Open Access Article
Open Access ArticleProduction of 5-hydroxymethylfurfural (HMF) from rice-straw biomass using a HSO3–ZSM-5 zeolite catalyst under assistance of sonication†
Phan Huy Hoang *,
Nguyen Minh Dat,
Thai Dinh Cuong and
Duong Thanh Tung
*,
Nguyen Minh Dat,
Thai Dinh Cuong and
Duong Thanh Tung
Hanoi University of Science & Technology, No.1 Dai Co Viet Street, Hanoi, Vietnam. E-mail: hoang.phanhuy@hust.edu.vn
First published on 2nd April 2020
Abstract
This work studied the application of sulfonated ZSM-5 zeolite, a bi-functional catalyst for conversion of biomass-derived glucose to HMF. Glucose hydrolysate was obtained by enzymatic hydrolysis of rice straw, that was pretreated by sodium hydroxide. Glucose hydrolysate was then subjected to a transformation reaction to achieve HMF using HSO3–ZSM-5 zeolite under the assistance of sonication. The reaction conditions including solvent, temperature, catalyst dosage and reaction time were studied. Suitable conditions, which gave the highest yield of HMF of 54.1% have been found. The HSO3–ZSM-5 zeolite presented a high catalytic efficiency for conversion of glucose to HMF, an important and useful intermediate in the chemical industry.
1. Introduction
Hydroxymethylfurfural (HMF) is an important platform in the chemical industry. It is used as valuable intermediate for chemical synthesis of biofuel, solvents, polymers, adhesives, plastic, pesticides, organic compounds, etc1–5. In general, HMF is produced from monosaccharides including fructose and glucose, catalyzed by acids in water or in biphasic solvents. It is highly valuable to produce HMF from lignocellulose or other nonedible polysaccharides, which are more abundant and low cost than edible glucose or fructose. Therefore, much attention has been paid to study and optimize the “green” process for production of HMF. Recently, there have been several researchers that reported the synthesis of HMF with high conversion yield from abundant and low cost lignocellulose biomass.5–13Vietnam is an agriculture country, annually produces about 50 million tons of rice and about 60 million tons of rice straw biomass.14–16 It is potential resource for bio-refinery process to achieve the valuable green-chemicals and fuel such as HMF. The utilization of these agricultural residues for production of valuable products such as green chemicals, fuel or platforms is effective solution as it brings us great benefit in economics and environment. It could not only resolve the environment issue but also help to improve the agricultural section by producing of valuable chemicals and fuel. Hence, this current work focuses on production of HMF from glucose hydrolysate, obtained by enzymatic hydrolysis of rice straw, using bi-function zeolite catalyst.
As known that, it is very important to choose the suitable catalyst for lignocellulosic biomass conversion to achieve high yield of green chemicals. The conversion of lignocellulose into HMF using liquid catalysts could give high yield of products. However, the application of liquid catalysts has many drawbacks in catalyst recycling and reproducing, as well as economic and environmental issues. Solid acid catalysts have several advantages over inorganic (liquid) acid catalysts6,7,17–23 such as recyclability, high selectivity leading to fewer by-products formed during the catalytic reactions, reducing reaction time and product decomposition; their surface acidity can be adjusted to improve selectivity, ability to work at higher temperatures the reusability of solid catalysts helps reduce energy consumption for the process. Among several different sorts of solid acid catalysts, zeolite ZSM-5 is one of the most popular ones, which is also effective in organic syntheses.21,22,24 ZSM-5, a solid acid catalyst, possess excellent shape selectivity and acidity, high thermal stability makes it an interesting material for catalyzing organic reactions. To enhance the catalytic activity and acidity, incorporation of other elements such as Cr, B, W and Ce into the lattice of ZSM-5 zeolite or adding chemical function into the pores of zeolites with organic groups should be carried out. ZSM-5 zeolite would contain two types of acid site: Brønsted and Lewis site after ion exchange process with cation Men+ or adding sulfonic acid function group into the structure of zeolites.24–27 There have been some reports on application of zeolite, especially ZSM-5 zeolite for conversion of glucose and carbohydrate into HMF.21,22 However, the efficiency of the process and yield of HMF was not high. Thus, in this work, ZSM-5 zeolite modified with sulfonic acid functional groups (HSO3–ZSM-5) with higher acidity and catalytic activity over traditional ZSM-5 was used as catalyst for conversion of lignocellulose into HMF. The use of bi-functional catalyst would enhance the conversion efficiency and yield of HMF from biomass.
