Open Access Article
Open Access ArticleEnhancing the water splitting performance via decorating Co3O4 nanoarrays with ruthenium doping and phosphorization†
Jiaqi Niu a,
Jian Yang
a,
Jian Yang a,
Ali Imran Channa
a,
Ali Imran Channa b,
Eric Ashalley
b,
Eric Ashalley b,
Jiachao Yanga,
Jie Jianga,
Handong Lia,
Haining Ji*a and
Xiaobin Niu
b,
Jiachao Yanga,
Jie Jianga,
Handong Lia,
Haining Ji*a and
Xiaobin Niu *a
*a
aSchool of Materials and Energy, University of Electronic Science and Technology of China, Chengdu, 610054, PR China. E-mail: hainingji@uestc.edu.cn; xbniu@uestc.edu.cn
bInstitute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China, Chengdu, 610054, PR China
First published on 21st July 2020
Abstract
Hydrogen is the most promising renewable energy source to replace traditional fossil fuels for its ultrahigh energy density, abundance and environmental friendliness. Generating hydrogen by water splitting with highly efficient electrocatalysts is a feasible route to meet current and future energy demand. Herein, the effects of Ru doping and phosphorization treatment on Co3O4 nanoarrays for water splitting are systemically investigated. The results show that a small amount of phosphorus can accelerate hydrogen evolution reaction (HER) and the trace of Ru dopant can significantly enhance the catalytic activities for HER and oxygen evolution reaction (OER). Ru-doped cobalt phosphorous oxide/nickel foam (CoRuPO/NF) nanoarrays exhibit highly efficient catalytic performance with an overpotential of 26 mV at 10 mA cm−2 for HER and 342 mV at 50 mA cm−2 for OER in 1 M KOH solution, indicating superior water splitting performance. Furthermore, the CoRuPO/NF also exhibits eminent and durable activities for alkaline seawater electrolysis. This work significantly advances the development of seawater splitting for hydrogen generation.
1. Introduction
In recent years, the excessive consumption of fossil fuels has brought various environmental problems, such as greenhouse effect, smog or acid rain. Therefore, it is necessary to develop clean renewable energy sources as viable alternatives to fossil-based energy. Among all the known alternative sources of energy, hydrogen is a clean and renewable energy source with no by-products affecting the environment and no carbon release, promising to address future energy demand.1–6A promising method for generating hydrogen is direct electrolysis of water using electricity derived from solar or wind energy.7,8 This method is not only environmental friendly, but also cost-effective, as water exists in abundant quantity in nature.9–11 For the process of electrolyzing water, the electrocatalysts for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) play a critical role to improve the conversion efficiency.12,13 At present, platinum and ruthenium/iridium are ideal for HER and OER respectively.14,15 However, the high cost and scarcity of noble metals limit the large-scale application and commercialization of hydrogen energy.16 Thus, it is important to develop highly efficient electrocatalysts with low-cost and abundant sources in nature. Group VIII transition metals (TMs) and their oxides (TMOs) can serve as efficient catalysts for water electrolysis owing to their unique 3d electronic structure. Additionally, their low-cost and abundant reservoirs in nature makes them ideal for the large-scale water-splitting.17 Nickel foam (NF) is able to be introduced as the substrate for the growth of TMOs due to their strong interaction with each other and prominent specific surface area.18 The unique TMO/NF heterostructure can facilitate the charge transfer from NF electrode to surface active site.19 Moreover, the incorporating heteroatoms such as P, S, and Se in the TMOs can further improve the overall performance of the TMOs based catalysts by modulating the electronic structures of active sites.2,11,20 The transition metal phosphides (TMPs) exhibit outstanding performance of water splitting in alkaline solution. Wang et al. reported that the P-doped Co3O4/NF nanowires exhibit superior performance in HER.21 In addition, doping with trace of noble metal in TMPs can further improve the catalytic activities during water splitting process. Studies have shown that decorating TMOs with Ru as a dopant is an effective way to further enhance the catalytic activity of TMO's for its significantly similar hydrogen bond strength as Pt, which can considerably boost the catalytic activity in an alkaline solution.22–24 Furthermore, most investigations of water splitting are carried out in the aqueous solution originated from ultrapure water, which would increase the difficulty of developing hydrogen energy in freshwater-deficient area. In order to overcome this issue, Dionigi and co-workers exploited abundant seawater instead of ultrapure water as electrolyte. This can not only save fresh water resources, but also obtain clean potable water from seawater.25,26 Unfortunately, the current electrocatalysts have exhibited poor performance in seawater solutions i.e. high overpotentials and poor stability compared to ultrapure water as electrolyte.27–29 Therefore, it is important to find out new catalysts to address these problems.
