laura Schmolkea,
Beatriz Giesen a,
Carsten Schlüsenera,
Paul Simond,
Yuri Grind,
Roland A. Fischer
a,
Carsten Schlüsenera,
Paul Simond,
Yuri Grind,
Roland A. Fischer e,
Christina Scheu
e,
Christina Scheu c,
Alfred Ludwig
c,
Alfred Ludwig b and
Christoph Janiak
b and
Christoph Janiak *a
*a
aInstitut für Anorganische Chemie und Strukturchemie, Heinrich-Heine-Universität Düsseldorf, 40204 Düsseldorf, Germany. E-mail: janiak@uni-duesseldorf.de; Fax: +49-211-81-12287; Tel: +49-211-81-12286
bMaterials Discovery and Interfaces, Institut für Werkstoffe, Fakultät für Maschinenbau, Ruhr-Universität Bochum, Universitätsstr. 150, D-44801 Bochum, Germany
cMax-Planck-Institut für Eisenforschung GmbH, Max-Planck-Straße 1, D-40237 Düsseldorf, Germany
dMax-Planck-Institut für Chemische Physik fester Stoffe, Nöthnitzer Straße 40, D-01187 Dresden, Germany
eDepartment of Chemistry, Technische Universität München, D-85748 Garching, Germany
First published on 31st March 2020
Abstract
Bottom-up and top-down approaches are described for the challenging synthesis of Fe/Al nanoparticles (NPs) in ionic liquids (ILs) under mild conditions. The crystalline phase and morphology of the metal nanoparticles synthesized in three different ionic liquids were identified by powder X-ray diffractometry (PXRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), selected-area electron diffraction (SAED) and fast Fourier transform (FFT) of high-resolution TEM images. Characterization was completed by scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDX) for the analysis of the element composition of the whole sample consisting of the NPs and the amorphous background. The bottom-up approaches resulted in crystalline FeAl NPs on an amorphous background. The top-down approach revealed small NPs and could be identified as Fe4Al13 NPs which in the IL [OPy][NTf2] yield two absorption bands in the green-blue to green spectral region at 475 and 520 nm which give rise to a complementary red color, akin to appropriate Au NPs.
Introduction
Bimetallic or alloyed nanoparticles (NPs) have attracted increasing interest for a wide range of applications,1–8 especially showing tuneable properties in terms of their catalytic behaviour.9 However, most of the studied bimetallic NPs are based on the combination of noble or coinage metals and non-noble transition metals (e.g. Pd/Ga,10 Cu/Fe,11 Pt/Fe,12 Ni/Fe,13 Pd/Fe12). The main disadvantage of these combinations is the high costs associated with the use of the noble metals, leading to a strong scientific and industrial desire towards non-noble metal-based catalysts. Accordingly, Cokoja et al. described the synthesis of non-noble bimetallic NPs, e.g. CoAl,14 NiAl,15 CuAlx (x = 1, 2)16 and CuE2 (E = Al, Ga)17 by the soft wet-chemical hydrogenolysis of organometallic precursors. Schütte et al. reported the synthesis of Ni/Ga18 and Cu/Zn19 NPs which were shown to be active catalysts for the semihydrogenation of alkynes and methanol formation, respectively. Recently, Schmolke et al. also showed the application of intermetallic Co/Al NPs in alkyne hydrogenation.20 With regard to potentially low-cost nano catalysts, iron aluminide NPs represent a particularly desirable option given that Fe and Al are both cheap and ubiquitous metals. Furthermore, Armbrüster et al. have already shown that bulk Fe4Al13 presents a possible low-cost alternative for Pd in the heterogenous semihydrogenation of acetylene, referring to high conversion and a very high ethylene-selectivity, which was only 6% lower than that of an industrial benchmark catalyst.21Furthermore, bulk FeAl and Fe3Al alloys are well known for their good corrosion, sulfidation, carburization resistance,22,23 making them a sought-after material for aggressive and corrosive environments.24 The corrosion resistance is caused by an oxide layer on their surface, thus protecting the bimetallic core from oxidation.14 This amorphous oxide layer mostly consists of alumina and iron oxide.25 Potential applications for Fe/Al alloys include heating elements, insulation, tools, textiles, use as pipe material and hot gas filtration.24 Yet, further applications of Fe/Al materials are very limited as they exhibit a poor room-temperature ductility, low high-temperature strength and strong embrittlement caused by reactions with water vapor.24
Kim et al. have shown that FeAl NPs can positively influence the otherwise low ductility of aluminum steel by using them as a second phase in the Al-rich steel when precipitated at grain boundaries and shear bands. As a result, the steel becomes stronger because the NPs facilitate shearing akin to a ball bearing.26 Deevi et al. have shown that in microstructured alloys with the size of 4–10 μm the mechanical properties of the material can be enhanced in terms of room-temperature ductility and high temperature strength.27 Accordingly, these results as well as catalytic applications of iron aluminides indicate a high interest in the systematic synthesis of well-defined Fe/Al NPs.