Ultrasound technology is considered a green technique for use in chemical processes as it can reduce reaction times and chemical loading.28–30 Recently, several researches reported on using of sonication for lignocellulosic biomass conversion. For bio-refinery, ultrasound has been currently used as pre-treatment process to destroy the recalcitrant matrix of cellulose–lignin–hemicellulose in biomass. Sonication has been proven to enhance the separation and hydrolysis of lignocellulosic materials for the use in biofuel production by providing physical augmentation via shear forces, mass and heat transfer as well as chemical effects of producing oxidizing radicals.
Therefore, we were motivated that a combination process for conversion of rice straw derived glucose into HMF could be prepared by taking fully advantages offered by both sulfonic acid-functionalized mesoporous ZSM-5 zeolite and sonication. Strong sulfonic acid group could produce a new catalyst with higher acidity, higher catalytic activity as well as many advantages of a solid acid catalyst for production of HMF. Lewis acid site would promote the isomerization of glucose to fructose and Brønsted acid site would promote the dehydration of fructose to HMF. Besides, the ultrasound energy would help to enhance the mixing of reaction mixture, heat and mas transfer inside the reaction thus enhance the biomass conversion efficiency and yield of HMF. HSO3–ZSM-5 zeolite catalyst showed very good catalytic activity in conversion of rice straw derived glucose into HMF with high yield of 54.1%.
2. Experimental
2.1. Preparation of HSO3–ZSM-5 zeolite
Sulfonic acid-functionalized mesoporous ZSM-5 zeolite was synthesized by a method following a report.26 In typical, first mesoporous ZSM-5 was synthesized by hydrothermal method using hexadecyl trimethyl ammonium bromide (C16H33(CH3)3NBr, CTAB) as a mesogenous templates. The synthetic solution was hydrothermally heated at 170 °C for 24 h in an autoclave. Subsequently, the product was collected by centrifugation, washed with DI water for three times and dried at 100 °C for several hours. Finally, it was calcined in air at 550 °C for 6 h to obtain mesoporous ZSM-5 zeolite. Then, HSO3–ZSM-5 zeolite was prepared by a two-stage process. The as-synthesized mesoporous ZSM-5 particles were added in a solution containing 3-mercaptopropyltrimethoxysilane (MPTS) and 5 ml of toluene solvent. The mixture was maintained at 60 °C with continuous stirring for 8 h. After the reaction time, solid part was collected and washed with toluene and DI water, then dried at 100 °C for 6 h. Subsequently, an oxidation was conducted by adding the solid part to 30 wt% hydrogen peroxide solution with solid/liquid ratio of 1/10 (w/v) and stirred at room temperature and for 12 h. HSO3–ZSM-5 particles obtained from the reaction were washed for 3 times with DI water, then dried at 100 °C for 6 h.2.2. Rice straw biomass conversion into HMF using solid acid catalyst HSO3–ZSM-5 zeolite
Dry rice straw collected from the North of Vietnam was used for preparation of biomass-derived glucose feedstock. The rice straw was ground to 2 mm size for the sodium hydroxide pretreatment. The condition for pretreatment is: temperature of about 100 °C, pretreatment time of 2 hours and solid/liquid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 with active alkali dosage of 20% of dry rice straw.14
10 with active alkali dosage of 20% of dry rice straw.14
For enzymatic saccharification, samples of pretreated biomass solid was taken and placed in 250 ml flask for enzymatic hydrolysis with concentration of 10% at 50 °C in 120 h. 0.05 M sodium citrate solution was used as buffer. Cellic® Ctec2 enzyme (Novozymes, Denmark) dosage is 35 FPU g−1 dry biomass matters.14,15 After hydrolysis the slurry was filtered to get the solid residue and the liquid glucose hydrolysate.
The glucose liquid was then subjected for conversion process using the as-synthesized solid acid catalyst in an autoclave. Prior to the reaction, the mixture was sonicated by placing in an ultrasonic bath at 60 °C for 15 min. The autoclave was then heated to reach desired temperature (120–140 °C) and maintained at that temperature for a specific duration (2–8 h). After the reaction, the slurry was centrifugated to separate the solid catalyst and the liquid hydrolysate. The liquid hydrolysate was used for HPLC analysis to determine the conversion yield. The solid acid catalyst that recovered from the mixture was washed 3 times with ethanol, then DI water and dry at 105 °C.