In this work, P and Ru co-doped cobalt oxide nanoarrays supported on NF (CoRuPO/NF) is synthesized via hydrothermal reaction along with solid phase method. CoRuPO/NF used as a bifunctional catalyst for overall water electrolysis exhibits a very low overpotential of 26 mV at 10 mA cm−2 for HER and an overpotential of 342 mV at 50 mA cm−2 for the OER in the alkaline electrolyte. In addition, the electrocatalytic performance of CoRuPO/NF in alkaline solution prepared by real seawater (Huangyan Island Beach, South China Sea) are also investigated. The CoRuPO/NF shows enviable chemical stability and catalytic activities in the alkaline seawater electrolyte, making CoRuPO/NF heterostructure a promising electrocatalyst for long-term and sustainable water splitting.
2. Experimental
2.1 Materials
NF was bought from Alantum New Material Technology Co. Ltd. Sodium hypophosphite (NaH2PO2), cobaltous nitrate hexahydrate (Co(NO3)2·6H2O), ruthenium trichloride (RuCl3), KOH and KCl were supplied by Adamas-beat. Urea was purchased from Aladdin Ltd. (Shanghai, China). Ammonium fluoride (NH4F), HCl as well as ethanol was obtained from Chengdu Kelong Chemicals Co. Ltd. Pt/C (20 wt%) was purchased from Johnson Matthey company. 5 wt% of Nafion™ and RuO2 were from Sigma-Aldrich. Ultrapure water was further purified through a Millipore system. The seawater was obtained from near Huangyan Island Beach, South China Sea. All the chemical reagents used in this experiment were of analytical grade and applied as received without further processing.2.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v water/ethanol solvent by 30 min sonication to form an ink. Subsequently, 53.4 μL catalyst ink was loaded on NF (0.5 cm × 0.5 cm) and air-dried at room temperature.
1 v/v water/ethanol solvent by 30 min sonication to form an ink. Subsequently, 53.4 μL catalyst ink was loaded on NF (0.5 cm × 0.5 cm) and air-dried at room temperature.2.3 Characterization
3. Results and discussion
3.1 Morphology and structure characterization
Nanoarray CoRu(OH)F were grown on the surface of NF via a simple hydrothermal method with Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio of 1
Co ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. Subsequently, as-prepared CuRu(OH)F nanoarrays were given oxidation and phosphorylation treatment at a mild temperature to obtain CoRuPO/NF electrodes. The schematic illustrations in Fig. 1 demonstrate the synthesis route of CoRuPO/NF. The morphologies of synthesized samples and pristine NF were characterized using SEM. As shown in Fig. 2a, the pristine NF presents macroporous structure with smooth surface. After hydrothermal reaction and oxidation treatment, the Co3O4 nanowires grow and fully cover the surface of the NF (Fig. 2b). However, the porous structure of the NF was retained (Fig. S1†). Such a structure is beneficial for the distribution of active sites. When incorporating Ru or P dopants in Co3O4, the morphologies of CoRuPO/NF show no obvious variation and maintain the shape of urchin in Fig. 2c–f. The existence of such a porous nanostructure contributes to expose more active sites and the rapid mass transfer of reactants and products.4,17,31,32