In general, NPs can be synthesized either by bottom-up or top-down processes. Bottom-up processes, in which NPs are generated by the reduction or decomposition of metal precursors, allow to achieve a variety of different NPs28–32 but are accompanied by the drawback of by-products from the decomposition.33 In the case of iron aluminide NPs an additional fundamental problem of such a synthetic approach is the formation of stable iron-centered clusters even at low Fe concentration, which suppresses the nucleation process and thus, the growth of crystalline particles.34 Therefore, even larger concentrations of Fe led to amorphous nanomaterials, which may be interesting in terms of catalysis but are much more difficult to analyze.34 Dutta et al. and Pithawalla et al., who described the wet-chemical synthesis of FeAl or Fe3AlC0.5 nanoparticles by reducing anhydrous iron(II) chloride with lithium aluminum hydride, showed that a subsequent thermal treatment at temperatures of over 500 °C is required for the crystallization of the initial amorphous material.35,36
Therefore, ball-milling, as a top-down process, is the most reported way for the preparation of Fe/Al NPs.36,37 This rather time-consuming method, however, leads to a relatively high number of defects and an often very wide particle size distribution in addition to particle impurities.22,37 As a result, other synthetic methods have to be used to achieve high quality Fe/Al NPs. An example of this is the laser vaporization-controlled condensation (LVCC) method. Here, a macroscopic Fe/Al alloy is vaporized with a laser and subsequently, the vapor is condensed in a controlled manner in order to achieve a narrow particle size distribution and high purity.22,35 For smaller batch sizes on a laboratory scale, the LVCC method is well suited, but not for desired larger industrial scales.36 Hence, the controlled synthesis of defined Fe/Al NPs, possibly even as dispersion in a liquid medium, remains a challenge.
In this work we aimed for the synthesis of Fe/Al NPs via bottom-up and top-down processes, whereby the synthesis was performed either by soft wet-chemical reaction of organometallic precursors in ionic liquids (ILs) or by co-sputter deposition from respective metal targets into the ILs. The use of ILs for the stabilization of NPs as dispersion has proven to be promising in numerous cases.38–43 ILs offer unique stabilizing properties for NPs due to their high polarity, dielectric constant, thermal stability, relatively low chemical reactivity.44–46 Especially since no further additives (ligands, polymers, surfactants) are needed for the stabilization, and their negligible vapor pressure, ILs enable the sputter deposition of multinary NPs.47
Experimental section
Materials
All synthesis experiments were carried out under nitrogen or argon atmosphere using Schlenk techniques, since the organometallic precursors and the NPs are air and moisture sensitive. Solvents were dried using an MBRAUN solvent purification system and stored over molecular sieves. The acetonitrile (ACN) used for the NP precipitation was dried, degassed and stored under nitrogen. Lithium aluminum hydride (95%), lithium bis(trimethylsilyl)amide, iron(II) chloride (98%) and iron pentacarbonyl were obtained from Sigma Aldrich, diiron nonacarbonyl (99%) from abcr chemicals. All commercial chemicals were used without further purification. The precursors (AlCp*)4 (ref. 48) and (CO)4FeAlCp* (ref. 49) were prepared according to literature.| Ionic liquid [g] | Fe-Precursor [mg] (Fea [mmol]) | Al-Precursor [mg] (Ala [mmol]) | |||
|---|---|---|---|---|---|
a Fe and Al [mmol] refer to the metal amount and were set for the intended molar 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Fe 1 Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio. Al ratio. |
|||||
| [OPy][NTf2] | 1.02 | FeCl2 | 23 (0.22) | LiAlH4 | 8 (0.18) |
| [OPy][NTf2] | 1.01 | [LiFe(btsa)3] | 97 (0.18) | LiAlH4 | 8 (0.18) |
| [BMIm][NTf2] | 1.02 | Fe(CO)5 | 24 (0.12) | (AlCp*)4 | 21 (0.13) |
| [BMIm][NTf2] | 1.1 | Fe2(CO)9 | 22 (0.12) | (AlCp*)4 | 21 (0.13) |
| Ionic liquid [g] | Fe/Al-precursor [mg] (Fe and Al each [mmol]) | ||
|---|---|---|---|
| [BMIm][NTf2] | 1.01 | (CO)4FeAlCp* | 20 (0.06) |
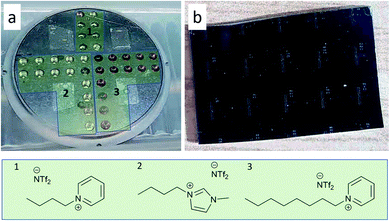
A multiple cavity holder was applied for sputtering into the ILs with a volume of 35 μL per cavity as described elsewhere.47 With special designed covers, between 6 or a maximum of 64 cavities are available (Fig. 1). For cleaning, the holder was ultrasonicated 20 minutes each in technical-grade acetone and isopropanol. The cavities were filled with the ILs [BMIm][NTf2], [OPy][NTf2], [BPy][NTf2] under Ar atmosphere (Fig. 1). Pieces of a patterned Si/SiO2 wafer (2 cm × 3 cm), photolithographically structured with a photoresist lift-off cross pattern for film thickness determination, were placed in each quadrant of the cavity holder (Fig. 1).
The as-such prepared cavity-holder was stored in the vacuum chamber overnight under a vacuum of 1.3 × 10−4 Pa. DC sputter deposition of Fe/Al NPs was performed as follows: After filling with Ar to a pressure of 1.33 Pa and plasma ignition at 20 W for a 2 min precleaning step, the Ar pressure was reduced to 0.5 Pa. A cathode tilt of 12° relative to the normal of the cavity holder was used for deposition (Fig. S1, ESI†). The deposition was performed at 25 W (305 V, 82 mA) for Fe and 46 W (342 V, 134 mA) for Al over a period for 2 h. After sputter deposition, the holder was removed and transported under Ar atmosphere to a glove box. There the NP/IL dispersions were collected and stored under Ar in the glove box.
Methods
Thermal analyses were performed on a NETZSCH TG Tarsus 209 F3 with a heating rate of 5 K min−1 under N2 atmosphere up to a temperature of 1000 °C.