2.3. Characterization
X-ray diffraction (XRD) data were obtained on a Rigaku D/max lllC (3 kW) with a θ/θ goniometer equipped with a Cu KR radiation generator. Scanning electron microscopy/energy dispersive X-ray spectroscopy (SEM/EDS) was performed using a JSM-7000F, JEOL (Japan). Mesoporous properties of modified zeolite catalysts were examined by N2 adsorption–desorption isotherms at 77 K with a Quantachrome NOVA 4200 E Porosimeter analyzer. Fourier transform infrared (FT-IR) absorption spectra were recorded at room temperature on a Nicolet Nexus 670 FT-IR spectrometer. Amount and strength of acid sites were measured by ammonia temperature programmed desorption (NH3-TPD) using Micrometrics 2900 instrument with a thermal conductivity detector. Approx. 0.20 g of sample was saturated with NH3 at 120 °C, then flushed with helium to remove all physically adsorbed NH3. Finally, the desorption of NH3 was carried out from 100 to 550 °C with heating rate of 10 °C min−1. The HMF concentrations in the hydrolysate were analyzed by a high-performance liquid chromatography system using a ProStar 210 HPLC system equipped with a UV detector and a reversed-phase C18 column (200 × 4.6 mm) at a wavelength of 280 nm. 1.75 × 10−2 M acetic acid aqueous solution and acetonitrile, volume ratio 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, was used as the mobile phase, at a flow rate of 1.0 ml min−1. The content of HMF in samples was quantified by external standard calibration curve method, which were based on the standard compounds.
85, was used as the mobile phase, at a flow rate of 1.0 ml min−1. The content of HMF in samples was quantified by external standard calibration curve method, which were based on the standard compounds.
The yields of HMF were calculated using the following equation:
| Yield = mole of product formed/mole of glucose utilized × 100% |
3. Results and discussions
3.1. Synthesis of mesoporous HSO3–ZSM-5 zeolite catalyst
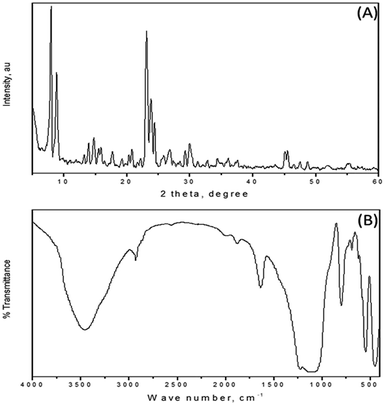
Mesoporous ZSM-5 zeolite was prepared by a hydrothermal method at 170 °C for 24 h. The as-synthesized ZSM-5 was subjected to a 2-step reaction to attach sulfonic acid group onto its structure. The reaction resulted in a sulfonated zeolite (HSO3–ZSM-5 zeolite), whose structure was characterized by XRD, IR spectroscopy, while EDS technique helped confirm the presence of HSO3 groups based on S content of the zeolite.As seen from X-ray diffraction (XRD) patterns of the mesoporous HSO3–ZSM-5 zeolite in Fig. 1A, the sample showed relatively high intensity peaks corresponding to dominant (011), (200), (031), and (051) reflections, which present a crystalline MFI-type zeolitic structure with high structural order. There were almost no changes in framework structure and crystallinity of the zeolite, which were, after the sulfonation, confirmed by XRD analysis. IR spectra of the as-synthesized HSO3–ZSM-5 zeolite particles (Fig. 1B) and of parent ZSM-5 zeolite (Fig. S1, ESI†) also showed several typical bands of ZSM-5 zeolite type with a peak at 1100 cm−1 assigned to an intermolecular vibration of SiO4 or AlO, and band at 1229 cm−1 assigned to an intramolecular vibration of these groups. After the sulfonation, new peaks observed at 693, 1068, 2589 and 2932 cm−1 indicate the presence of S–O, S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds and strong hydrogen bond in HSO3 groups.26,27,31 This suggests that sulfonic (HSO3) group was successfully added to the ZSM-5 zeolite structure. These results are once again confirmed by EDS data as shown in Fig. 2.
O bonds and strong hydrogen bond in HSO3 groups.26,27,31 This suggests that sulfonic (HSO3) group was successfully added to the ZSM-5 zeolite structure. These results are once again confirmed by EDS data as shown in Fig. 2.
