Open Access Article
Open Access ArticleProperties of PEDOT nanowire/Te nanowire nanocomposites and fabrication of a flexible thermoelectric generator
Haihui
Liu
 *,
Pengfei
Liu
,
Mengqi
Zhang
,
Zihan
Tian
,
Ning
Wang
,
Yanxin
Liu
and
Xingxiang
Zhang
*,
Pengfei
Liu
,
Mengqi
Zhang
,
Zihan
Tian
,
Ning
Wang
,
Yanxin
Liu
and
Xingxiang
Zhang
Tiangong University, No. 399, West Binshui Road, Xi Qing District, Tianjin, 300387, China. E-mail: haihui_liu@aliyun.com
First published on 14th September 2020
Abstract
Light-weight, mechanically flexible, transparent thermoelectric devices are promising as portable, and easy-to-integrate energy sources. Poly(3,4-ethylenedioxythiophene) nanowires (PEDOT NWs) possessing high electrical conductivity were synthesized by a facile self-assembled micellar soft-template method. And then, Te nanowires (Te NWs) with high Seebeck coefficient were easily synthesized by the solution process and then added as an inorganic filler to form the PEDOT NW/Te NW nanocomposite films via a simple and convenient vacuum filtration method. The thermoelectric (TE) properties of the nanocomposites were characterized in this research. A maximum power factor of 58.03 μW m−1 K−2 is obtained from the film containing 90 wt% Te NWs at room temperature, which is dozens of times that of the pure PEDOT NW film. This work uses the as-prepared PEDOT NWs/Te NW (90 wt%) nanocomposite film to fabricate a flexible thermoelectric generator and an output voltage of 2.8 mV was generated at a temperature difference of 13.5 K between the environment and human body.
1. Introduction
In recent years, thermoelectric generator (TEG) devices have gradually been recognized as a viable alternative to power generating applications.1–3 Thermoelectric generator devices can directly convert thermal energy that passes through them into electricity, which is a pollution-free, convenient and safe conversion technology, and plays an irreplaceable role in environmental friendliness and sustainable development. With the continuous development of portable/wearable and self-powered electronic devices, flexible thermoelectric generators have become a promising power source.4,5 The advantage of thermoelectric generators is that they have no moving parts and can run continuously without any need for supplementary materials.6 And flexible thermoelectric generators benefit wearable applications due to their good fit with human skin, thus helping to collect human heat with minimal energy loss.7 The efficiency of a TE generator is governed by the thermoelectric materials' figure of merit ZT, ZT = S2σ/κ, where T is the absolute temperature, S is the Seebeck coefficient, σ is the electrical conductivity, κ is the thermal conductivity and the power factor (PF) is S2σ. Applied to the field of thermoelectric, materials should exhibit a large power factor and low thermal conductivity.8 However, for a given material, the S, σ and κ of thermoelectric materials are highly interdependent and conflict with each other, which causes the difficulty in obtaining high ZT.9,10Conventional inorganic materials such as Bi2Te3,11 PbTe12 and Sb2Te3 (ref. 13) have attracted wide attention due to their outstanding thermoelectric properties. However, the expensive raw materials, poor processability and the toxicity restrict the application of inorganic materials in the field of thermoelectrics.14–16 Conducting polymers are now believed to be potential candidates for TE materials.17–19 Conducting polymers possess many advantages of light-weight, low cost, mechanical flexibility and enabling the development of portable and wearable thermoelectric devices.20–22 The development of portable/wearable TE devices has become an area of increasing interest because this device has the potential to use the temperature difference between the human body and its environment to continuously convert human body heat into electrical energy.23 Moreover, their thermal conductivity is also much lower than that