10. Subsequently, as-prepared CuRu(OH)F nanoarrays were given oxidation and phosphorylation treatment at a mild temperature to obtain CoRuPO/NF electrodes. The schematic illustrations in Fig. 1 demonstrate the synthesis route of CoRuPO/NF. The morphologies of synthesized samples and pristine NF were characterized using SEM. As shown in Fig. 2a, the pristine NF presents macroporous structure with smooth surface. After hydrothermal reaction and oxidation treatment, the Co3O4 nanowires grow and fully cover the surface of the NF (Fig. 2b). However, the porous structure of the NF was retained (Fig. S1†). Such a structure is beneficial for the distribution of active sites. When incorporating Ru or P dopants in Co3O4, the morphologies of CoRuPO/NF show no obvious variation and maintain the shape of urchin in Fig. 2c–f. The existence of such a porous nanostructure contributes to expose more active sites and the rapid mass transfer of reactants and products.4,17,31,32
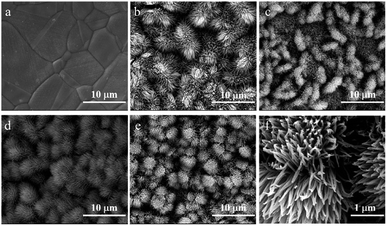 | ||
| Fig. 2 SEM images of (a) blank NF, (b) Co3O4/NF, (c) CoRuO/NF, (d) CoPO/NF, and (e and f) CoRuPO/NF film at different magnifications. | ||
The elemental composition of the samples was studied by EDS. As shown in Fig. 3a, the EDS spectrum of CoRuPO/NF demonstrates the presence of all the necessary elements such as Co, P, Ru and O in the CoRuPO/NF, and the composition percentage of the main elements is summarized in Table S1.† The results indicate that Ru and P have been successfully incorporated in the Co3O4 nanoarrays (Fig. S2†). XRD was employed to study the crystal structure of samples. As shown in Fig. 3b, the sharp diffraction peaks of CoRuPO/NF at 44.5°, 51.8° and 76.4° correspond to (111), (200) and (220) planes of nickel, owing to the NF substrate. The diffraction peaks located at 31.2°, 38.5°, 59.4°and 67.3° correspond to the (220), (311), (511) and (440) planes of Co3O4 respectively.21,33,34
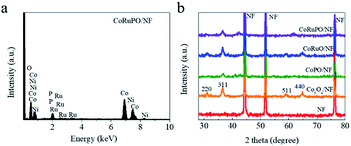 | ||
| Fig. 3 (a) EDS spectrum of as synthesized CoRuPO/NF catalyst, and (b) XRD patterns of pure NF, Co3O4/NF, CoPO/NF, CoRuO/NF, and CoRuPO/NF. | ||
To further determine the elemental composition and chemical states of the samples, XPS was performed. The full survey XPS spectrum of the CoRuPO/NF sample displayed in Fig. 4a clearly demonstrates the presence of Co, Ni, O, C, P and Ru elements, which is consistent with the EDS results in Fig. 3a. Furthermore, the high resolution XPS (HRXPS) spectrum of Co 2p is deconvoluted into four peaks (Fig. 4b). The corresponding subpeaks at 780.9 eV and 796.5 eV can be attributed to Co 2p3/2 and Co 2p1/2, respectively. The satellite peaks observed at 795.0 eV and 781.1 eV can be ascribed to Co with oxidation states Co3+ and Co2+.35–37 The HRXPS spectrum of O 1s reveals three peaks at binding energies of 529.45 eV, 530.18 eV and 531.0 eV, which are attributed to metal–oxygen bonds in the spinel lattice, surface adsorbed oxygen, and O from the surface of absorbed H2O due to P doping, respectively (Fig. S3†).38 The HRXPS spectrum of P (Fig. 4c) exhibits two peaks at 126.6 eV and 128.0 eV corresponding to P 2p3/2 and P 2p1/2 respectively, and the peak at 132.8 eV can be ascribed to the oxidized phosphate species.35,39 The deconvolution of HRXPS spectrum of Ru 3d obtained two peaks at 281.4 eV and 284.2 eV (Fig. 4d), originated from Ru 3d5/2 and Ru 3d3/2 respectively.40,41 Comparing the Co 2p and O 1s peaks of CoRuO/NF, CoPO/NF and CoRuPO/NF (Fig. S4†), it can be seen that after the Ru or P is doped, the binding energy changes, which may be due to the alteration in the electron density caused by doping.21 The XPS analysis infers the successful synthesis of CoRuPO/NF.