Atomic absorption spectroscopy (AAS) for metal analysis were performed on a PerkinElmer PinAAcle 900T, equipped with flame and graphite furnace and with automatic sampler for the graphite-furnace mode. This AAS instrument has a transversely heated graphite atomizer with longitudinal Zeeman-effect background correction. Flame-AAS was used for Fe and graphite-furnace AAS for Al. Samples were digested in hot aqua regia two times. The residues were re-dissolved in aqua regia (8 mL), filtered and brought with water to a total volume of 50 mL. For the iron measurements the samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and for aluminum 1
10 and for aluminum 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
Scanning-electron microscope (SEM) images were recorded with a JEOL JSM-6510LV QSEM advanced electron microscope equipped with a LaB6 cathode. The microscope was equipped with a Bruker Xflash 410 silicon drift detector and Bruker ESPRIT software for EDX analysis. The samples were prepared for SEM microscopy by coating them with gold using a JEOL JFC 1200 fine-coater. The samples were placed on brass sample holders fixed with a carbon tape.
Powder X-ray diffractometry (PXRD) were measured at ambient temperature on a Bruker D2 Phaser using a flat sample holder and Cu Kα radiation (λ = 1.54182 Å, 35 kV). PXRDs were measured for 30 min.
Initial characterization of the NP/IL dispersion from DC sputter deposition was performed with a FEI Tecnai F20 S/TEM (Institut für Werkstoffe, Fakultät für Maschinenbau, Ruhr-Universität Bochum). For these TEM investigations a holey carbon-coated gold grid (200 mesh) was used and 2.5 μL of the NP/IL sample was dropped on the C-coated side and left for NP adhesion for 2 h. Dried ACN was used to wash the grid dropwise for 1 h under inert conditions. The grid was stored in Ar atmosphere. Further information can be found elsewhere.47
The as-deposited NPs in the ILs were crystallized ex situ under vacuum (30 Pa) at 100 °C for 2, 5 and 10 h in a silicone oil bath and then cooled down to room temperature in order to verify a possible crystallization.
HR-TEM characterization of the sputter-deposited and heat-treated sample in [OPy][NTf2] for 10 h was performed in a Titan Themis 60-300 X-FEG (Max-Planck-Institut für Eisenforschung GmbH, Düsseldorf) equipped with an image corrector, operated at 300 kV. TEM images were recorded on a metal-oxide-semiconductor (CMOS) camera with 4k · 4k pixels. Beam-induced in situ crystallization was achieved at 300 kV with a dose rate ∼6 × 105 e nm−2 s−1, for a total time of 30 min, with a magnification of 490 k×, spot size 3 and a screen current of 9.47 nA.
HR-TEM analysis was also carried out by the FEI Tecnai F30-G2 (Max-Planck-Institut für Chemische Physik fester Stoffe, Dresden) with super-twin and a field emission gun at an acceleration voltage of 300 kV. The point resolution amounted to 2.0 Å, and the information limit amounted to about 1.2 Å. The microscope is equipped with a slow scan CCD camera (MultiScan, 2k·2k pixels; Gatan Inc., Pleasanton, CA, USA).
X-ray photoelectron spectroscopy (XPS) was performed with an ULVAC-PHI VersaProbe II microfocus X-ray photoelectron spectrometer. The spectra were recorded using a polychromatic aluminum Kα X-ray source (1486.8 eV) and referenced to the carbon 1s orbital with a binding energy of 284.8 eV. Fitting of the experimental XP spectra was done with the program CasaXPS (version 2.3.19PR1.0, copyright 1999–2018, Casa Software Ltd).
Results and discussion
The synthesis of iron aluminide alloy NPs was attempted from different Fe and Al starting materials by two methods and in three ILs with an overview given in Table 2. The three ILs [BMIm][NTf2], [BPy][NTf2] and [OPy][NTf2] (Fig. 1, Scheme S1†) were used for the Fe/Al nanoparticle syntheses. The [NTf2]− anion was chosen because of its hydrophobic character and its hydrothermal stability, since the anion [BF4]− is well known to form metal-fluoride nanoparticles.53,54| Method | Ionic liquid | Metal source | Crystalline phaseb | Size [nm] |
|---|---|---|---|---|
| a 1 wt% metal-NP/IL dispersions obtained by microwave-assisted heating for 30 min at 230 °C.b The identity of the crystalline fraction of NPs was determined by powder X-ray diffractometry (PXRD), selected area electron diffraction (SAED) or fast Fourier transformation (FFT) in the TEM.c No separated particles were found.d Average diameter (Ø). See Experimental section for transmission electron microscopy (TEM) measurement conditions, at least 100 particles were used for the size analysis.e Phase determination after 10 h annealing at 100 °C.f Range of particle size from various TEM images.g n.a. = not available but the same synthesis conditions as for the sample in [OPy][NTf2] were used, albeit without annealing so that the low crystallinity prevented a phase determination. | ||||
| Bottom-up | [OPy][NTf2] | FeCl2, LiAlH4 | FeAl | —c |
| [LiFe(btsa)3], LiAlH4 | FeAl | 10 ± 2d | ||
| [BMIm][NTf2] | Fe(CO)5, (AlCp*)4 | FeAl2O4 | 1.0 ± 0.5d | |
| Fe2(CO)9, (AlCp*)4 | None | —c | ||
| (CO)4FeAlCp* | None | —c | ||
| Top-down | [OPy][NTf2] | Fe, Al target | Fe4Al13e | 2–4f |
| [BPy][NTf2] | n.a.g | 2–4f | ||
| [BMIm][NTf2] | n.a.g | 2–4f | ||
The IL cations can have a profound effect on the stabilization, size and size distribution of the obtained metal nanoparticles.55,57 Imidazolium-ILs, in particular with the cation [BMIm]+ are well established for the synthesis of small NPs.18,19,54,56–60 However, [BMIm]+ contains a slightly acidic proton in the C-2 position which can lead to metal-carbene formation.61 The cation [BPy]+ was included here because of the absence of such acidic protons. The cation [OPy]+ was used because it is even more hydrophobic than [BPy]+ and offers a better steric stabilization of the nanoparticles through its long alkyl chain.18
Bottom-up approach
Generally, our bottom-up soft wet-chemical syntheses of Fe/Al NPs in ILs yielded crystalline FeAl NPs either agglomerated or only together with an amorphous background.Following the literature,22,24,35,36 first iron(II) chloride and lithium aluminum hydride were suspended in IL and reacted by microwave irradiation (Fig. S2†). According to the TEM images, EDX analyses and PXRD, strongly agglomerated but crystalline FeAl NPs could be found together with amorphous regions (Fig. S3†). The latter probably consist of amorphous aluminum and iron oxides, which is confirmed by a distinct oxygen signal in the EDX spectra. Since it is known that the presence of halide anions (here from FeCl2) induces the agglomeration of NPs, other synthetic routes were pursued.62
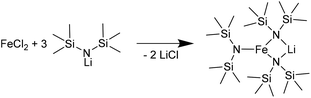
Kelsen et al. and Amiens et al. were able to produce small pure Fe NPs by hydrogenation of bis[bis(trimethylsilyl)amido]iron(II) [[Fe(btsa)2]2].63,64 Our reaction of [LiFe(btsa)3] with LiAlH4 (Fig. S4†) led to crystalline FeAl NPs on an amorphous background (SAED in Fig. S5, ESI†). We note that the btsa ligand is decomposed by heating to 170 °C to volatile products, which can easily be removed from the reaction mixture.