of inorganic materials, which is beneficial to the enhancement of the ZT value. Poly(3,4-ethylenedioxythiophene) (PEDOT) is one of the most promising polymer materials for practical applications in thermoelectric device due to the stability and high conductivity of the p-doped state.24,25 In order to improve the solubility, PEDOT needs to be emulsified with PSS in water to form PEDOT:PSS. But the PEDOT:PSS film prepared from its aqueous solution has the electrical conductivity of 0.15 ± 0.01 S cm−1,26 because in the mixture of PEDOT and PSS, PEDOT is responsible for conducting, whereas PSS is insulated. In general, there are two strategies to optimize the thermoelectric properties of the PEDOT:PSS. In one strategy, it can be secondly doped by a range of chemicals, including ethylene glycol (EG),27 dimethyl sulfoxide (DMSO)28,29 or sulfuric acid (H2SO4).30–32 The excess insulating PSS could be removed by treatments process and the PEDOT component transforms from a coil to a expanded-coil or linear conformation, leading to an enhancement of electrical conductivity while the Seebeck coefficient remained essentially constant.33 In the another strategy, PEDOT:PSS can be mixed with inorganic components which possess large Seebeck coefficients to form PEDOT:PSS/inorganic composites, such as PEDOT:PSS/Bi2Te3,34 PEDOT:PSS/PbTe35 and PEDOT:PSS/SnSe.36 One-dimensional (1D) nanostructures of inorganic materials were typically used as filers to improve the TE properties. One-dimensional Te nanostructures are widely used as inorganic filers to mix with polymer because of the high Seebeck coefficients, the interface phonon scattering effect and the quantum size effect.37,38 In addition to the “energy-filtering” process,39 low-energy carriers are preferentially scattered by the potential barrier at the more interface between polymer matrix and inorganic nanostructures, whereas high-energy carriers easily through cross these barriers, resulting in an increase in S. See et al.40 reported the hybrid materials of PEDOT:PSS/Te nanorods exhibit a large room temperature ZT of 0.1. Song et al.41 prepared PEDOT:PSS/PF-Te composites and a maximum power factor of 51.4 μW m−1 K−2 was obtained for the composite film containing 70 wt% PF-Te.
In this work, we have firstly prepared one-dimensional PEDOT NWs, which does not need to be mixed with PSS but have good water solubility, and then, we choose Te NWs as inorganic filler to mixed with PEDOT NWs to optimized the TE properties of materials by taking the advantages of the high S of the Te NWs and the low κ of the polymer. The nanocomposite film has excellent room temperature power factor. The Seebeck coefficient and electrical conductivity of the PEDOT NWs/Te NWs (90 wt%) from 100 to 300 K are also reported. The prepared thermoelectric film device shows excellent power generation capacity and the novel method for manufacturing thermoelectric device with PEDOT NWs/Te NWs (90 wt%) composite film provides a good application prospect of the polymer/inorganic flexible materials in the field of portable/wearable thermoelectric devices.
2. Experimental
2.1 Materials
3,4-Ethylenedioxythiophene (EDOT, purity > 99%), sodium dodecyl sulfate (SDS, AR), anhydrous ferric chloride (FeCl3, AR), tellurium dioxide (TeO2, 99.99%), polyvinyl pyrrolidone (PVP, MW ∼ 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and L-ascorbic acid (AA, 99%) were purchased from Aladdin. Potassium hydroxide (KOH, ≥85%), and ethyl alcohol (AR) were provided by Tianjin Fengchuan Chemical Reagents Factory. Ethylene glycol (EG, ≥99%) was provided by Guangfu Fine Chemical Research Institute. All chemical reagents were used as received.
000) and L-ascorbic acid (AA, 99%) were purchased from Aladdin. Potassium hydroxide (KOH, ≥85%), and ethyl alcohol (AR) were provided by Tianjin Fengchuan Chemical Reagents Factory. Ethylene glycol (EG, ≥99%) was provided by Guangfu Fine Chemical Research Institute. All chemical reagents were used as received.