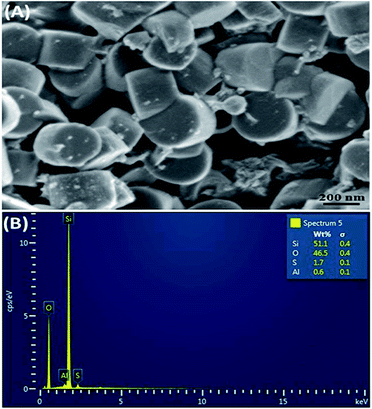
The obtained product, HSO3–ZSM-5, was elementally analyzed using Energy-dispersive X-ray spectroscopy (EDS) for determination of S (in HSO3) attached on ZSM-5 particles. According to EDS spectrum in Fig. 2B, 1.7% of S was present in the sample, while no S was detected in the non-modified zeolite (data not shown). Morphology and size of the HSO3–ZSM-5 zeolites (Fig. 2A) and of parent ZSM-5 zeolite (Fig. S2, ESI†) were relatively uniform with a diameter of 350 ± 50 nm as seen from SEM image. Moreover, after sulfonation, there were almost no change in morphology and size of zeolite.
On the other hand, NH3-TPD was utilized to investigate the concentration and strength of acid sites by the amount of desorbed NH3 and the location of desorption peak (Fig. S3, ESI†). The acidity of zeolite catalyst was remarkably improved after the sulfonation. As seen, the acid concentration of the HSO3–ZSM-5 is 2.78 mmol g−1 (corresponds to S content of the sulfonated zeolite of approx. 1.7%), while the acid concentration of the parent ZSM-5 zeolite was of 0.29 mmol g−1. This indicated that sulfonic groups were successfully incorporated into the zeolite structure (surface and/or pores), which reflected in a significant enhancement in acidity of the as-obtained HSO3–ZSM-5 zeolite.
After sulfonation of zeolite, both surface area and pore size of the sulfonated zeolite are slightly lower than those of the parent zeolite. In typical, surface area and pore size of HSO3–ZSM-5 zeolite is 470 m2 g−1 and 5.5 nm, respectively. While these parameters of parent zeolite are 486 m2 g−1 and 5.7 nm, respectively. It is attributed to the presence of the bulky sulfonic groups on the pore surface of the HSO3–ZSM-5 zeolite. This suggests that the incorporation of sulfonic groups into the zeolite structure does not change the pore structure of zeolite.
3.2. Conversion of biomass-derived glucose into HMF using HSO3–ZSM-5 zeolite catalyst
HSO3–ZSM-5 zeolite catalyst, which contains both of Lewis and Brønsted acid site, was used to replace conventional liquid acid catalyst in the conversion of glucose obtained from hydrolysis process of rice straw biomass. The first attempt studied in this work was the effect of solvent on conversion of glucose. It is well known that solvent plays an important and sometimes decisive role in the catalytic behavior of a catalyst. In most of organic reactions, solvents must be used as reaction medium; to dissolve reactants making them able to contact well with each other as well as with catalyst if available, and therefore facilitate the reaction; to dilute reactants to control side reactions; and prevent the catalyst deactivation. As reported in a reference32 that aqueous medium cannot provide high HMF yields comparing to other organic solvent or two-component solvent. Hence, in this work, DMSO and THF were used alternately to add to sugar hydrolysate in a volume ratio to aqueous of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while other parameters were kept constant.
1, while other parameters were kept constant.
As obtained results in Fig. 3, changing of solvent would induce the change of HMF yield, indicating that suitable solvent used is very important for conversion of glucose to HMF. At the same temperature condition, the yield of HMF when using DMSO/H2O (24%) was higher than when using THF/H2O (15.8%). Using DMSO/H2O resulted in higher yield of HMF than using THF/H2O. It could be attributed to low solubility of the reaction mixture in THF/H2O due to low polarity of the two components results in low reaction efficiency. With higher polarity than THF, DMSO is a good solvent for dissolving reagents like glucose, can facilitate the mixing of glucose molecules and catalyst.33,34 The formation of hydrogen bonds between the acidic site of the zeolite and THF were also believed to be the reason that induce the lower HMF yield in the case of THF/H2O solvent. These hydrogen bonds would reduce the acidity and catalytic activity of acidic zeolite catalyst.33 Moreover, DMSO can absorb water to promote the dehydration reaction and suppresses the hydrolysis of HMF to levulinic acid and formic acid resulting in high conversion efficiency of glucose to HMF.32,35 According to the obtained results, DMSO was chosen for further investigations.
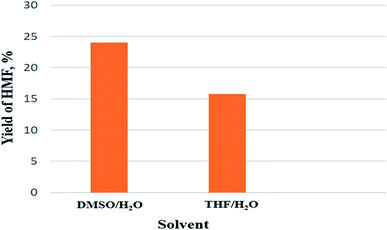 | ||
| Fig. 3 Effect of solvent on yield of HMF (reaction condition: temperature of 130 °C in 2 h with 20% catalyst). | ||
As known that temperature is important factors that influences the efficiency of chemical transformation reactions. Thus, effect of reaction temperature on the conversion yield was studied by varying from 120 to 140 °C, while other conditions were maintained constant. Effect of reaction temperature on HMF yield was summarized in Fig. 4.