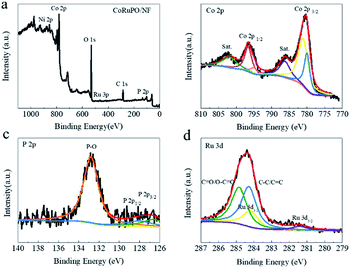 | ||
| Fig. 4 (a) XPS survey spectrum, high resolution XPS spectra of (b) Co 2p, (c) P 2p, and (d) Ru 3d for CoRuPO/NF. | ||
3.2 Electrochemical performance characterization
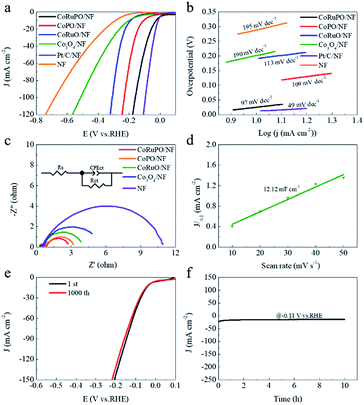
The electrochemical tests of the samples were carried out in a 1.0 M KOH solution utilizing a standard three-electrode system. Fig. 5a displays the polarization curves of as prepared electrodes with a scan rate of 10 mV s−1, and the corresponding overpotential (η10) obtained at 10 mA cm−2 for HER. As expected, the LSV curves of the synthesized electrodes are positively shifted after modification by the Ru and P dopants compared with bare NF. For example, as a substrate material, the η10 of NF is 288 mV. The η10 of Co3O4/NF becomes 202 mV after growing Co3O4 nanoarray on the surface NF, suggesting that inducing Co3O4 nanoarray is beneficial to improve the activity of electrode. There can be two reasons for this result. First, the intrinsic catalytic activity of Co3O4 is higher than that of pristine NF. The second is that nanoarray of Co3O4 can magnify the specific surface area of electrodes and lead to more active sites participation for the HER. After decorating Co3O4 nanoarray with doping Ru or phosphorization, the catalytic performances of CoRuO/NF and CoPO/NF are improved with a η10 of 191 mV and 106 mV, respectively. Surprisingly, the η10 of CoRuPO/NF is only 26 mV. This value is higher than the Pt/C/NF (13 mV), but its mass activity is more excellent (as seen in Table S2†). This result is attributed to the strong hydrogen bond strength of Ru atoms, which slows down the hydrogen release and promotes the adsorption and desorption of hydrogen.42,43 The reaction mechanism and kinetics of as-synthesized catalytic electrodes towards the HER are also evaluated by the Tafel slopes, as depicted in Fig. 5b. CoRuPO/NF exhibited a Tafel slope of 97 mV dec−1, which is higher than Pt/C (49 mV dec−1), suggesting that it is still slightly inferior to Pt/C/NF in kinetics. However, compared with the CoPO/NF and CoRuO/NF, the Tafel slope is lower-indicating the favorable HER kinetics for the CoRuPO/NF based electrode is a Volmer–Heyrovsky route. The incorporation of Ru and P changes the electron density around Co, causing a part of Co2+ to Co3+, thereby generating enriched empty d orbitals. The empty d orbitals can promote the adsorption of OH groups and H atoms to improve the kinetics of the Volmer step.44,45 Doping Ru and P in Co3O4 nanoarray is an effective way to enhance the HER performance.In order to investigate the interfacial reactions and the electrode kinetics for HER, we performed EIS for the samples. The Nyquist diagram in Fig. 5c presents semicircles and the corresponding electrochemical parameters are summarized in Table 1. Moreover, the equivalent circuit is represented in the inset of Fig. 5c. The charge transfer resistance (Rct) between the CoRuPO/NF catalyst based electrode and electrolyte interface is lowest (2.021 ohm cm2), indicating a fast electron transfer rate and rapid electrocatalytic kinetics for HER.46 Fig. 5d displays the CV curves of CoRuPO/NF with different scan rates, and the corresponding double layer capacitance (Cdl) is 12.12 mF cm−2 via an archetypical CV method.47,48 The electrochemical active surface area (ECSA) was estimated by Cdl according to the formula presented in eqn (1) below.49
| ECSA = Cdl/Cs | (1) |
| Electrodes | Rs/ohm cm2 | Rct/ohm cm2 |
|---|---|---|