2.2 Synthesis of PEDOT NWs and Te NWs
PEDOT NWs were synthesized by EDOT monomer with cylindrical SDS micellar as template. First, desired amounts of 1.08 g SDS were dissolved in distilled water to form micellar solution. Then, an aqueous solution of FeCl3 (0.304 g FeCl3 dissolved in 10 mL water) was injected into the above micellar solution to promote the transformation from aspherical micelles to cylindrical micellar with stirring at property temperature. Finally, EDOT monomer was slowly added into the micellar solution and the polymerization was subsequently induced by FeCl3. The whole reaction was carried out at 50 °C with continuous stirring for 6 h. Finally, the obtained products were centrifuged and washed repeatedly with ethanol and deionized water.For synthesis of Te NWs, 15 mL EG, 1.5 g KOH, 0.5 g PVP and 0.16 g TeO2, were added into a three-neck flask. And then the mixture was heated to 120 °C under nitrogen. Meanwhile, 1.89 M L-ascorbic acid aqueous solution was prepared in a beaker by stirring and heating to 80 °C. 3 mL of 1.89 M L-ascorbic acid solution was rapidly injected as a reducing agent into the mixture solution when it reached to 120 °C. The Te NWs were successfully synthesized after the reaction process was performed at 120 °C for 24 h. The as-synthesized Te NWs were separated from solution by centrifugation at 8000 rpm for 40 min. And the Te NWs were then redissolved in 1 M potassium hydroxide aqueous to remove the residual PVP. Finally, the obtained Te NWs were centrifuged and washed several times with deionized water and ethanol.
2.3 Sample preparation
The PEDOT NWs aqueous solution was mixed with the Te NWs aqueous solution at the designed ratios. Then the mixture solution was ultrasonically dispersed for 10 min. The porous PTFE membrane (0.45 μm) as a base and the simple vacuum filtration was used to prepare PEDOT NWs/Te NWs nanocomposite thin films. Finally, the as-prepared nanocomposite films were dried at 50 °C under vacuum for 12 h.2.4 Characterization
Transmission electron microscopy (TEM) images were observed by a Hitachi H7650 transmission electron microscope operating at 120 kV. Scanning Electron Microscope (SEM) images were obtained by a ZEISS Gemini SEM500 scanning electron microscope operating at 20 kV. The wide-angle X-ray patterns were collected by a Bruker D8 ADVANCE X-ray diffractometer with Cu Kα1 (λ = 1.541 Å) radiation. The Seebeck coefficient of nanocomposite films was measured by a portable Seebeck coefficient tester (PTM-demo). For electrical conductivity, Hall effect testing instrument (Swin HALL8800) was used. The characterization of thermal conductivity was tested by the thin film material testing equipment produced by Beijing Multi-field Technology Company.3. Results and discussion
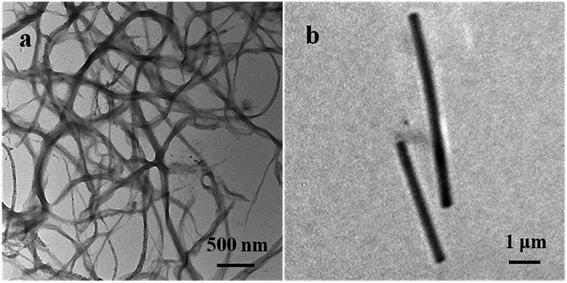
The morphology of the prepared PEDOT NWs and Te NWs are characterized by TEM. Fig. 1a shows the clearly microscopic morphology of the prepared PEDOT NWs. A nanowire bundle consists of many individual nanowires. The average diameter of a single PEDOT NWs is about 100 nm and the length of nanowires can reach several micrometers. As the Fig. 1b shown, it can be observed that the surface of Te NWs is relatively smooth and uniform in size with a diameter of about Te NWs 400 nm and a length of up to 10 μm.The XRD peaks are shown in Fig. 2a, all diffraction peaks of the synthesized Te NWs are consistent with the crystal structure of pure Te phase, and it can be indexed to a trigonal structure Te phase with lattice parameters of a = 4.4328 Å and c = 5.9466 Å, which are consistent with the standard PDF card (JCPDS card no. 36-1452). No diffraction peaks of other phases were detected, indicating that we obtained the pure Te NWs by solution process. As showed in Fig. 2b, the broad peak at 2θ = 25.8° corresponds to the structure of PEDOT chain.42,43 The diffraction peaks of both PEDOT NWs and Te NWs can be observed in the XRD spectrum of the nanocomposite films. When the content of Te NWs content in the composite film is low, the diffraction peaks are not easily observed. As the content of Te NWs increases, the diffraction peaks of Te NWs become more obvious and they are very strong for the nanocomposite with 90 wt% Te NWs.