Given the observed unsatisfactory results from the reduction of Fe(II) salts with LiAlH4, we decided to follow a fully organometallic approach avoiding the addition of a separate reducing agent. Organometallic precursor compounds with labile ligands, e.g. with CO and Cp* (pentamethylcyclopentadienyl) have been frequently used successfully for the synthesis of metal NPs.14,15,18,61,65-67 A recent related example was the synthesis of CoAl NPs from Co2(CO)8 and AlCp*.20 Therefore, we applied this route to the synthesis of Fe/Al NPs through the reaction of the iron carbonyls [Fe(CO)5 or Fe2(CO)9] and AlCp*(Fig. S6 and S8†). In case of Fe(CO)5, small FeAl2O4 NPs could be identified which are immobilized on an amorphous background material (SAED in Fig. S7, ESI†). From the reaction with Fe2(CO)9 only amorphous products could be found according to PXRD, EDX and FFT analyses (Fig. S9, ESI†).
The above dual-source approaches were complemented by a single-source approach using the intermetallic complex (CO)4FeAlCp* (Fig. S10, ESI†). The formation of bimetallic FeAl NPs should be promoted by the already existing intermetallic Fe–Al bond.68,69 Yet, the decomposition of (CO)4FeAlCp* (ref. 49) yielded only amorphous phases (Fig. S11, ESI†) as seen before in the dual source approach with Fe2(CO)9 and AlCp*.
The bottom-up NP/IL samples were also analyzed by TGA, AAS and SEM-EDX for the amount of metal and content of the adhering IL. This composition analysis showed more than 50 wt% of IL still adhering to the precipitated, separated and washed samples from the NP/IL dispersions. At the same time the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al ratio deviated from the 1
Al ratio deviated from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 expectation (Fig. S12–S15, Table S1 and S2, ESI†).
1 expectation (Fig. S12–S15, Table S1 and S2, ESI†).
In summary, the attempted synthesis of the Fe/Al NPs in ILs using a bottom-up method yielded unclear samples with a presumably amorphous metal-oxide background.
Top-down approach
As a top-down method, magnetron co-sputtering using elemental metal targets and Ar as sputter gas enables the synthesis of multinary NPs libraries without limits, e.g. related to the chemical reactivity of the used metal precursors.47,70,71 This approach has led to the discovery of noble metal free eletrocatalysts.71–73The synthesis of the Fe/Al NPs was carried out in a commercial co-sputter system with 1.5-inch diameter magnetron sputter cathodes using DC power on the Fe and Al targets. A cavity holder was used for the deposition of metals into ILs (Fig. 1a). Each cavity was filled with 35 μL of IL. Next to the cavities, photolithographically structured wafer pieces were placed on the cavity holder lid for the thin film analysis (Fig. 1b).
The chemical composition of the thin films on the Si wafer piece was analyzed by SEM-EDX on the patterned reference regions of the Si/SiO2 wafer to yield a composition of 24 at% Fe and 76 at% Al. However, the composition of the thin film and the NPs can deviate.74
XPS analysis of the film on the Si substrate was done for further compositional analysis (Table S3, Fig. S16–S18, ESI†). The XPS survey spectrum (Fig. S16, ESI†) identifies aluminum, carbon, silicon, oxygen and iron in the sputtered sample in [OPy][NTf2]. The O 1s orbital indicates two different oxygen species (Table S3 and Fig. S18, ESI†). The first species at 530.7 eV can be ascribed to a metal oxide and the second at 532.0 eV to SiO2.75 The Al 2p orbital binding energy of 74.3 eV indicates Al2O3 (ref. 75) and at 72.0 eV Al0.76 For the Fe 2p3/2 orbital the binding energy at 706.2 eV can be assigned to Fe0 and the bands between 707.1 eV and 710.1 eV to a mixture of FeII and FeIII.77
A possible partial oxidation of the sample before XPS cannot be excluded. The reason is a contact with air after the synthesis to transfer the samples into the glovebox. Since XPS is very surface-sensitive, it shows an oxidation of the Fe/Al film. The quantification of Fe and Al over the entire substrate gave a Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio of 1
Al molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in agreement with the EDX results.