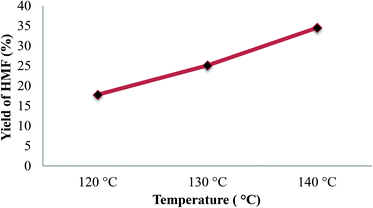 | ||
| Fig. 4 Effect of solvent on yield of HMF (reaction condition: reaction time of 2 h with 20% catalyst). | ||
It is seen that, the yield of HMF increased with the increase of reaction temperature. At 120 °C the yield of HMF was 17.5%, increasing reaction temperature to 130 and 140 °C resulted in a significant increase in HMF yield to 24.2 and 34.6%, respectively. At low temperature, diffusivity as well as number of effective collisions between reactant molecules with reactant molecules and between reactants with catalyst acid sites were low. Hence, reaction velocity and yield of HMF was low. When reaction temperature increased, these above factors are improved and reaction velocity and yield of HMF thus increased. However, temperature was further increased (over 150 °C) the yield of HMF decreased due to the degradation of HMF to form levulinic acid, formic acid, humins.22 The formation of these undesired by-products can be visually observed by the change of reaction mixture color from brown to dark brown. Therefore, reaction temperature was chosen at 140 °C for further study.
The influence of catalyst loading on the yield of HMF obtained by glucose conversion using the HSO3/ZSM-5 zeolite was investigated and shown in Fig. 5. Catalyst dosage was 20, 30 and 40% based on weight of the glucose, while other parameters were maintained. It can be seen from Fig. 5, the amount of HSO3/ZSM-5 zeolite affected clearly the conversion of glucose into HMF. Increased the catalyst dosage from 20 to 30%, the yield of HMF was significantly increased from 34.6% to 43.5%. This indicated that HSO3–ZSM-5 zeolite showed high catalytic efficiency in glucose conversion into HMF. When catalyst dosage was further increased from 30 to 40%, the yield slightly decreased to 39.4%. It could be explained that at large amount of catalyst, the catalyst particles could not disperse well in solution. They tended to aggregate and flocculate to form larger particles and fell down to the bottom of the reactor. Thus it would reduce the efficiency of catalyst. Moreover, due to large amount of catalyst the side reactions could be enhanced or product tended to be adsorbed more into pores of catalyst resulting in decrease of HMF yield.
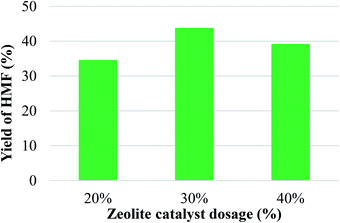 | ||
| Fig. 5 Effect of catalyst dosage on yield of HMF (reaction condition: temperature of 140 °C in 2 h). | ||
To prove the efficiency of sulfonic acid groups, two experiments: without catalyst and using unmodified ZSM-5 zeolite were conducted while other reaction conditions were kept unchanged. According to the results, the reaction carried out in absence of catalyst showed very low yield of HMF (about 3%). The result indicated that the contribution of the non-catalyzed reactions is negligible. While the reaction carried out using unmodified ZSM-5 zeolite resulted in yield of HMF of 22.8%. ZSM-5 is an acidic zeolite catalyst, able to support the conversion of glucose into HMF. However, the acidity of ZSM-5 zeolite is not as high as sulfonated ZSM-5 zeolite thus unmodified ZSM-5 zeolite catalyst gave a lower conversion efficiency than HSO3–ZSM-5 zeolite catalyst. From these obtained results, the HSO3–ZSM-5 catalyst dosage was selected to be 30% as suitable catalyst amount for further investigation.