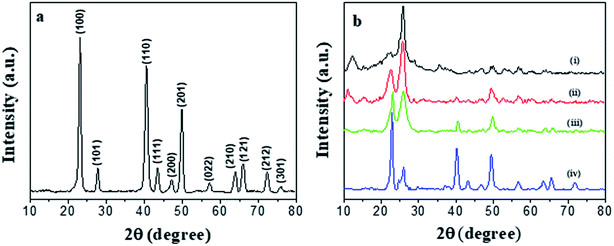 | ||
| Fig. 2 XRD patterns of Te NWs (a), XRD patterns of different Te NWs contents (b), PEDOT NWs (i), and PEDOT NWs/Te NWs nanocomposite films with 10 wt% (ii), 50 wt% (iii) and 90 wt% (iv) Te NWs. | ||
Fig. 3a shows SEM images of pure PEDOT NWs film. PEDOT NWs in the film formed by vacuum filtration is interwoven together, which may be for this reason that our PEDOT NWs film exhibit high electric conductivity. Fig. 3b and c show SEM images of composite films with different Te NWs contents. Randomly distributed Te NWs can be observed, indicating that Te NWs are well dispersed in PEDOT matrix and form a homogeneous film structure. Clearly, due to the higher electron density of inorganic Te NWs than PEDOT NWs, the Te NWs of the composite films appear bright while the PEDOT NWs matrix appear black or grey in the SEM images. In the Fig. 3d, a higher magnification SEM image clearly shows that Te NWs and PEDOT NWs interweave to form a network microstructure and many polymer/inorganic interfaces.
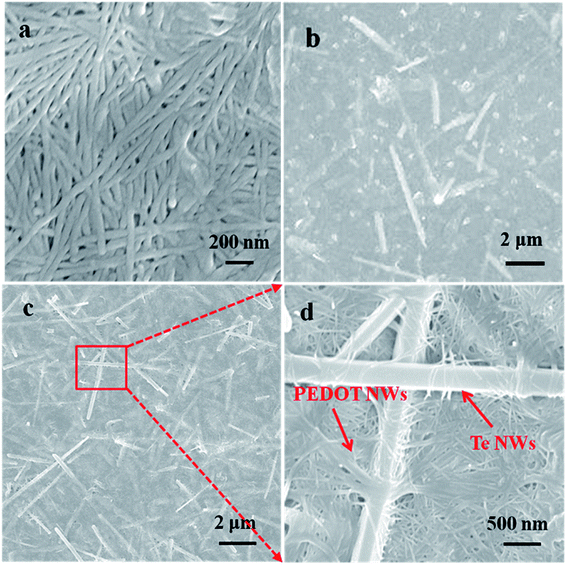 | ||
| Fig. 3 SEM image of pure PEDOT NWs film (a), PEDOT NWs/Te NWs thin film with 50 wt% Te NWs (b) and 90 wt% Te NWs (c), the high magnification SEM shown in (d) is the red squared portion of the (c). | ||
The room-temperature TE properties of the PEDOT NWs/Te NWs nanocomposite films with different contents of Te NWs are shown in Fig. 4. As observed, pure PEDOT NWs film exhibited power factor of 2.54 μW m−1 K−2 which is 3 orders of magnitude higher than that of the PEDOT:PSS (0.0027 μW m−1 K−2),26 due to the high σ value of 249.5 S cm−1, and the Seebeck coefficient of 10.08 μV K−1 just a slight drop, which may be due to the absence of PSS and the effect of our PEDOT NWs special 1D conductive network structure. As the content of Te NWs increases, the electrical conductivity of the composite films decreases gradually while the Seebeck coefficient shows an opposite tendency. The enhanced Seebeck coefficient is mainly due to the inherent high Seebeck coefficient of resultant Te NWs (330.04 μV K−1) and “energy filtering” effect44–47 at the interface between PEDOT NWs and Te NWs. Because in the “energy filtering” process,48–50 low-energy carriers are scattered by the potential barrier formed by the organic/inorganic interfaces of PEDOT NWs and Te NWs, only high-energy carriers can pass through the barrier. High-energy carriers can transfer more heat than low-energy carriers, which increase the Seebeck coefficient.51 Since Te NWs has a relatively low electrical conductivity compared to PEDOT NWs, the electrical conductivity of the nanocomposites decreased with increasing Te NWs content. However, the electric conductivity of the nanocomposite film decreased