3 in agreement with the EDX results.
Fig. 2 shows the NPs/IL suspensions directly after the magnetron sputter synthesis. All NPs have a size between 2 and 4 nm as determined from TEM images (Fig. 4a, b, S19 and S23, ESI†).
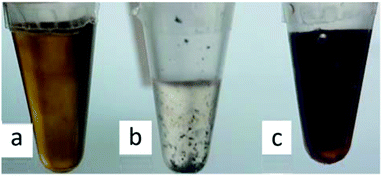 | ||
| Fig. 2 Magnetron sputter synthesized Fe/Al NPs dispersions, (a) in [BMIm][NTf2], (b) in [BPy][NTf2] and (c) in [OPy][NTf2] in 1.5 mL Eppendorf caps. | ||
We exemplarily describe here the analysis of the Fe/Al NPs obtained from the magnetron sputter synthesis in [OPy][NTf2]. For the characterization of the samples in [BMIm][NTf2] and [BPy][NTf2] see ESI (Fig. S19–S23†).
The sputtered Fe/Al NPs in [OPy][NTf2] showed an unexpected red color, which remained after thermal annealing (vide infra) and which was examined by UV-vis spectroscopy (Fig. 2c and 3). The NPs in the [OPy][NTf2] IL exhibit two absorption bands in the green-blue to green spectral region at 475 and 520 nm which give rise to the complementary red color. This absorption maximum at 520 nm and the resulting red color is akin to the surface plasmon resonance of Au NPs,78 which suggest that Fe/Al NPs of the right size mimic the electronic surface structure of Au NPs including a similar band gap width.79 At present there are limited reports on the electronic band structure of bulk Fe/Al alloys80 but none on the nanoscale. Even for Au NPs accurate computational approaches with good agreement to experiments and a correct description of the electronic situation appears still challenging.78,81
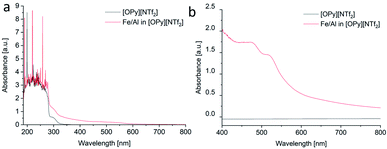 | ||
| Fig. 3 (a and b) UV-vis spectra from the Fe/Al NP/IL dispersion in [OPy][NTf2] measured over different wavelength regions. | ||
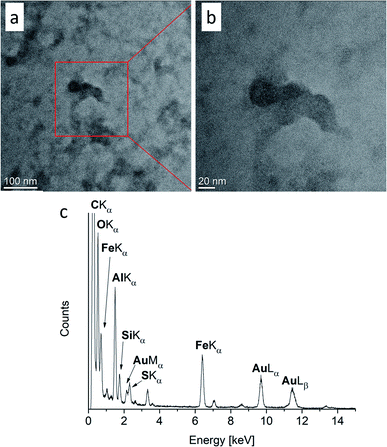
The characterization of Fe/Al NPs in [OPy][NTf2] by TEM indicated the formation of small NPs together with amorphous material (Fig. 4). The elemental analysis from the EDX spectrum of the image region gives a Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al molar ratio of 20
Al molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80. When sample areas with a large amount of amorphous material were investigated in the TEM, crystalline NPs were spontaneously formed under the electron beam.82 In order to promote the crystallization of the NPs outside of the TEM, the Fe/Al@[OPy][NTf2] sample was heated (annealed) for altogether 10 h at 100 °C in vacuum (see ESI for analyses of intermediate annealing times with Fig. S24 and S25†).47
80. When sample areas with a large amount of amorphous material were investigated in the TEM, crystalline NPs were spontaneously formed under the electron beam.82 In order to promote the crystallization of the NPs outside of the TEM, the Fe/Al@[OPy][NTf2] sample was heated (annealed) for altogether 10 h at 100 °C in vacuum (see ESI for analyses of intermediate annealing times with Fig. S24 and S25†).47
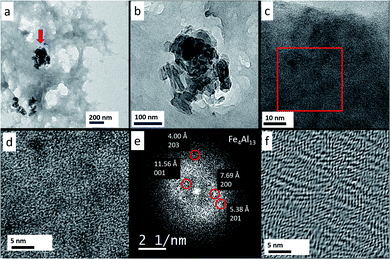
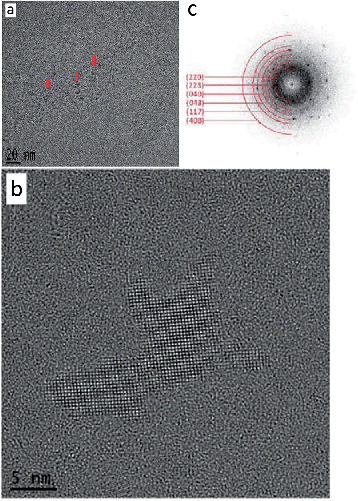
After a total annealing time of 10 h, embedded Fe/Al nanoaggregates in the ionic liquid matrix (Fig. 5a and b) were susceptible to fast Fourier transform (FFT) analysis of the HR-TEM images (Fig. 5e) and gave a clear indication of a Fe4Al13 phase (see ESI, Fig. S26 and S27† for further HR-TEM images from other sample areas with FFT analysis and Fe4Al13 phase assignment).
Also, the Fe/Al NPs were continued to be crystallized in situ in the HR-TEM at 300 kV and a magnification of 490 kx. Every 10 minutes a picture was taken (Fig. S28, ESI†) and a FFT was generated to verify the crystallinity of the NPs. After 30 min sufficiently crystalline NPs could be obtained (Fig. 6a and b).
From the sample an FFT was generated and the reflexes could be assigned to the Fe4Al13 phase with the space group C12/m1 (Table 3 and Fig. 6c).