The reaction time was varied from 2 to 8 h to estimate the influence of reaction time on the conversion of glucose into HMF. As seen from Fig. 6, the HMF yield were increased with the increase of the reaction time. When reaction time increased from 2 h to 4 h the yield of HMF increased from 43.5% to 54.1%. Obviously, at short reaction time the reaction occurs incompletely resulting in low yield of HMF. When lengthening the reaction time, high HMF yield could be observed. However, when conducting the reaction for longer than 4 h, HMF yield increased slightly (58% and 58.3% at 6 h and 8 h, respectively), which can be considered as a sign of saturation after 6 h reaction time. While the conversion of glucose kept rising from 69.5% to 85.1% when increased the reaction time from 4 h to 8 h. This phenomenon can be attributed to the degradation of HMF at high temperature after long reaction time to form by-products such as: levulinic acid, formic acid, humins, etc. The obtained result by using sulfonated ZSM-5 zeolite catalyst is comparable or higher than HMF yield obtained in previous reports using other solid acid catalyst.10,22 The high acidity supplied by strong sulfonic acid group could give the higher acidity, higher catalytic activity to the ZSM-5 zeolite catalyst. The bi-functional catalyst with Lewis and Brønsted acid site would enhance the conversion efficiency and yield of HMF. Furthermore, the high yield of HMF obtained in our work could be attributed to the assistance of sonication which help to enhance the mixing of reaction mixture, dispersion of catalyst particles and heat and mas transfer inside the reaction hence improve the conversion efficiency and yield of HMF. According to the result and considering both efficiency and economic issue, 4 h reaction time was chosen as suitable condition for the catalytic conversion of glucose.
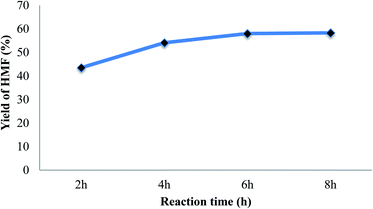 | ||
| Fig. 6 Effect of reaction time on yield of HMF (reaction condition: temperature of 140 °C with catalyst dosage of 30%). | ||
To evaluate the recyclability and durability of catalyst, HSO3–ZSM-5 zeolite was subject to several runs of carbohydrate conversion (data are shown in Table S1, ESI†). Reaction conditions were used as: 140 °C; 4 h and 30 wt% of catalyst loading in co-solvent of DMSO/H2O. The obtained results showed that there was no significant decrease in catalytic activity of sulfonated ZSM-5 after several runs. HSO3–ZSM-5 zeolite could be recycled and reused efficiently for at least 5 times. The HMF yield was about 51.9% after fifth run, quite close to the result of first run with about 54.1% HMF yield. This indicated that the HSO3–ZSM-5 is highly stable in catalytic reactions and is more resilient to catalytic deactivation.
Interestingly, in the product mixture after reaction, a small amount of furfural was observed. It can be attributed to the conversion of xylose sugar presented in the reducing sugar hydrolysate after enzymatic hydrolysis of rice straw. Furthermore, the reactions were conducted at the suitable condition except using pure glucose for comparison. The HMF yield obtained from conversion of pure sugar was slightly higher that the yield obtained from saccharification hydrolysate. It could be explained by the influence of other components in the hydrolysate.
4. Conclusion
The sulfonic acid functional ZSM-5 zeolite showed high catalytic activity in the conversion of biomass-derived glucose and gave high yield of HMF. Several factors that affect the yield of HMF were studied to determine the suitable condition to obtain HMF. The suitable parameters were found to be: solvent: DMSO/H2O; reaction temperature: 140 °C; dosage of HSO3–ZSM-5 catalyst: 30%; reaction time: 4 h. Prior to the reaction, the reaction mixture (glucose hydrolysate and zeolite catalyst) should be sonicated at 60 °C for 15 min. When the conversion of rice straw derived glucose conducted under the suitable conditions, about 54.1% of HMF was achieved from glucose hydrolysate. The results indicated that HSO3–HZSM-5 zeolite can be considered as a promising catalyst for conversion of glucose to HMF, and further for lignocellulosic biomass conversion to chemicals and fuel.Conflicts of interest
There are no conflicts to declare.References
- Z. Hu, B. Liu, Z. Zhang and L. Chen, Conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by acidic ionic liquids in dimethyl sulfoxide, Ind. Crops Prod., 2013, 50, 264–269 CrossRef CAS.
- Z. Fang, B. Liu, J. Luo, Y. Ren and Z. Zhang, Efficient conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by the chromium-exchanged montmorillonite K-10 clay, Biomass Bioenergy, 2014, 60, 171–177 CrossRef CAS.
- I. K. M. Yu and D. C. W. Tsang, Bioresource Technology Conversion of biomass to hydroxymethylfurfural: a review of catalytic systems and underlying mechanisms, Bioresour. Technol., 2017, 238, 716–732 CrossRef CAS PubMed.
- R. Karinen, K. Vilonen and M. Niemelä, Biorefining: Heterogeneously catalyzed reactions of carbohydrates for the production of furfural and hydroxymethylfurfural, ChemSusChem, 2011, 4, 1002–1016 CrossRef CAS PubMed.
- L. Zhou, Y. He, Z. Ma, R. Liang, T. Wu and Y. Wu, One-step degradation of cellulose to 5-hydroxymethylfurfural in ionic liquid under mild conditions, Carbohydr. Polym., 2015, 117, 694–700 CrossRef CAS PubMed.
- L. Hu, G. Zhao, X. Tang, Z. Wu, J. Xu, L. Lin and S. Liu, Bioresource Technology Catalytic conversion of carbohydrates into 5-hydroxymethylfurfural over cellulose-derived carbonaceous catalyst in ionic liquid, Bioresour. Technol., 2013, 148, 501–507 CrossRef CAS PubMed.
- M. Zhang, X. Tong, R. Ma and Y. Li, Catalytic transformation of carbohydrates into 5-hydroxymethyl furfural over tin phosphate in a water-containing system, Catal. Today, 2016, 264, 131–135 CrossRef CAS.
- C. Van Nguyen, D. Lewis, W. Chen, H. Huang, Z. Abdullah, Y. Yamauchi and K. C. Wu, Combined treatments for producing 5-hydroxymethylfurfural (HMF) from lignocellulosic biomass, Catal. Today, 2016, 278, 344–349 CrossRef CAS.
- S. Xiao, B. Liu, Y. Wang, Z. Fang and Z. Zhang, Bioresource Technology Efficient conversion of cellulose into biofuel precursor 5-hydroxymethylfurfural in dimethyl sulfoxide – ionic liquid mixtures, Bioresour. Technol., 2014, 151, 361–366 CrossRef CAS PubMed.
- Z. Fang, B. Liu, J. Luo, Y. Ren and Z. Zhang, ScienceDirect Efficient conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by the, Biomass Bioenergy, 2013, 60, 171–177 CrossRef.
- X. Zhang, D. Zhang, Z. Sun, L. Xue and X. Wang, Applied Catalysis B: Environmental Highly efficient preparation of HMF from cellulose using temperature-responsive heteropolyacid catalysts in cascade reaction, Appl. Catal., B, 2016, 196, 50–56 CrossRef CAS.
- I. Jiménez-Morales, A. Teckchandani-Ortiz, J. Santamaría-González, P. Maireles-Torres and A. Jiménez-López, Selective dehydration of glucose to 5-hydroxymethylfurfural on acidic mesoporous tantalum phosphate, Appl. Catal., B, 2014, 144, 22–28 CrossRef.
- O. Yemis and G. Mazza, Bioresource Technology Optimization of furfural and 5-hydroxymethylfurfural production from wheat straw by a microwave-assisted process Oktay Yemis, Bioresour. Technol., 2012, 109, 215–223 CrossRef CAS PubMed.
- L. Q. Dien, N. T. Minh Phuong, P. H. Hoang and D. T. Hoa, Efficient Pretreatment of Vietnamese Rice Straw by Soda and Sulfate Cooking Methods for Enzymatic Saccharification, Appl. Biochem. Biotechnol., 2015, 175, 1536–1547 CrossRef CAS PubMed.
- N. T. M. Phuong, P. H. Hoang, L. Q. Dien and D. T. Hoa, Optimization of sodium sulfide treatment of rice straw to increase the enzymatic hydrolysis in bioethanol production, Clean Technol. Environ. Policy, 2017, 19, 1313–1322 CrossRef.
- P. H. Hoang, A. T. Hoang, N. H. Chung, X. P. Nguyen and X. D. Pham, The efficient lignocellulose-based sorbent for oil spill treatment from polyurethane and agricultural residue of Vietnam, Energy Sources, Part A, 2017, 40, 1–8 Search PubMed.
- Z. Fang, B. Liu, J. Luo, Y. Ren and Z. Zhang, Efficient conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by the chromium-exchanged montmorillonite K-10 clay, Biomass Bioenergy, 2014, 60, 171–177 CrossRef CAS.
- J. Li, Y. Ma, L. Wang, Z. Song, H. Li, T. Wang, H. Li and W. Eli, Catalytic Conversion of Glucose into 5-Hydroxymethylfurfural by Hf(OTf)4 Lewis Acid in Water, Catalysts, 2015, 6, 1 CrossRef.
- X. Li, K. Peng, Q. Xia, X. Liu and Y. Wang, Efficient conversion of cellulose into 5-hydroxymethylfurfural over niobia/carbon composites, Chem. Eng. J., 2018, 332, 528–536 CrossRef CAS.
- H. Xia, S. Xu, X. Yan and S. Zuo, High yield synthesis of 5-hydroxymethylfurfural from cellulose using FePO 4 as the catalyst, Fuel Process. Technol., 2016, 152, 140–146 CrossRef CAS.