slowly, especially before Te NWs content was 70 wt%. This is explained by the fact that the conductive link between the PEDOT NWs was not completely broken when the content of PEDOT NWs is low. In addition, many Te NWs–PEDOT NWs–Te NWs junctions are established in PEDOT NWs/Te NWs nanocomposite films. Therefore carriers can also transport through the Te–PEDOT–Te junctions with low energy barrier (in Fig. 3d), so that the decrease of electric conductivity caused by the increase of Te content is hindered.52 It is worth noting that carrier filtering and other mechanisms of improving the thermoelectric properties are mainly related to the interface and surface area, rather than weight fraction of each component.53 An optimized power factor (PF = S2σ) of 58.03 μV m−1 K−2 is obtained for PEDOT NWs/Te NWs composites with 90 wt% Te NWs content at room temperature, which is about dozens of times that of the pure PEDOT NWs film and pure Te NWs film. Moreover, the its thermal conductivity was measured and the value of thermal conductivity for the hybrid ranges from 0.423 to 0.502 W (m K)−1. Thus, the optimized ZT value is 0.041 at room temperature. All the performance values are comparable with the previous works as showed in Table 1.
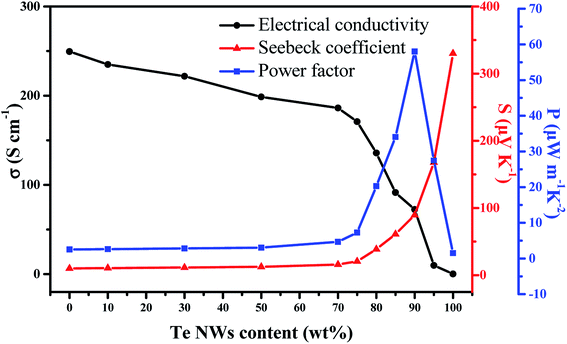 | ||
| Fig. 4 Thermoelectric properties of PEDOT NWs/Te NWs nanocomposite films with different contents of Te NWs. | ||
| System | σ (cm−1) | S (μV K−1) | S 2 σ (μW m−1 K−2) | Reference |
|---|---|---|---|---|
| PEDOT:PSS/Te nanorods treated with H2SO4 | — | — | 42.1 | 33 |
| PEDOT:PSS/Te nanorod | ∼700 | ∼27 | 51.4 | 41 |
| PEDOT:PSS/Te NWs | — | — | 28.5 | 52 |
| PEDOT:PSS/Te NWs | 163(±4) | 19.3(±2.3) | 70.9 | 54 |
| PEDOT:PSS/Te NWs doping with EG or DMSO | — | — | 100 | 55 |
| PEDOT:PSS/Te NWs | ∼150 | ∼0.2 | ∼4.5 | 56 |
| PEDOT NWs/Te NWs | 72.41 | 89.52 | 58.03 | This work |
Furthermore, it is insightful to consider the temperature dependent thermoelectric properties of the PEDOT NWs/Te NWs (90 wt%) nanocomposite film (as showed in Fig. 5). At a temperature between 100 and 300 K, the Seebeck coefficient of the nanocomposite exhibit p-type semiconductor characteristics and gradually increases strongly with increasing temperature. The electric conductivity shows a negative TCR where the electric conductivity increases with increasing temperature below 300 K. As described by the Mott's variable-range hopping model, the electrical conductivity and Seebeck coefficient would simultaneously increase with increasing temperature,57 which is consistent with the results we tested. This characteristic is different from the band-like conduction theory in which the Seebeck coefficient and electrical conductivity show anti-correlated with temperature changes.