| NF | 0.635 | 8.884 |
| Co3O4/NF | 0.251 | 4.995 |
| CoRuO/NF | 0.264 | 3.483 |
| CoPO/NF | 0.278 | 2.686 |
| CoRuPO/NF | 0.206 | 2.021 |
The long-term stability is another key factor to evaluate an electrocatalyst for the sustainable energy conversion. Firstly, we probed the stability of the CoRuPO/NF based electrode by performing CV scan between −0.22 and +0.12 V vs. RHE at a scan rate of 100 mV s−1 in 1.0 M KOH as electrolyte. After 1000 CV cycles, the polarization curve is almost overlapping the initial ones (Fig. 5e). Moreover, the current vs. time (i–t) curve of CoRuPO/NF electrode is carried out by chronoamperometry measurement for 10 hours at a constant voltage of −0.11 V vs. RHE. As shown in Fig. 5f, the current density has no significant phase change compared with the initial current density after 10 hours of continuous large current operation, demonstrating excellent stability of CoRuPO/NF electrode.
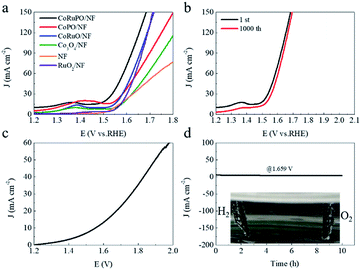
The OER catalytic performance of prepared catalysts were also analyzed for estimating the bifunctional activities for water electrolysis. Although the LSV curves did not display the same behavior for OER compared to HER, it can be seen that the overpotential of CoRuPO/NF for OER is only 342 mV to reach 50 mA cm−2 (see Fig. 6a), which is better than RuO2/NF (397 mV). Meanwhile, the mass activity of CoRuPO/NF is as high as 1192 mA mg−1 at the overpotential of 250 mV vs. RHE, which is 4 times relative to RuO2/NF (Table S3†). Doping Ru and P is an effective way to adjust the electronic structure and enhance the electrochemical performance. Moreover, the synergistic effect between Ru and Co can ameliorate the conductivity of Co3O4 to improve the catalytic activities for OER.56,57 In addition, the LSV curve of CoRuPO/NF changes slightly after treated by CV scan with 1000 cycles. Based on the above results, it can be concluded that CoRuPO/NF can serve as the bifunctional electrocatalyst for overall water electrolysis. A two-electrode system in which CoRuPO/NF sheets deployed both as anode and cathode is assembled to further monitor the performance of full water splitting. As shown in Fig. 6c, a current density of 10 mA cm−2 is obtained at a voltage of 1.56 V, indicating a low potential in favour of water electrolysis.58 This result is attributed to the superior catalytic activities for HER and OER. The stability measurements in Fig. 6d for 10 hours by chronoamperometry exhibit no obvious loss of current density, revealing the outstanding durability of CoRuPO/NF.
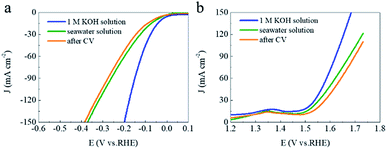
In most of previous researches related to water splitting, the electrolytes were based on ultrapure water or fresh water and rarely from the seawater. Therefore, investigating the seawater splitting is a significant work for developing hydrogen energy in freshwater deficient areas. As shown in Fig. 7a and b, the LSV curves of CoRuPO/NF for HER and OER have obvious shifts in seawater electrolyte compared to those of ultrapure water electrolytes, which is possibly due to the poisoning of impurities in seawater. But its overpotential is superior to that of most previously reported catalysts in natural and alkaline seawater solution (As shown in Tables 2 and 3). In addition, the electrocatalyst CoRuPO/NF displays superior durability in seawater even after 1000 cycles of CV without obvious shift for HER and OER, illustrating that the CoRuPO/NF is an outstanding candidate for direct seawater splitting.
4. Conclusions
The Ru and P decorating Co3O4 nanoarrays catalyst supported in NF (CoRuPO/NF) was synthesized using hydrothermal method and post-treatments. SEM images showed nanowire morphology of CoRuPO uniformly distributed on NF substrate. EDS and XPS revealed the presence of all the elements such as Co, Ru, P and O in CoRuPO/NF. XRD revealed high crystalline quality of synthesized CoRuPO/NF. The electrochemical results indicated that doping Ru and P is an effective way to improve the catalytic performance towards HER and OER for their synergistic effect. The CoRuPO/NF exhibited superior HER and OER catalytic activities in alkaline solution as well as marvelous stability. Moreover, the seawater splitting performance of CoRuPO/NF was also studied, and the result indicated that CoRuPO/NF possesses the potential of catalysis for efficient seawater splitting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 61974014), the National Program on Key Basic Research Project (Grant No. SQ2019YFB220038, 2018YFA0306102), and the Sichuan Science and Technology Program under (Grant No. 2019YJ0202).References
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- S. Anantharaj, S. R. Ede, K. Sakthikumar, K. Karthick, S. Mishra and S. Kundu, ACS Catal., 2016, 6, 8069–8097 CrossRef CAS.
- H. Wang, Y. Li, R. Wang, B. He and Y. Gong, Electrochim. Acta, 2018, 284, 504–512 CrossRef CAS.
- J. Yang, C. Cai, Y. Li, L. Gao, H. Guo, B. Wang, B. Pu and X. Niu, Electrochim. Acta, 2018, 262, 48–56 CrossRef CAS.
- M. S. Faber and S. Jin, Energy Environ. Sci., 2014, 7, 3519–3542 RSC.
- M. Zhou, H. L. Wang and S. Guo, Chem. Soc. Rev., 2016, 45, 1273–1307 CAS.
- J. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri and X. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS PubMed.
- A. J. Esswein, M. J. McMurdo, P. N. Ross, A. T. Bell and T. D. Tilley, J. Phys. Chem. C, 2009, 113, 15068–15072 CrossRef CAS.
- M. T. Koper, Nat. Chem., 2013, 5, 255–256 CrossRef CAS PubMed.
- B. D. Stubbert, J. C. Peters and H. B. Gray, J. Am. Chem. Soc., 2011, 133, 18070–18073 CrossRef CAS PubMed.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- Y. Yan, B. Y. Xia, B. Zhao and X. Wang, J. Mater. Chem. A, 2016, 4, 17587–17603 RSC.
- J. Yang, H. Guo, S. Chen, Y. Li, C. Cai, P. Gao, L. Wang, Y. Zhang, R. Sun, X. Niu and Z. Wang, J. Mater. Chem. A, 2018, 6, 13859–13866 RSC.
- S. Bai, C. Wang, M. Deng, M. Gong, Y. Bai, J. Jiang and Y. Xiong, Angew. Chem., Int. Ed., 2014, 53, 12120–12124 CrossRef CAS PubMed.
- D.-S. Liu, J. Wu, Y. Wang, H. Ji, L. Gao, X. Tong, M. Usman, P. Yu and Z. Wang, RSC Adv., 2017, 7, 11987–11997 RSC.
- S. Yang, L. Chen, W. Wei, X. Lv and J. Xie, Appl. Surf. Sci., 2019, 476, 749–756 CrossRef CAS.
- P. Babar, A. Lokhande, H. H. Shin, B. Pawar, M. G. Gang, S. Pawar and J. H. Kim, Small, 2018, 14, 1702568 CrossRef PubMed.
- X. Hu, Q. Zhou, P. Cheng, S. Su, X. Wang, X. Gao, G. Zhou, Z. Zhang and J. Liu, Appl. Surf. Sci., 2019, 488, 326–334 CrossRef CAS.
- C. Zhang, J. Xiao, X. Lv, L. Qian, S. Yuan, S. Wang and P. Lei, J. Mater. Chem. A, 2016, 4, 16516–16523 RSC.
- J.-T. Ren, L. Chen, G.-G. Yuan, C.-C. Weng and Z.-Y. Yuan, Electrochim. Acta, 2019, 295, 148–156 CrossRef CAS.
- Z. Wang, H. Liu, R. Ge, X. Ren, J. Ren, D. Yang, L. Zhang and X. Sun, ACS Catal., 2018, 8, 2236–2241 CrossRef CAS.
- J. Mahmood, F. Li, S. M. Jung, M. S. Okyay, I. Ahmad, S. J. Kim, N. Park, H. Y. Jeong and J. B. Baek, Nat. Nanotechnol., 2017, 12, 441–446 CrossRef CAS PubMed.
- K. Yang, P. Xu, Z. Lin, Y. Yang, P. Jiang, C. Wang, S. Liu, S. Gong, L. Hu and Q. Chen, Small, 2018, 14, 1803009 CrossRef PubMed.
- J. Zhang, P. Liu, G. Wang, P. Zhang, x. zhuang, M. CHEN, I. Weidinger and X. Feng, J. Mater. Chem. A, 2017, 5, 25314–25318 RSC.
- F. Dionigi, T. Reier, Z. Pawolek, M. Gliech and P. Strasser, ChemSusChem, 2016, 9, 962–972 CrossRef CAS PubMed.
- S. Dresp, F. Dionigi, S. Loos, J. Ferreira de Araujo, C. Spöri, M. Gliech, H. Dau and P. Strasser, Adv. Energy Mater., 2018, 8, 1800338 CrossRef.
- F. Cheng, X. Feng, X. Chen, W. Lin, J. Rong and W. Yang, Electrochim. Acta, 2017, 251, 336–343 CrossRef CAS.
- J. Zheng, Electrochim. Acta, 2017, 247, 381–391 CrossRef CAS.
- L. Yu, Q. Zhu, S. Song, B. McElhenny, D. Wang, C. Wu, Z. Qin, J. Bao, Y. Yu, S. Chen and Z. Ren, Nat. Commun., 2019, 10, 1–10 CrossRef PubMed.
- R. Yuan, L. Hu, P. Yu, H. Wang, Z. Wang and J. Fang, Chemosphere, 2018, 198, 204–215 CrossRef CAS PubMed.
- L. Li, L. Song, H. Xue, C. Jiang, B. Gao, H. Gong, W. Xia, X. Fan, H. Guo, T. Wang and J. He, Carbon, 2019, 150, 446–454 CrossRef CAS.
- W. Chang, W. Xue, E. Liu, J. Fan and B. Zhao, Chem. Eng. J., 2019, 362, 392–401 CrossRef CAS.
- S. Liu, Y. Jiang, M. Yang, M. Zhang, Q. Guo, W. Shen, R. He and M. Li, Nanoscale, 2019, 11, 7959–7966 RSC.
- Y. Gong, Y. Lin, Z. Yang, F. Jiao, J. Li and W. Wang, Appl. Surf. Sci., 2019, 476, 840–849 CrossRef CAS.
- Y.-P. Zhu, Y.-P. Liu, T.-Z. Ren and Z.-Y. Yuan, Adv. Funct. Mater., 2015, 25, 7337–7347 CrossRef CAS.
- H. Schäfer, D. M. Chevrier, K. Kuepper, P. Zhang, J. Wollschlaeger, D. Daum, M. Steinhart, C. Heß, U. Krupp, K. Müller-Buschbaum, J. Stangl and M. Schmidt, Energy Environ. Sci., 2016, 9, 2609–2622 RSC.
- L. Fu, Z. Liu, Y. Liu, B. Han, P. Hu, L. Cao and D. Zhu, Adv. Mater., 2005, 17, 217–221 CrossRef CAS.
- X. Wu and K. Scott, Int. J. Hydrogen Energy, 2013, 38, 3123–3129 CrossRef CAS.
- C. Tang, R. Zhang, W. Lu, Z. Wang, D. Liu, S. Hao, G. Du, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2017, 56, 842–846 CrossRef CAS PubMed.
- P.-F. Zhang, Y.-Q. Lu, Y.-J. Wu, Z.-W. Yin, J.-T. Li, Y. Zhou, Y.-H. Hong, Y.-Y. Li, L. Huang and S.-G. Sun, Chem. Eng. J., 2019, 363, 224–233 CrossRef CAS.
- E. Serra-Pérez, S. Álvarez-Torrellas, V. Ismael Águeda, J. A. Delgado, G. Ovejero and J. García, Appl. Surf. Sci., 2019, 473, 726–737 CrossRef.
- J. Yang, B. Chen, X. Liu, W. Liu, Z. Li, J. Dong, W. Chen, W. Yan, T. Yao, X. Duan, Y. Wu and Y. Li, Angew. Chem., Int. Ed., 2018, 57, 9495–9500 CrossRef CAS PubMed.
- Z. Pu, I. S. Amiinu, Z. Kou, W. Li and S. Mu, Angew. Chem., Int. Ed., 2017, 56, 11559–11564 CrossRef CAS PubMed.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chorkendorff and J. K. Norskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed.
- Y. Yang, K. Zhang, H. Lin, X. Li, H. C. Chan, L. Yang and Q. Gao, ACS Catal., 2017, 7, 2357–2366 CrossRef CAS.
- H. Zhang, X. Li, A. Hähnel, V. Naumann, C. Lin, S. Azimi, S. L. Schweizer, A. W. Maijenburg and R. B. Wehrspohn, Adv. Funct. Mater., 2018, 28, 1706847 CrossRef.
- L. Peng, J. Shen, L. Zhang, Y. Wang, R. Xiang, J. Li, L. Li and Z. Wei, J. Mater. Chem. A, 2017, 5, 23028–23034 RSC.
- Y.-Y. Ma, C.-X. Wu, X.-J. Feng, H.-Q. Tan, L.-K. Yan, Y. Liu, Z.-H. Kang, E.-B. Wang and Y.-G. Li, Energy Environ. Sci., 2017, 10, 788–798 RSC.
- V. Kannan, A. I. Inamdar, S. M. Pawar, H. S. Kim, H. C. Park, H. Kim, H. Im and Y. S. Chae, ACS Appl. Mater. Interfaces, 2016, 8, 17220–17225 CrossRef CAS PubMed.
- C. C. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- H. Li, Q. Tang, B. He and P. Yang, J. Mater. Chem. A, 2016, 4, 6513–6520 RSC.
- Q. Lv, J. Han, X. Tan, W. Wang, L. Cao and B. Dong, ACS Appl. Energy Mater., 2019, 2, 3910–3917 CrossRef CAS.
- Y. Zhang, P. Li, X. Yang, W. Fa and S. Ge, J. Alloys Compd., 2018, 732, 248–256 CrossRef CAS.
- H. J. Song, H. Yoon, B. Ju, D.-Y. Lee and D.-W. Kim, ACS Catal., 2019, 10, 702–709 CrossRef.
- A. J. Esswein, Y. Surendranath, S. Y. Reece and D. G. Nocera, Energy Environ. Sci., 2011, 4, 499–504 RSC.
- D. Yang, D. Bhattacharjya, S. N. Inamdar, J. Park and J. Yu, J. Am. Chem. Soc., 2012, 134, 16127–16130 CrossRef CAS PubMed.
- H. Liu, G. Xia, R. Zhang, P. Jiang, J. Chen and Q. Chen, RSC Adv., 2017, 7, 3686–3694 RSC.
- C. Xuan, Z. Peng, K. Xia, J. Wang, W. Xiao, W. Lei, M. Gong, T. Huang and D. Wang, Electrochim. Acta, 2017, 258, 423–432 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02128h |
| This journal is © The Royal Society of Chemistry 2020 |