Open Access Article
Open Access ArticleNitrileimines as an alternative to azides in base-mediated click [3 + 2] cycloaddition with methylene active nitriles†
Mykola A. Tupychak *,
Olga Ya. Shyyka
*,
Olga Ya. Shyyka ,
Nazariy T. Pokhodylo
,
Nazariy T. Pokhodylo and
Mykola D. Obushak
and
Mykola D. Obushak
Department of Organic Chemistry, Ivan Franko National University of Lviv, Kyryla i Mefodiya St. 6, Lviv 79005, Ukraine. E-mail: tupychakmykola@gmail.com
First published on 3rd April 2020
Abstract
Nitrileimines were implemented in practical click protocols with oxopropanenitriles for the straightforward 5-amino-1H-pyrazole synthesis via 1,3-dipolar cycloaddition. The reaction proceeds at room temperature in a short time with base catalysis and no chromatographic purification. High purity products were isolated by simple filtration. The selectivity of the reaction was observed.
Introduction
Synthetic protocols meeting click- and green chemistry principles and allowing the reactions proceed quickly with high yields at room temperature with easy workup, simple isolation without additional purification and in short-step automated format for the construction of diverse molecular libraries, are nowadays key to organic synthesis.1 Environmental safety and economic attractiveness of such protocols2 stimulate the implementation of new reactions and reagents, and determine their limitations with the aim to solve urgent tasks (Scheme 1).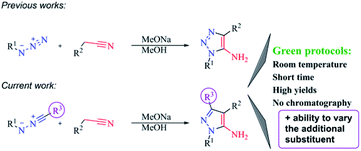 | ||
| Scheme 1 Overview of the developed green protocols giving libraries of amino-functionalized azole derivatives. | ||
1,3-Dipolar cycloaddition is one of the powerful click chemistry tools that allow the formation of a wide variety of five-membered heterocycles desirable for biological activity screening.3 However, organic azides remain the most studied 1,3-dipoles in click reactions.4 Much less attention is paid to other 1,3-dipolar reagents, for example, nitrileimines, which are insufficiently explored in similar reactions.5 Notably, the cycloaddition of nitrileimines usually occurs at room temperature in the presence of base catalysts, nonetheless, such transformations typically last from a few hours to several days.6 Application of other types of dipolarophiles different from alkynes remains poorly studied even in well-established click protocols. Evidently, several dipolarophiles look more perspective over alkynes as many reactions of alkynes usually require heavy metal catalysis and do not always lead to the formation of the target triazoles.7 Thus, methylene active nitriles could be used as convenient partners in the reaction with 1,3-dipoles. The main advantage for such reactions is the employment of bases (including organic bases) as a catalyst.8
Previously, we have shown that methylene active nitriles readily react with azides in the base-solvent system NaOMe–MeOH at room temperature giving triazoles with excellent yields in short time (in some cases during the reagents mixing).9 Moreover, the formed azoles usually contain the amino group, a convenient function for further modifications.10 Specifically, we revealed that the formation of the amino group during the reaction could cause the flow of domino reactions. As a result, the formation of polycyclic derivatives, bioisosteric to natural purine bases,11 was observed and their anticancer activity was evaluated.12 Therefore, it becomes apparent that development of synthetic approaches, including the promising reaction of nitrileimines with activated acetonitriles that fully fit click- and green chemistry concepts, are of current interest and demand further investigation. Another growing appreciation of pyrazole derivatives determines the need for increasing their molecular diversity as such derivatives may hold significant potential in medicinal chemistry. Moreover, the utility of nitrileimines allows to vary the additional substituent in pyrazole core, what would broaden combinatorial library of biologically suitable azole derivatives.
Results and discussion
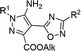
In the current work, we investigated the click protocol involving the reactions of methylene active nitriles with nitrileimines as alternatives to highly reactive organic azides. As found previously, the reaction of electron-deficient hetaryl acetonitriles with arylazides in sodium methylate solution at room temperature led to 1,2,3-triazole derivatives with high yields without by-products.11 Herein, we implemented nitrileimines instead of azides as 1,3-dipoles in [3 + 2] cycloaddition with activated dipolarophiles – (3-het(aryl)-1,2,4-oxadiazol-5-yl)acetonitriles.It should be mentioned that (1,2,4-oxadiazol-5-yl)-1H-1,2,3-triazol-5-amines – the products of the reaction of (1,2,4-oxadiazol-5-yl)acetonitriles with alkyl azides were found to be in focus due to promising potent activity against the T. cruzi parasite (Trypanosoma cruzi).13 Moreover, 1,2,4-oxadiazole ring could be introduced into designed molecules to enhance or tune some of its physico-chemical properties including biological activity.14 Therefore, preparation of a new library of novel pyrazoles with 1,2,4-oxadiazole ring via green protocol of 1,3-dipolar cycloaddition is inquired.