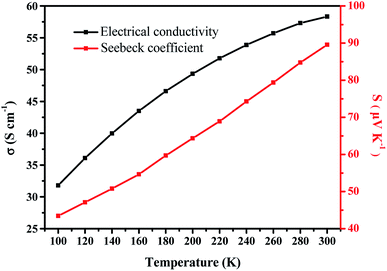 | ||
| Fig. 5 Temperature-dependent thermoelectric properties of PEDOT NWs/Te NWs (90 wt%) nanocomposite film. | ||
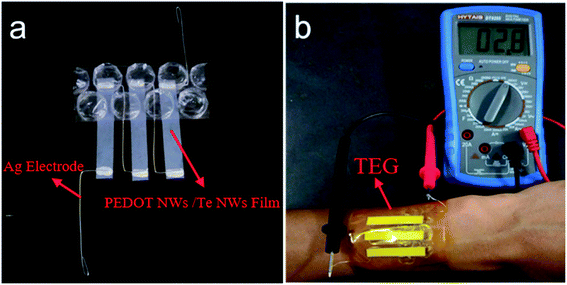
Fig. 6a shows a prototype of a flexible power generator with PEDOT NWs/Te NWs nanocomposite film as thermoelectric element. Three single-leg of PEDOT NWs/Te NWs (90 wt%) nanocomposite film (length 36 mm, width 6 mm) were adhered to the polyimide tape and the silver wires were used as electrode. The film is fixed to the silver wires with conductive silver paste to ensure good electrical conductivity. One side of the device is in contact with the bubble film. The output performance of the power generator at a temperature difference of 13.5 K is shown in Fig. 6b. The temperature difference is obtained by contacting one end of the device with the wrist and the other end separated from the skin by a bubble film as a thermal insulator. The output voltage of the module is 2.8 mV at a temperature difference of approximately 13.5 K. The output voltage V0 of the power generator is defined as: V0 = NSΔT, where S is the Seebeck coefficient, N is the number of TE elements and the ΔT is temperature difference. For this flexible device, N = 3, S = 89.52 mV K−1, ΔT = 13.5 K, the V0 calculated based on these parameters is 3.63 mV, which is higher than the value obtained by the experiment, probably because the actual temperature of the device's hot end is lower than that of the wrist and the inevitable contact resistance in the device. We firstly used PEDOT NWs/Te NWs composite film as raw material to produce flexible wear-resistant thermoelectric devices with high power factors. The preparation process is simple without PSS, which saves cost and reduces environmental pollution. This experimental result provides attempt for the application of flexible TE generators based on PEDOT NWs/Te NWs films in the wearable field.
4. Conclusions
The water soluble PEDOT NWs were synthesized successfully without insulated PSS. Te NWs with high Seebeck coefficient were prepared by solution process. An optimized power factor of 58.03 μW m−1 K−2 has been obtained for the PEDOT NWs/Te NWs (90 wt%) nanocomposite film. The flexible TE generator exhibits an acceptable output voltage of 2.8 mV at an about 13.5 K temperature difference between environment and human body. It promotes research advances in organic materials in the field of thermoelectric and provides great inspiration for the development of flexible and wearable devices. The obtained films are flexible and are ideal for wearable electronic devices. This study provides a new approach to facilitate practical application of flexible TE generator for low-grade energy harvesting.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was financially supported by the National Key Research and Development Program of China (Grant no. 2016YFB0303000) and the New Materials Research Key Program of Tianjin (Grant no. 16ZXCLGX00090).References
- W. He, G. Zhang, X. Zhang, J. Ji, G. Li and X. Zhao, Appl. Energy, 2015, 143, 1–25 CrossRef.
- R. J. Stevens, S. J. Weinstein and K. S. Koppula, Appl. Energy, 2014, 133, 80–88 CrossRef.
- M. K. Kim, M. S. Kim, S. Lee, C. Kim and Y. J. Kim, Smart Mater. Struct., 2014, 23, 105002 CrossRef.
- H. Shang, T. Li, D. Luo, L. Yu, Q. Zou, D. Huang, L. Xiao, H. Gu, Z. Ren and F. Ding, ACS Appl. Mater. Interfaces, 2020, 12, 7358–7365 CrossRef CAS PubMed.
- H. Shang, C. Dun, Y. Deng, T. Li, Z. Gao, L. Xiao, H. Gu, D. J. Singh, Z. Ren and F. Ding, J. Mater. Chem. A, 2020, 8, 4552–4561 RSC.
- A. R. M. Siddique, S. Mahmud and B. V. Heyst, Renewable Sustainable Energy Rev., 2017, 73, 730–744 CrossRef.