- L. Hu, Z. Wu, J. Xu, Y. Sun, L. Lin and S. Liu, Zeolite-promoted transformation of glucose into 5-hydroxymethylfurfural in ionic liquid, Chem. Eng. J., 2014, 244, 137–144 CrossRef CAS.
- M. Moreno-recio, J. Santamaría-gonzález and P. Maireles-torres, Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural, Chem. Eng. J., 2016, 303, 22–30 CrossRef CAS.
- M. L. Sawkmie, D. Paul, G. Kalita, K. Agarwala, P. K. Maji and P. N. Chatterjee, Synthesis and characterization of active cuprous oxide particles and their catalytic application in 1,2,3-triazole synthesis via alkyne-azide cycloaddition reaction in water, J. Heterocycl. Chem., 2019, 56, 3277–3288 CrossRef CAS.
- P. H. Hoang, N. T. Nhung and L. Q. Dien, Synthesis of mesoporous Cr/ZSM-5 and W-Cr/ZSM-5 zeolite catalysts for oxidation of unsaturated fatty acid, AIP Adv., 2017, 7, 105311–105318 CrossRef.
- P. H. Hoang, B. Van Don and N. H. Chung, Cleavage of double bond using metal-loaded ZSM-5 zeolite catalysts for renewable biochemical application, Can. J. Chem. Eng., 2018, 9999, 1–7 Search PubMed.
- N. H. Chung, L. Q. Dien, T. D. Cuong, N. Van Lieu and P. H. Hoang, Influence of the acidity of solid catalyst HSO3-ZSM-5 on the hydrolysis of pretreated corncob, RSC Adv., 2018, 8, 41776–41781 RSC.
- P. H. Hoang, N. H. Chung and L. Q. Dien, Porous ZSM-5 zeolite catalyst modified with sulfonic acid functional groups for hydrolysis of biomass, J. Iran. Chem. Soc., 2019, 16, 2203–2210 CrossRef CAS.
- J. Luo, Z. Fang and R. L. Smith, Ultrasound-enhanced conversion of biomass to biofuels, Prog. Energy Combust. Sci., 2014, 41, 56–93 CrossRef.
- A. Sarwono, Z. Man, N. Muhammad, A. Sada, W. Suzaini, W. Hamzah, A. Hanim, A. Rahim, Z. Ullah and C. Devi, Ultrasonics Sonochemistry A new approach of probe sonication assisted ionic liquid conversion of glucose, cellulose and biomass into 5-hydroxymethylfurfural, Ultrason. Sonochem., 2017, 37, 310–319 CrossRef CAS PubMed.
- D. Paul, A. Borah, S. Khatua and P. Nath Chatterjee, para-Toluenesulfonic Acid-Catalyzed, Ultrasound-Promoted, One-Pot, Three-Component Coupling of Aldehydes, β-Dicarbonyls/Amides, and Electron-Rich Arenes, Asian J. Org. Chem., 2019, 8, 1870–1878 CrossRef CAS.
- S. Suganuma, K. Nakajima, M. Kitano, D. Yamaguchi, H. Kato, S. Hayashi and M. Hara, Hydrolysis of Cellulose by Amorphous Carbon Bearing SO 3 H, COOH, and OH Groups, J. Am. Chem. Soc., 2008, 130, 12787–12793 CrossRef CAS PubMed.
- A. Mukherjee, M.-J. Dumont and V. Raghavan, ScienceDirect Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities, Biomass Bioenergy, 2015, 72, 143–183 CrossRef CAS.
- N. H. Chung, V. T. Oanh, L. K. Thoa and P. H. Hoang, Catalytic Conversion of Glucose into 5-Hydroxymethyl Furfural Over Cu–Cr/ZSM-5 Zeolite, Catal. Lett., 2020, 150, 170–177 CrossRef CAS.
- C. Zhou, J. Zhao, A. E. G. A. Yagoub, H. Ma, X. Yu, J. Hu, X. Bao and S. Liu, Conversion of glucose into 5-hydroxymethylfurfural in different solvents and catalysts: Reaction kinetics and mechanism, Egypt. J. Pet., 2017, 26, 477–487 CrossRef.
- A. S. Amarasekara, L. D. Williams and C. C. Ebede, Mechanism of the dehydration of D-fructose to 5-hydroxymethylfurfural in dimethyl sulfoxide at 150 °C: an NMR study, Carbohydr. Res., 2008, 343, 3021–3024 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02037k |
| This journal is © The Royal Society of Chemistry 2020 |