Conclusions
We prepared Fe/Al NPs via a bottom-up and a top-down approach. The bottom-up approach yielded FeAl NPs, albeit with an amorphous background from the dual-source approach with FeCl2 and LiAlH4 or with [LiFe(btsa)3] and LiAlH4. Very small Fe/Al NPs from the dual-source approach with the iron carbonyl Fe(CO)5 and (AlCp*)4 were apparently almost completely oxidized and analyzed as FeAl2O4 NPs. The top-down synthesis by magnetron co-sputtering using elemental metal targets and Ar yielded very small Fe4Al13 NPs with a size of 2–4 nm. Unequivocal phase determination was possible after thermal and electron-beam annealing which was exemplarily carried out in the ionic liquid [OPy][NTf2]. The Fe/Al NPs in [OPy][NTf2] showed a red color, before and after thermal annealing and the absorption maximum in the visible region at 520 nm is akin to the plasmon resonance of Au NPs, which suggests a similar electronic structure for both Fe/Al and Au NPs. We will continue to investigate the properties of Fe/Al NPs in further studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by the German Science Foundation (DFG) within the priority program SPP 1708 “Material Synthesis Near Room Temperature” (grant JA 466/31-1/2 for C. J., grant Fi502/32-1/2 for R. A. F) and by the BMBF project NEMEZU. M. M. and A. L. acknowledge DFG funding of the project LU1175/23-1. A. G. M. and C. S. acknowledge DFG funding of the project SCHE 634/21-1. We thank Mr Tim-Oliver Knedel for the UV-vis spectra.Notes and references
- P. Migowski and J. Dupont, Chem.–Eur. J., 2007, 13, 32–39 CrossRef CAS PubMed.
- J. Conde, G. Doria and P. Baptista, J. Drug Delivery, 2012, 2012, 751075 Search PubMed.
- Q. A. Pankhurst, J. Connolly, S. K. Jones and J. Dobson, J. Phys. D: Appl. Phys., 2003, 36, R167 CrossRef CAS.
- A. L. Stepanov, in Glass Nanocomposites, ed. B. Karmakar, K. Rademann and A. L. Stepanov, William Andrew Publishing, Boston, 2016, pp. 165–179 Search PubMed.
- M. Li, S. K. Cushing and N. Wu, Analyst, 2014, 140, 386–406 RSC.
- W. Wu, Nanoscale, 2017, 9, 7342–7372 RSC.
- J. D. Scholten, B. C. Leal and J. Dupont, ACS Catal., 2012, 2, 184–200 CrossRef CAS.
- W. J. Stark, P. R. Stoessel, W. Wohlleben and A. Hafner, Chem. Soc. Rev., 2015, 44, 5793–5805 RSC.
- P. M. Humbert and J. G. Chen, J. Catal., 2008, 257, 297–306 CrossRef.
- M. Armbrüster, G. Wowsnick, M. Friedrich, M. Heggen and R. Cardoso-Gil, J. Am. Chem. Soc., 2011, 133, 9112–9118 CrossRef PubMed.
- R. Hudson, C.-J. Li and A. Moores, Green Chem., 2012, 14, 622–624 RSC.
- H. L. Ngyuen, L. E. M. Howard, S. R. Giblin, B. K. Tanner, I. Terry, A. K. Hughes, I. M. Ross, A. Serres, H. Bürckstümmer and J. S. O. Evans, J. Mater. Chem., 2005, 15, 5136–5143 RSC.
- Y. Chen, X. Luo, G.-H. Yue, X. Luo and D.-L. Peng, Mater. Chem. Phys., 2009, 113, 412–416 CrossRef CAS.
- M. Cokoja, H. Parala, A. Birkner, R. A. Fischer, O. Margeat, D. Ciuculescu, C. Amiens, B. Chaudret, A. Falqui and P. Lecante, Eur. J. Inorg. Chem., 2010, 1599–1603 CrossRef CAS.
- M. Cokoja, H. Parala, A. Birkner, O. Shekhah, M. W. E. van Berg and R. A. Fischer, Chem. Mater., 2007, 19, 5721–5733 CrossRef CAS.
- M. Cokoja, H. Parala, M. K. Schröter, A. Birkner, M. W. E. van den Berg, W. Grünert and R. A. Fischer, Chem. Mater., 2006, 18, 1634–1642 CrossRef CAS.
- M. Cokoja, B. J. Jagirdar, H. Parala, A. Birkner and R. A. Fischer, Eur. J. Inorg. Chem., 2008, 3330–3339 CrossRef CAS.
- K. Schütte, A. Doddi, C. Kroll, H. Meyer, C. Wiktor, C. Gemel, G. van Tendeloo, R. A. Fischer and C. Janiak, Nanoscale, 2014, 6, 5532–5544 RSC.
- K. Schütte, H. Meyer, C. Gemel, J. Barthel, R. A. Fischer and C. Janiak, Nanoscale, 2014, 6, 3116–3126 RSC.
- L. Schmolke, B. Gregori, B. Giesen, A. Schmitz, J. Barthel, L. Staiger, R. A. Fischer, A. J. von Wangelin and C. Janiak, New J. Chem., 2019, 43, 16583–16594 RSC.
- M. Armbrüster, K. Kovnir, M. Friedrich, D. Teschner, G. Wowsnick, M. Hahne, P. Gille, L. Szentmiklósi, M. Feuerbacher, M. Heggen, F. Girgsdies, D. Rosenthal, R. Schlögl and Y. Grin, Nat. Mater., 2012, 11, 690–693 CrossRef PubMed.
- Y. B. Pithawalla, M. S. El Shall and S. C. Deevi, Intermetallics, 2000, 8, 1225–1231 CrossRef CAS.
- C. T. Liu, E. P. George, P. J. Maziasz and J. H. Schneibel, Mater. Sci. Eng., A, 1998, 258, 84–89 CrossRef.
- Y. B. Pithawalla, M. S. El-Shall, S. C. Deevi, V. Ström and K. V. Rao, J. Phys. Chem. B, 2001, 105, 2085–2090 CrossRef CAS.
- T. Liu, Y. Pang, H. Kikuchi, Y. Kamada and S. Takahashi, J. Mater. Chem. C, 2015, 3, 6232–6239 RSC.
- S.-H. Kim, H. Kim and N. J. Kim, Nature, 2015, 518, 77–79 CrossRef CAS PubMed.
- S. C. Deevi, V. K. Sikka and C. T. Liu, Prog. Mater. Sci., 1997, 42, 177–192 CrossRef CAS.
- P. Migowski, G. Machado, S. R. Texeira, M. C. M. Alves, J. Morais, A. Traverse and J. Dupont, Phys. Chem. Chem. Phys., 2007, 9, 4814–4821 RSC.
- C. C. Cassol, A. P. Umpierre, G. Machado, S. I. Wolke and J. Dupont, J. Am. Chem. Soc., 2005, 127, 3298–3299 CrossRef CAS PubMed.
- K.-S. Kim, D. Demberelnyamba and H. Lee, Langmuir, 2004, 20, 556–560 CrossRef CAS PubMed.
- C. N. R. Rao, H. S. S. Ramakrishna Matte, R. Voggu and A. Govindaraj, Dalton Trans., 2012, 41, 5089–5120 RSC.
- I. S. Helgadottir, P. P. Arquillière, P. Bréa, C. C. Santini, P.-H. Haumesser, K. Richter, A.-V. Mudring and M. Aouine, Microelectron. Eng., 2003, 107, 229–232 CrossRef.
- T. Torimoto, K.-i. Okazaki, T. Kiyama, K. Hirahara, N. Tanak and S. Kuwabata, Appl. Phys. Lett., 2006, 89, 243117 CrossRef.
- S. Xiao, X. Li, H. Deng, L. Deng and W. Hu, Phys. Chem. Chem. Phys., 2015, 17, 6511–6522 RSC.
- D. P. Dutta, G. Sharma, A. K. Rajarajan, S. M. Yusuf and G. K. Dey, Chem. Mater., 2007, 19, 1221–1225 CrossRef CAS.
- Y. B. Pithawalla and S. Deevi, Mater. Res. Bull., 2004, 39, 2303–2316 CrossRef CAS.
- S. Mende, F. Stenger, W. Peukert and J. Schwedes, Chem. Ing. Tech., 2002, 74, 994–1000 CrossRef CAS.
- P. S. Campbell, M. H. G. Prechtl, C. C. Santini and P.-H. Haumesser, Curr. Org. Chem., 2013, 17, 414–429 CrossRef CAS.
- D. Freudenmann, S. Wolf, M. Wolff and C. Feldmann, Angew. Chem., Int. Ed., 2011, 50, 11050–11060 CrossRef CAS PubMed.
- E. Ahmed, J. Breternitz, M. F. Groh and M. Ruck, CrystEngComm, 2012, 14, 4874–4885 RSC.
- H. Kaper, F. Endres, I. Djerdj, M. Antonietti, B. M. Smarsly, J. Maier and Y.-S. Hu, Small, 2007, 3, 1753–1763 CrossRef CAS PubMed.
- M. Antonietti, D. Kuang, B. Smarsly and Y. Zhou, Angew. Chem., Int. Ed., 2004, 43, 4988–4992 CrossRef CAS PubMed.
- D. Marquardt and C. Janiak, Nachr. Chem., 2013, 61, 754–757 CrossRef CAS.
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS PubMed.
- J. Dupont and J. D. Scholten, Chem. Soc. Rev., 2010, 39, 1780–1804 RSC.
- C. S. Consorti, P. A. Z. Suarez, R. F. de Souza, R. A. Burrow, D. H. Farrar, A. J. Lough, W. Loh, L. H. M. da Silva and J. Dupont, J. Phys. Chem. B, 2005, 109, 4341–4349 CrossRef CAS PubMed.
- H. Meyer, M. Meischein and A. Ludwig, ACS Comb. Sci., 2018, 20, 243–250 CrossRef CAS PubMed.
- C. Ganesamoorthy, S. Loerke, C. Gemel, P. Jerabek, M. Winter, G. Frenking and R. A. Fischer, Chem. Commun., 2013, 49, 2858–2860 RSC.
- J. Weiss, D. Stetzkamp, B. Nuber, R. A. Fischer, C. Boehme and G. Frenking, Angew. Chem., Int. Ed., 1997, 36, 70–72 CrossRef CAS.
- C. Rutz, L. Schmolke, V. Gvilava and C. Janiak, Z. Anorg. Allg. Chem., 2017, 643, 130–135 CrossRef CAS.
- M. Deetlefs and K. R. Seddon, Green Chem., 2003, 5, 181–186 RSC.
- Ernst Ruska-Centre for Microscopy and Spectroscopy with Electrons, FEI Tecnai G2 F20, Journal of large-scale research facilities, 2016, 2, A77 CrossRef.
- A. Schmitz, K. Schütte, V. Ilievski, J. Barthel, L. Burk, R. Mülhaupt, J. Yue, B. Smarsly and C. Janiak, Beilstein J. Nanotechnol., 2017, 8, 2474–2483 CrossRef PubMed.
- M. Siebels, L. Mai, L. Schmolke, K. Schütte, J. Barthel, J. Yue, J. Thomas, B. M. Smarsly, A. Devi, R. A. Fischer and C. Janiak, Beilstein J. Nanotechnol., 2018, 9, 1881–1894 CrossRef CAS PubMed.
- P. Migowski, D. Zanchet, G. Machado, M. A. Gelesky, S. R. Teixeira and J. Dupot, Phys. Chem. Chem. Phys., 2010, 12, 6826–6833 RSC.
- K. Klauke, I. Gruber, T.-O. Knedel, L. Schmolke, J. Barthel, H. Breitzke, G. Buntkowsky and C. Janiak, Organometallics, 2018, 37, 298–308 CrossRef CAS.
- L. Schmolke, S. Lerch, M. Bülow, M. Siebels, A. Schmitz, J. Thomas, G. Dehm, C. Held, T. Strassner and C. Janiak, Nanoscale, 2019, 11, 4073–4082 RSC.
- K. Schütte, J. Barthel, M. Endres, M. Siebels, B. M. Smarsly, J. Yue and C. Janiak, ChemistryOpen, 2017, 6, 137–148 CrossRef PubMed.
- M. Siebels, C. Schlüsener, J. Thomas, Y.-X. Xiao, X.-Y. Yang and C. Janiak, J. Mater. Chem. A, 2019, 7, 11934–11943 RSC.
- S. Wegner, C. Rutz, K. Schütte, J. Barthel, A. Bushmelev, A. Schmidt, K. Dilchert, R. A. Fischer and C. Janiak, Chem.–Eur. J., 2017, 23, 6330–6340 CrossRef CAS PubMed.
- C. Vollmer and C. Janiak, Coord. Chem. Rev., 2011, 255, 2039–2057 CrossRef CAS.
- S. Watanabe, H. Seguchi, K. Yoshida, K. Kifune, T. Tadaki and H. Shiozaki, Tetrahedron Lett., 2005, 46, 8827–8829 CrossRef CAS.
- V. Kelsen, B. Wendt, S. Werkmeister, K. Junge, M. Beller and B. Chaudret, Chem. Commun., 2003, 49, 3416–3418 RSC.
- C. Amiens, B. Chaudret, D. Ciuculescu-Pradines, V. Collière, K. Fajerwerg, P. Fau, M. Kahn, A. Maisonnat, K. Soulantica and K. Philippot, New J. Chem., 2013, 37, 3374–3401 RSC.
- J. Krämer, E. Redel, R. Thomann and C. Janiak, Organometallics, 2008, 27, 1976–1978 CrossRef.
- C. Janiak, in Ionic Liquids (ILs) in Organometallic Catalysis, ed. J. Dupont and L. Kollár, Springer-Verlag Berlin Heidelberg, 2015, pp. 38–42 Search PubMed.
- K. Soulantica, A. Maisonnat, M.-C. Fromen, M.-J. Casanove, P. Lecante and B. Chaudret, Angew. Chem., Int. Ed., 2001, 40, 448–451 CrossRef CAS PubMed.
- Z. Tshemese, M. D. Khan, S. Mlowe and N. Revaprasadu, Mater. Sci. Eng., B, 2018, 227, 116–121 CrossRef CAS.
- K. Klauke, B. Hahn, K. Schütte, J. Barthel and C. Janiak, Nano-Struct. Nano-Objects, 2015, 1, 24–31 CrossRef CAS.
- D. König, K. Richter, A. Siegel, A.-V. Mudring and A. Ludwig, Adv. Funct. Mater., 2014, 24, 2049–2056 CrossRef.
- T. Löffler, H. Meyer, A. Savan, P. Wilde, A. Garzón Manjón, Y. T. Chen, E. Ventosa, C. Scheu, A. Ludwig and W. Schuhmann, Adv. Energy Mater., 2018, 8, 1802269 CrossRef.
- T. Löffler, A. Savan, A. Garzón Manjón, M. Meischein, C. Scheu, A. Ludwig and W. Schuhmann, ACS Energy Lett., 2019, 4, 1206–1214 CrossRef.
- T. Löffler, A. Savan, H. Meyer, M. Meischein, V. Strotkötter, A. Ludwig and W. Schuhmann, Angew. Chem., Int. Ed., 2020, 59, 5844–5850 CrossRef PubMed.
- M. Meischein, A. Garzón-Manjón, T. Frohn, H. Meyer, S. Salomon, C. Scheu and A. Ludwig, ACS Comb. Sci., 2019, 21, 743–752 CrossRef CAS PubMed.
- G. Beamson and D. Briggs, J. Chem. Educ., 1993, 70, A25 Search PubMed.
- B. R. Strohmeier, Surf. Interface Anal., 1990, 15, 51–56 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. S. T. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- V. Amendola, R. Pilot, M. Frasconi, O. M Maragò and M. A. Iatì, J. Phys.: Condens. Matter, 2017, 29, 203002 CrossRef PubMed.
- V. G. Yarzhemskii, M. A. Kazaryan and E. N. Murav’ev, Electronic structure and optical properties of gold nanoparticles, Bull. Lebedev Phys. Inst., 2012, 39, 254–256 CrossRef.
- Y. Li, Y. Liu and J. Yang, Opt. Laser Technol., 2020, 122, 105875 CrossRef CAS.
- J. Zhao, A. O. Pinchuk, J. M. McMahon, S. Li, L. K. Ausman, A. L. Atkinson and G. C. Schatz, Acc. Chem. Res., 2008, 41, 1710–1720 CrossRef CAS PubMed.
- A. Garzón-Manjón, H. Meyer, D. Grochla, T. Löffler, W. Schuhmann, A. Ludwig and C. Scheu, Nanomaterials, 2018, 8, 903–914 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of ionic liquids, details of bottom-up and top-down synthesis of Fe/Al NPs with their analysis. See DOI: 10.1039/d0ra01111h |
| This journal is © The Royal Society of Chemistry 2020 |