- J. Yuan and R. Zhu, Appl. Energy, 2020, 271, 115250 CrossRef.
- R. Yue and J. Xu, Synth. Met., 2012, 162, 912–917 CrossRef CAS.
- M. Zebarjadi, K. Esfarjani, M. S. Dresselhaus, Z. F. Ren and G. Chen, Energy Environ. Sci., 2012, 5, 5147–5162 RSC.
- J. Yang, H. L. Yip and A. K. Y. Jen, Adv. Energy Mater., 2013, 3, 549–565 CrossRef CAS.
- J. W. G. Bos, H. W. Z. Bergen, M. H. Lee, N. P. Ong and R. J. Cava, Phys. Rev. B, 2007, 75, 195203 CrossRef.
- J. Dong, W. Liu, H. Li, X. Su, X. Tang and C. Uher, J. Mater. Chem. A, 2013, 1, 12503 RSC.
- W. Shi, L. Zhou, S. Song, J. Yang and H. Zhang, Adv. Mater., 2008, 20, 1892–1897 CrossRef CAS.
- Y. Du, S. Z. Shen, K. Cai and P. S. Casey, Prog. Polym. Sci., 2012, 37, 820–841 CrossRef CAS.
- Y. Chen, Y. Zhao and Z. Liang, Energy Environ. Sci., 2015, 8, 401–422 RSC.
- R. Kroon, D. A. Mengistie, D. Kiefer, J. Hynynen, J. D. Ryan, L. Yu and C. Müller, Chem. Soc. Rev., 2016, 45, 6147–6164 RSC.
- F. Yakuphanoglu, B. F. Şenkal and A. Saraç, J. Electron. Mater., 2008, 37, 930–934 CrossRef CAS.
- K. Chatterjee, S. Ganguly, K. Kargupta and D. Banerjee, Synth. Met., 2011, 161, 275–279 CrossRef CAS.
- R. Yue and J. Xu, Synth. Met., 2012, 162, 912–917 CrossRef CAS.
- Z. Song and H. Zhou, Energy Environ. Sci., 2013, 6, 2280–2301 RSC.
- O. Bubnova and X. Crispin, Energy Environ. Sci., 2012, 5, 9345–9362 RSC.
- Q. Yao, L. Chen, W. Zhang, S. Liufu and X. Chen, ACS Nano, 2010, 4, 2445–2451 CrossRef CAS PubMed.
- C. Li, F. Jiang, C. Liu, P. Liu and J. Xu, Appl. Mater. Today, 2019, 15, 543–557 CrossRef.
- M. Dietrich, J. Heinze, G. Heywang and F. Jonas, J. Electroanal. Chem., 1994, 369, 87–92 CrossRef CAS.
- H. Yamato, M. Ohwa and W. Wernet, J. Electroanal. Chem., 1995, 397, 163–170 CrossRef.
- J. Kim, R. Patel, B. J. Jung and J. Kwak, Macromol. Res., 2017, 26, 61–65 CrossRef.
- W. Lee, Y. H. Kang, J. Y. Lee, K. S. Jang and S. Y. Cho, RSC Adv., 2016, 6, 53339–53344 RSC.
- Y. Xia and J. Ouyang, J. Mater. Chem., 2011, 21, 4927–4936 RSC.
- G. H. Kim, L. Shao, K. Zhang and K. P. Pipe, Nat. Mater., 2013, 12, 719–723 CrossRef CAS PubMed.
- N. Kim, S. Kee, S. H. Lee, B. H. Lee, Y. H. Kahng, Y. R. Jo, B. J. Kim and K. Lee, Adv. Mater., 2014, 26, 2268–2272 CrossRef CAS PubMed.
- N. Massonnet, A. Carella, G. A. De, J. Faurevincent and J. P. Simonato, Chem. Sci., 2015, 6, 412–417 RSC.
- J. Liu, X. Wang, D. Li, N. E. Coates, R. A. Segalman and D. G. Cahill, Macromolecules, 2015, 48, 585–591 CrossRef CAS.
- H. Song, K. Cai and S. Shen, J. Nanopart. Res., 2016, 18, 386 CrossRef.