Starting 2-(3-aryl-1,2,4-oxadiazol-5-yl)acetonitriles 1a–e were obtained by the interaction of aromatic amidoximes with 3-(3,5-dimethyl-1H-pyrazol-1-yl)-3-oxopropanenitrile in dioxane with good yields.15 Readily available arylchlorohydrazones 2, as nitrileimines precursors generated in situ, were allowed to react with nitriles 1 in methanol solution in the presence of two equivalents of sodium methylate as a base at room temperature (Scheme 2). In the control parallel experiments, 2-(3-aryl-1,2,4-oxadiazol-5-yl)acetonitriles 1a–e were tested in the reaction with arylazides 3a–e under the same conditions using just one equivalent of sodium methylate. The 1,2,3-triazoles 5a–e were precipitated from the reaction medium in the form of white powders with good yields (Table 1). Similarly, nitrileimines demonstrated excellent reactivity leading to functionalized pyrazoles 4a–e during 5–15 min in high yields and without by-products. It was found that replacement of the 1,3-dipoles did not change the direction of the reaction and the yields of products were comparable. The influence of the substituent in the aryl fragment of 1,3 dipole (azide or imine nitrile) was not meaningful as in all cases desired products were obtained with high yields. In addition, nitrileimines generally gave slightly higher yields. Furthermore, the utility of 1,2,4-oxadiazolyl acetonitriles with a donor substituent also increased the yield of products in both cases (nitrileimines or azides). The established high purity of products also proved the tolerance of this procedure for compounds with the 1,2,4-oxadiazole ring.
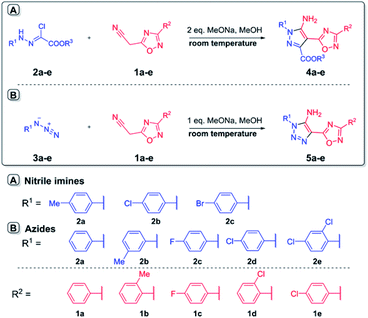 | ||
| Scheme 2 Synthesis of 5-amino-pyrazoles 4a–e and 5-amino-1,2,3-triazoles 5a–e with 1,2,4-oxadiazole ring. | ||
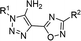
Lately, we have shown that bifunctional 3-(1H-pyrrol-2-yl)/(1H-indol-3-yl)-3-oxopropanenitriles reacted with azides to yield aminotriazoles.16 Selective attack on nitrile moiety instead of carbonyl carbon by azide was observed. However, the 3-hetaryl-3-oxopropanenitriles in reactions with chlorohydrazones have not been investigated. It was found that their reactions with 3-aryl-3-oxopropanenitrile proceeded with the formation of pyrazole-4-carbonitrile.17 In order to study the selectivity and scope of application of the proposed method bifunctional oxopropanenitrile compounds were tested in such green “click chemistry” protocol with nitrileimines.
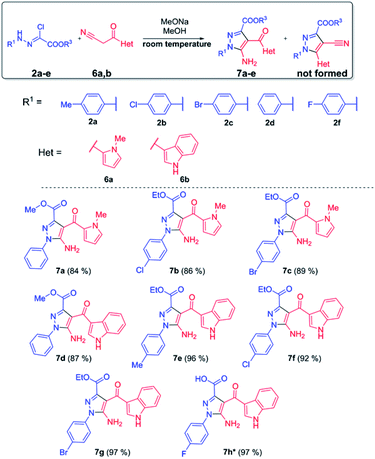
We found out that reaction of bifunctional 3-(1-methyl-1H-pyrrol-2-yl)-3-oxopropanenitrile 6a and 3-(1H-indol-3-yl)-3-oxopropanenitrile 6b with chlorohydrazones in methanol solution in the presence of two equivalents of sodium methylate occurred selectively with the formation of 5-aminopyrazoles 7a–g in high yields (Scheme 3). The formation of pyrazole-4-carbonitrile 8 was not observed. One equivalent of the base is spent on the formation of nitrileimine and another one on the formation of 3-oxopropanenitrile carbonanion. In the first reaction stage, the carbanion attacks the nucleophilic carbon atom of the nitrileimine. It is obvious that the nature of the substituent in the 3-position of 3-oxopropanenitrile influences the further direction of cyclization. The presence of electron-donor pyrrole or indole rings deactivates the carbonyl group and increases the likelihood attack of a nitrogen atom on the nitrile group, leading to the formation of 5-amino-pyrazole 7a–g. The reaction occurs similarly to azides, the replacement of 1,3-dipole reagent with nitrileimine does not affect its regioselectivity.16 Besides, the reaction occurs quickly, within 5–15 minutes at room temperature and the obtained products do not need additional purification, which fits well into the concept of click-reactions.
In the case of fluoro-substituted chlorohydrazone 2f, the formation of 5-aminopyrazole was followed by the subsequent hydrolysis of the ester group to form a corresponding sodium salt. The further treatment of last with a solution of acetic acid led to the formation of pyrazole-3-carboxylic acid 7h with a high yield.
Conclusions
Consequently, having established 1,3-dipolar cycloaddition of a number of methylene active nitriles, the scope of their synthetic application and limitations of the click reaction were extended. Chlorohydrazones as nitrileimines precursors generated in situ declared to be a convenient alternative to highly-reactive azides in such [3 + 2] cycloaddition. The excellent selectivity of chlorohydrazones reaction with 3-hetaryl-3-oxopropionitriles was reported. New examples of pyrazoles and triazoles with 1,2,4-oxadiazole, indole and pyrrole moieties were obtained with high yields. The proposed green synthetic protocols make it possible to synthesize a wide range of functionalized heterocyclic compounds suitable for screening for biological activity in cost and time more effective manner and from available reagents.Conflicts of interest
The authors have declared no conflict of interest.Notes and references
- (a) A. S. Barrow, C. J. Smedley, Q. Zheng, S. Li, J. Dong and J. E. Moses, Chem. Soc. Rev., 2019, 48, 4731 RSC; (b) T. Hong, W. Liu, M. Li and C. Chen, Analyst, 2019, 144, 1492 RSC.
- H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929 RSC.
- K. Martina, S. Tagliapietra, V. V. Veselov and G. Cravotto, Front. Chem., 2019, 7, A95 CrossRef PubMed.
- (a) R. S. Gomes, G. A. M. Jardim, R. L. de Carvalho, M. H. Araujo and E. N. da Silva Júnior, Tetrahedron, 2019, 75, 3697 CrossRef CAS; (b) M. S. Singh, S. Chowdhury and S. Koley, Tetrahedron, 2016, 72(35), 5257 CrossRef CAS.
- (a) B. A. Thaher, M. Arnsmann, F. Totzke, J. E. Ehlert, M. H. G. Kubbutat, C. Schachtele, M. O. Zimmermann, P. Koch, F. M. Boeckler and S. A. Laufer, J. Med. Chem., 2012, 55, 961 CrossRef PubMed; (b) I. Yavari, M. Nematpour, S. Yavari and F. Sadeghizadeh, Tetrahedron Lett., 2012, 53, 1889 CrossRef CAS; (c) J. S. S. Neto and G. Zeni, Chem.–Eur. J., 2020 DOI:10.1002/chem.201905276 , accepted author manuscript.
- (a) I. Yavari, G. Khalili and A. Mirzaei, Helv. Chim. Acta, 2010, 93(2), 277 CrossRef CAS; (b) M. A. Raslan, S. M. Sayed and M. A. Khalil, J. Heterocycl. Chem., 2016, 53, 727 CrossRef CAS; (c) T. A. Farghaly, M. A. Abdallah and M. R. Abdel Aziz, J. Heterocycl. Chem., 2016, 54(1), 699 CrossRef.
- N. T. Pokhodylo, O. Ya. Shyyka, M. A. Tupychak, Y. I. Slyvka and M. D. Obushak, Chem. Heterocycl. Compd., 2019, 55, 374 CrossRef CAS.
- For the most recent reviews about organocatalytic 1,2,3-triazole synthesis, see: (a) H. B. Jalani, A. Ç. Karagöz and S. B. Tsogoeva, Synthesis, 2017, 49, 29 CAS; (b) J. John, J. Thomas and W. Dehaen, Chem. Commun., 2015, 51, 10797 RSC.
- (a) N. T. Pokhodylo, V. S. Matiychuk and M. D. Obushak, Chem. Heterocycl. Compd., 2009, 45, 483 CrossRef CAS; (b) N. T. Pokhodylo, V. S. Matiychuk and M. D. Obushak, Synthesis, 2009, 14, 2321 CrossRef; (c) N. T. Pokhodylo, V. S. Matiychuk and M. D. Obushak, Chem. Heterocycl. Compd., 2009, 45, 245 CrossRef CAS.
- (a) R. Aggarwal and S. Kumar, Beilstein J. Org. Chem., 2018, 14, 203 CrossRef CAS PubMed; (b) A. Shaabani, M. T. Nazeri and R. Afshari, Mol. Diversity, 2019, 23(3), 751 CrossRef CAS PubMed; (c) M. V. Murlykina, A. D. Morozova, I. M. Zviagin, Y. I. Sakhno, S. M. Desenko and V. A. Chebanov, Front. Chem., 2018, 6, 527 CrossRef PubMed.
- (a) N. T. Pokhodylo, O. Y. Shyyka, M. A. Tupychak and M. D. Obushak, Chem. Heterocycl. Compd., 2018, 54(2), 209 CrossRef CAS; (b) N. T. Pokhodylo and O. Y. Shyyka, Synth. Commun., 2017, 47(11), 1096 CrossRef CAS; (c) N. T. Pokhodylo, O. Y. Shyyka and M. D. Obushak, Synth. Commun., 2014, 44, 1002 CrossRef CAS; (d) N. T. Pokhodylo, O. Y. Shyyka, R. D. Savka and M. D. Obushak, Phosphorus, Sulfur Silicon Relat. Elem., 2010, 185, 2092 CrossRef CAS; (e) N. T. Pokhodylo and V. S. Matiychuk, J. Heterocycl. Chem., 2010, 47, 415 CAS; (f) N. T. Pokhodylo, V. S. Matiychuk and M. D. Obushak, Chem. Heterocycl. Compd., 2009, 45, 881 CrossRef CAS; (g) N. T. Pokhodylo, V. S. Matiichuk and N. D. Obushak, Tetrahedron, 2009, 65, 2678 CrossRef CAS.
- N. T. Pokhodylo, O. Y. Shyyka and N. S. Finiuk, Biopolym. Cell, 2019, 35, 321 Search PubMed.
- S. Brand, E. J. Ko, E. Viayna, S. Thompson, D. Spinks, M. Thomas, L. Sandberg, A. F. Francisco, S. Jayawardhana, V. C. Smith, C. Jansen, M. De Rycker, J. Thomas, L. MacLean, M. Osuna-Cabello, J. Riley, P. Scullion, L. Stojanovski, F. R. C. Simeons, O. Epemolu, Y. Oko Shishikura, S. D. Crouch, T. S. Bakshi, C. J. Nixon, I. H. Reid, A. P. Hill, T. Z. Underwood, S. J. Hindley, S. A. Robinson, J. M. Kelly, J. M. Fiandor, P. G. Wyatt, M. Marco, T. J. Miles, K. D. Read and I. H. Gilbert, J. Med. Chem., 2017, 60, 7284 CrossRef CAS PubMed.
- (a) Z. Yang, M. Shen, M. Tang, W. Zhang, X. Cui, Z. Zhang, H. Pei, Y. Li, M. Hu, P. Bai and L. Chen, Eur. J. Med. Chem., 2019, 178, 116 CrossRef CAS PubMed; (b) W. Caneschi, K. B. Enes, C. Carvalho de Mendonça, F. á. de Souza Fernandes, F. B. Miguel, J. da Silva Martins, M. Le Hyaric, R. R. Pinho, L. M. Duarte, M. A. Leal de Oliveira, H. é. F. Dos Santos, M. T. Paz Lopes, D. Dittz, H. Silva and M. R. Costa Couri, Eur. J. Med. Chem., 2019, 165, 18 CrossRef CAS PubMed; (c) M. Mohammadi-Khanaposhtani, N. Ahangar, S. Sobhani, P. H. Masihi, A. Shakiba, M. Saeedi and T. Akbarzadeh, Bioorg. Chem., 2019, 89, 102989 CrossRef PubMed; (d) S. S. De, M. P. Khambetea and M. S. Degani, Bioorg. Med. Chem. Lett., 2019, 29, 1999 CrossRef CAS PubMed.
- L. W. Hardy, M. L. R. Heffernan, F. X. Wu, K. L. Spear and L. D. Saraswat, US Pat. WO2011075699A2, 2011.
- N. T. Pokhodylo, V. S. Matiychuk and M. D. Obushak, Synthesis, 2009, 8, 1297 Search PubMed.
- (a) H. Fan, E. Kotsikorou, A. F. Hoffman, H. T. Ravert, D. Holt, D. P. Hurst, C. R. Lupica, P. H. Reggio, R. F. Dannals and A. G. Horti, Eur. J. Med. Chem., 2009, 44, 593 CrossRef CAS PubMed; (b) M. Gao, M. Wang and Q.-H. Zheng, Bioorg. Med. Chem. Lett., 2012, 22, 3704 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra01417f |
| This journal is © The Royal Society of Chemistry 2020 |