- B. Zhang, J. Sun, H. E. Katz, F. Fang and R. L. Opila, ACS Appl. Mater. Interfaces, 2010, 2, 3170–3178 CrossRef CAS PubMed.
- Y. Wang, K. Cai and X. Yao, ACS Appl. Mater. Interfaces, 2011, 3, 1163–1166 CrossRef CAS PubMed.
- H. Ju and J. Kim, ACS Nano, 2016, 10, 5730–5739 CrossRef CAS PubMed.
- R. A. Horne, J. Appl. Phys., 1959, 30, 393–397 CrossRef.
- A. I. Boukai, Y. Bunimovich, J. Tahir-Kheli, J. K. Yu, W. A. Goddard and J. R. Heath, Nature, 2008, 451, 168–171 CrossRef CAS PubMed.
- J. Choi, J. Y. Lee, S. S. Lee, C. R. Park and H. Kim, Adv. Energy Mater., 2016, 6, 1502181 CrossRef.
- K. C. See, J. P. Feser, C. E. Chen, A. Majumdar, J. J. Urban and R. A. Segalman, Nano Lett., 2010, 10, 4664–4667 CrossRef CAS PubMed.
- H. Song and K. Cai, Energy, 2017, 125, 519–525 CrossRef CAS.
- T. Y. Kim, M. P. Chang, J. E. Kim and K. S. Suh, Synth. Met., 2005, 149, 169–174 CrossRef CAS.
- J. W. Choi, M. G. Han, S. Y. Kim, S. G. Oh and S. S. Im, Synth. Met., 2004, 141, 293–299 CrossRef CAS.
- D. K. Ko, Y. Kang and C. B. Murray, Nano Lett., 2011, 11, 2841–2844 CrossRef CAS PubMed.
- M. He, J. Ge, Z. Lin, X. Feng, X. Wang, H. Lu, Y. Yang and F. Qiu, Energy Environ. Sci., 2012, 5, 8351–8358 RSC.
- K. Zhang, Y. Zhang and S. Wang, Sci. Rep., 2013, 3, 3448 CrossRef PubMed.
- H. Ju and J. Kim, ACS Nano, 2016, 10, 5730–5739 CrossRef CAS PubMed.
- P. Pichanusakorn and P. Bandaru, Mater. Sci. Eng., R, 2010, 67, 19–63 CrossRef.
- Y. Zhang, J. H. Bahk, J. Lee, C. S. Birkel, M. L. Snedaker, D. Liu, H. Zeng, M. Moskovits, A. Shakouri and G. D. Stucky, Adv. Mater., 2014, 26, 2755–2761 CrossRef CAS PubMed.
- C. Gayner and Y. Amouyal, Adv. Funct. Mater., 2019, 30, 1901789 CrossRef.
- C. Meng, C. Liu and S. Fan, Adv. Mater., 2010, 22, 535–539 CrossRef CAS PubMed.
- C. Li, F. Jiang, C. Liu, W. Wang, X. Li, T. Wang and J. Xu, Chem. Eng. J., 2017, 320, 201–210 CrossRef CAS.
- J. Xiong, L. Wang, J. Xu, C. Liu, W. Zhou, H. Shi, Q. Jiang and F. Jiang, J. Mater. Sci.: Mater. Electron., 2016, 27, 1769–1776 CrossRef CAS.
- K. C. See, J. P. Feser, C. E. Chen, A. Majumdar, J. J. Urban and R. A. Segalman, Nano Lett., 2010, 10, 4664–4667 CrossRef CAS PubMed.
- S. K. Yee, N. E. Coates, A. Majumdar, J. J. Urban and R. A. Segalman, Phys. Chem. Chem. Phys., 2013, 15, 4024–4032 RSC.
- S. Ma, K. Anderson, L. Guo, A. Yousuf, E. C. Ellingsworth, C. Vajner, H. T. Wang and G. Szulczewski, Appl. Phys. Lett., 2014, 105, 073905 CrossRef.
- Q. Zhang, Y. Sun, F. Jiao, J. Zhang, W. Xu and D. Zhu, Synth. Met., 2012, 162, 788–793 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |