Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
g-C3N4 synthesized from NH4SCN in a H2 atmosphere as a high performance photocatalyst for blue light-driven degradation of rhodamine B†
Shuting Zhang,
Guoqiang Li *,
Liyuan Duan,
Hongyu Wang,
Yongle Zhao and
Yongfa Zhang
*,
Liyuan Duan,
Hongyu Wang,
Yongle Zhao and
Yongfa Zhang
Key Laboratory of Coal Science and Technology, Ministry of Education and Shanxi Province, Taiyuan University of Technology, Taiyuan 030024, China. E-mail: liguoqiang01@tyu.edu.cn
First published on 22nd May 2020
Abstract
Graphitic carbon nitride (g-C3N4) was prepared by a simple thermal polymerization method in this work. The effects of precursor type, thermal polymerization temperature, constant temperature time and atmosphere on the crystal structure, morphology, elemental composition, valence distribution, light absorption properties and photocatalytic activity of the prepared photocatalytic materials were investigated. Taking rhodamine B (RhB) as the target degradant, the blue light catalytic activity of the photocatalytic material was studied in detail. The experimental results showed that the final pyrolysis temperature and constant temperature time are positively related to the adsorption characteristics and photocatalytic ability of the prepared materials. In addition, the adsorption capacity and photocatalytic activity of the products obtained in Ar and H2 atmospheres are better than those produced in CO and CH4, which can be attributed to the combined effect of large specific surface area and structural defects of the materials. The sample's large specific surface area, wide band gap, and excellent photogenerated carrier separation and transfer capabilities make the adsorption performance and photocatalytic performance of the products obtained with ammonium thiocyanate and thiourea as precursors better than those prepared from melamine and dicyandiamide. g-C3N4 prepared by using ammonium thiocyanate as precursor at 550 °C for 5 h under a hydrogen atmosphere showed the best catalytic activity for the degradation of RhB under blue light. It was demonstrated that g-C3N4 prepared exhibited good stability and reusability after four repeat experiments. The active components that play major roles in the degradation of RhB described herein were holes and superoxide radicals, which was inferred by free radical trapping experiments. This work provides a theoretical basis for the idea of converting the mixed salts of desulfurization waste liquid containing ammonium thiocyanate into an excellent photocatalyst g-C3N4 with visible light response.
1 Introduction
With the rapid development of human society, problems of resources and ecological environment have become increasingly prominent, which has presented a severe test to mankind. As a renewable energy source, solar energy is rich in resources, cheap and clean, and is the basis for the sustainable development of human society.1 Therefore, how to efficiently use, transform and store solar energy is an important issue in scientific research. Semiconductor photocatalysis is a green technology that can drive low-density solar energy into high-density chemical energy or directly degrade and purify environmental pollutants by using sunlight to drive a series of important chemical reactions under mild conditions. It has shown great potential in solving problems such as energy shortages and environmental pollution.2 From the perspective of practical application of photocatalysis, metal-free photocatalysts have more advantages and have great potential in practical applications. In particular, g-C3N4 has received extensive attention at home and abroad because of its rich sources, easy availability, good thermal and chemical stability, and excellent performance without secondary pollution to the environment.3–5The development history of g-C3N4 could be dated back to 1834, when melamine, melam, melem, and melon were discovered by Liebig for the first time;6 since then, g-C3N4 based on triazine ring structure has attracted extensive attention as an ideal precursor for high-temperature and high-pressure synthesis of high-density carbon-nitrogen structures.7–10 The research on graphite phase carbonitride has quickly shifted from the initial superhard properties to its photocatalytic applications since Xinchen Wang11–13 first reported the excellent performance of g-C3N4, based on the heptazine ring structure in photocatalysis in 2009, the synthesis research of g-C3N4 once again become a focus.14–16 Pyrolysis of nitrogen-rich organic compounds is the most mature method for synthesizing g-C3N4 in the preparation of conventional graphite carbonitride17 such as melamine,14,18–24 dcyandiamide,25–28 and ammonium thiocyanate,29 melamine chloride (C3N3Cl3),14 cyanamide (N2H2),30,31 azide32 and guanidine carbonate,25 etc. As a metal-free photocatalyst, g-C3N4 offers many unique advantages, including a fascinating graphite-like phase layer structure, a suitable band gap of approximately 2.7 eV, excellent stability, nontoxicity, and facile synthesis.33,34 In addition, g-C3N4 products with different morphology structure, polymerization degrees, photoelectric properties and photocatalytic activities can be easily obtained by adding or doping other substances,35–38 changing preparation conditions,39–41 and coupling with other semiconductors.5,42,43
Based on the previous research regarding the pyrolysis of mixed salts obtained from evaporation and concentration of desulfurization waste liquid (rich in ammonium thiocyanate) and the pyrolysis of ammonium thiocyanate in H2 atmosphere,44 the following work was carried out. In this paper, we chose ammonium thiocyanate, thiourea, melamine and dicyandiamide as nitrogen-containing precursors with different structures to synthesize g-C3N4 by thermal polymerization. The aim is to systematically compare the different structures and properties of g-C3N4 produced by different raw materials. In addition to the precursor types, we also studied the effects of pyrolysis end temperature, pyrolysis constant temperature time and pyrolysis atmosphere on the morphology, elemental composition, element valence distribution, specific surface area and pore size distribution of the carbon nitride materials. The activity and stability of the prepared catalysts in photocatalytic degradation of RhB were investigated. The photocatalytic reaction mechanism was also investigated by free radical trapping experiments.
2 Experimental
2.1 Materials
Ammonium thiocyanate (NH4SCN), thiourea (CS(NH2)2), dicyanamide (C2H4N4), melamine (C3H6N6) and rhodamine B are all obtained from Shanghai Aladdin Biochemical Technology Co., Ltd.All reagents used in this work are of analytical grade and used as received without further purification. All the water used in this experiment is ultrapure water.
2.2 Preparation of g-C3N4
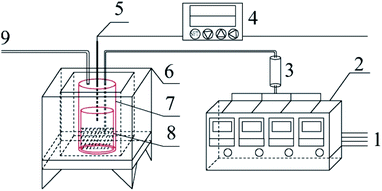
Graphite carbonitride (g-C3N4) was obtained by thermal polymerization using ammonium thiocyanate, thiourea, dicyandiamide and melamine as raw materials. The schematic diagram of the sample preparation device is shown in Fig. 1. Thirty g of the precursor was placed in the bottom of a cylindrical corundum crucible, which was placed in a stainless steel reaction tank. The reaction tank was placed in a temperature-programmed high-temperature pyrolysis furnace. The precursor was heated from room temperature to the specified temperature (400–550 °C) at a heating rate of 3 °C min−1 in a dynamic atmosphere (Ar, H2, CH4, CO), followed by thermal insulation for 1–5 h. The polymerization tail gas was vented after absorption by sodium hydroxide solution. After the pyrolysis furnace naturally cooled to room temperature, the obtained bulk sample was ground into powder and abbreviated as X–G–T-t (where X = AT, T, D, and M represents ammonium thiocyanate, thiourea, dicyandiamide and melamine, respectively; G = Ar, H2, CH4, and CO refers to the thermal polymerization atmosphere; T = 400, 450, 500, and 550 °C refers to the thermal polymerization temperature; t = 1, 2, 3, 4, and 5 h represents the thermopolymerization constant temperature time. For example, AT–H2-550-5 represents a sample of g-C3N4 prepared by heating ammonium thiocyanate in H2 atmosphere to 550 °C and maintaining the temperature for 5 h).2.3 Analytical methods
The phase analysis of residues was carried out using a Model ULTIMA IV X-ray diffractometer (Rigaku, Japan). It was carried out with an automated X-ray diffractometer using Cu Kα radiation (λ = 1.5418 Å), a 40 kV voltage, a 40 mA electrical current, and 2θ from 5° to 85°.The Fourier transform infrared spectra of all samples were obtained using a Vertex 70 Fourier transform infrared spectrometer (FTIR, Bruker, Germany) by the KBr tableting method in the wavelength range of 4000–400 cm−1 with a resolution of 4 cm−1.
The element at the near surface of the material and its chemical state were determined by X-ray photoelectron spectroscopy (XPS, Thermo ESCALAB 250XI).
The surface morphologies of the samples were characterized with SEM (S4800).
Ultraviolet visible (UV-Vis) diffuse reflectance spectra (DRS) of the samples were measured with a UV-Vis spectrophotometer (U-3900).
The surface area and pore structure of the samples were measured by nitrogen adsorption–desorption testing on a specific surface and pore size analysis instrument (3H–2000PS2). The nitrogen sorption isotherms were measured at 77 K with a 3H–2000PS2 analyzer after the samples were degassed in a vacuum at 190 °C for 10 h.
The elements such as C, H, N, and S in the prepared samples were quantitatively analyzed by the German Elementar analyzer (EA).
2.4 Photo catalytic activity evaluation
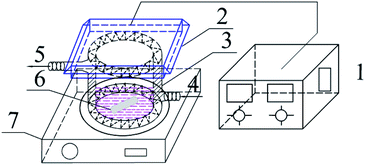
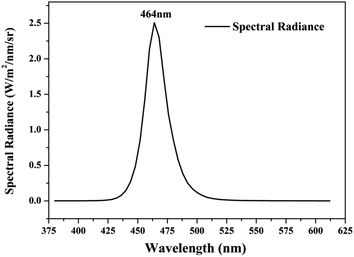
Photocatalytic activities of prepared samples were illustrated by the photodegradation of rhodamine B under visible light irradiation using a 10 W LED with a maximum emission line peak at 464 nm (L100-100-20, 425–525 nm, > 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Lux, Dongguan Yingshi Electronic Technology Co., Ltd., China). The schematic diagram of the photocatalytic evaluation experimental device is shown in Fig. 2. Fig. 3 shows the spectrum of the LED light source. In the photocatalytic test, 0.3 g of the prepared sample was dispersed in 300 mL of a rhodamine B solution having a concentration of 10 mg L−1. The suspension was magnetically stirred in the dark for 90 minutes to ensure an adsorption/desorption equilibrium between rhodamine B and the photocatalyst. After this, the reactant system was placed under blue light irradiation: during the process, 6 mL of suspension was removed from the reactor at a given time interval and centrifuged for 5 minutes at a rate of 10
000 Lux, Dongguan Yingshi Electronic Technology Co., Ltd., China). The schematic diagram of the photocatalytic evaluation experimental device is shown in Fig. 2. Fig. 3 shows the spectrum of the LED light source. In the photocatalytic test, 0.3 g of the prepared sample was dispersed in 300 mL of a rhodamine B solution having a concentration of 10 mg L−1. The suspension was magnetically stirred in the dark for 90 minutes to ensure an adsorption/desorption equilibrium between rhodamine B and the photocatalyst. After this, the reactant system was placed under blue light irradiation: during the process, 6 mL of suspension was removed from the reactor at a given time interval and centrifuged for 5 minutes at a rate of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to achieve solid–liquid separation. The concentration of RhB was determined by measuring the absorbance of the solution at a wavelength of 553 nm after centrifugation using a UV-vis spectrometer (UV-1500 Shanghai Macy Instrument Co., Ltd.), and then converting it by a standard curve.
000 rpm to achieve solid–liquid separation. The concentration of RhB was determined by measuring the absorbance of the solution at a wavelength of 553 nm after centrifugation using a UV-vis spectrometer (UV-1500 Shanghai Macy Instrument Co., Ltd.), and then converting it by a standard curve.
The degradation rate of RhB can be calculated according to formula (1):
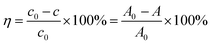 | (1) |
3 Results and discussion
3.1 Morphology and structural characterization of g-C3N4
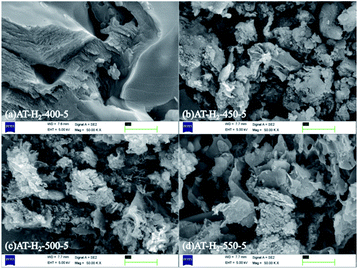
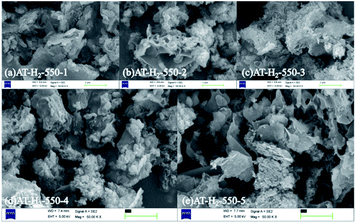
Fig. 5 shows the SEM images of g-C3N4 obtained by pyrolysis of ammonium thiocyanate at 550 °C in H2 atmosphere for 1, 2, 3, 4 and 5 h, respectively. It can be observed that, with the increase of the polymerization time, the products of 1 h exhibit some lamellar structures attached to the agglomerated particles; the products of 2 h and 3 h have similar apparent structures, similar to dry tremella and have a fine porous structure. There are some bubbly structures besides auricularia for the 4 h products, which are attributed to a large number of gases produced during the pyrolysis of ammonium thiocyanate, and porous nanosheets are formed in the 5 h product. g-C3N4 exhibits a two-dimensional nanosheet structure consisting of small plates with irregularly shaped pleats that provide larger specific surfaces and more reactive sites.
The SEM images (Fig. 6) of products obtained by thermal polymerization of ammonium thiocyanate at 550 °C in four different atmospheres showed that the products produced in the H2 atmosphere were nanosheets. The products in the CH4 atmosphere and in Ar and CO atmospheres were similar to those tremella-like thin-layer aggregates, and the products in the CH4 atmosphere looked more fluffy.
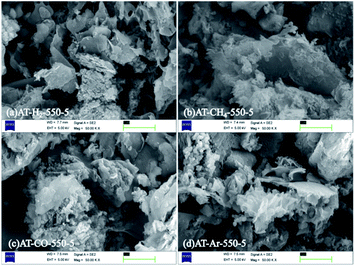 | ||
| Fig. 6 SEM images of products obtained by thermal polymerization of NH4SCN at 550 °C in different atmospheres. | ||
Graphite phase carbon nitride SEM images in Fig. 7 obtained by thermal polymerization of ammonium thiocyanate, thiourea, dicyandiamide and melamine at 550 °C in H2 atmosphere showed that thiourea products were stacked as cornflake-shaped nanosheets; dicyandiamide products were curved flakes and tremella-like; melamine thermal polymerization products were stacked as nanosheets and small and thin sheets. The sample prepared from melamine has a very smooth block surface with a large block size. The above obvious morphological and dimensional differences can be attributed to the inherent properties of different nitrogen-rich precursors, such as melting point, amino content, degree of polymerization, and preferential reaction pathways.45 Due to the release of NH3 and sulfur species (such as H2S and CS2) during the decomposition of ammonium thiocyanate and thiourea, the connectivity mode and topological structure of g-C3N4 are changed.51
 | ||
| Fig. 8 Nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves (inset) of the samples prepared at different temperature. | ||
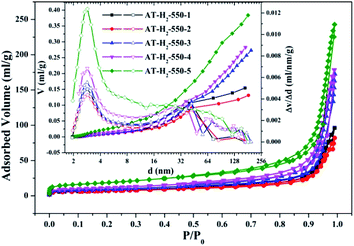 | ||
| Fig. 9 Nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves (inset) of the samples prepared in different temperature maintenance time. | ||
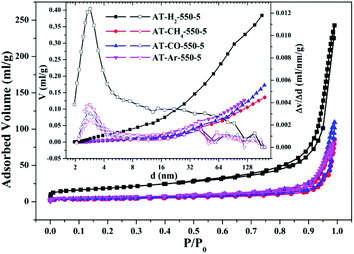 | ||
| Fig. 10 Nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves (inset) of the samples prepared in different atmospheres. | ||
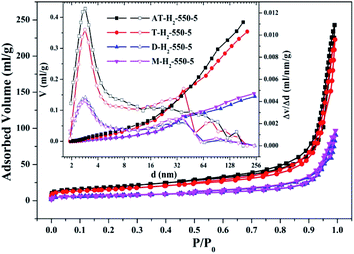 | ||
| Fig. 11 Nitrogen adsorption–desorption isotherms and the corresponding pore size distribution curves (inset) of the samples prepared by different precursors. | ||
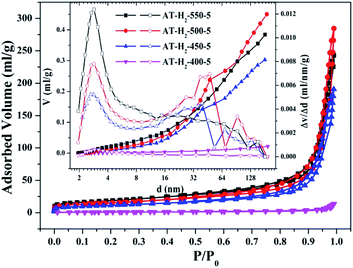
The specific surface area (SBET, m2 g−1), total pore volume (Vpore, cm3 g−1), and BJH average pore width (Dpore, nm) are shown in Table S1.† The data in the table clearly show that the specific surface area of the product obtained by thermal polymerization of ammonium thiocyanate at 550 °C under H2 increases from 24.05 m2 g−1 to 65.88 m2 g−1 with the pore volume gradually increased from 0.13 cm3 g−1 to 0.39 cm3 g−1 as the thermal polymerization constant time is extended from 1 h to 5 h. It means that with the extension of the pyrolysis isothermal time and as the degree of polymerization of the resulting product increases, the specific surface area of the product also increases significantly, which is advantageous for improving the photocatalytic activity of the prepared materials. Similarly, as the thermal polymerization temperature gradually increased from 400 °C to 550 °C, the structure of the obtained material changed from a bulk structure to a lamellar structure, and the degree of heat shrinkage of the sample was enhanced. At the same time, the specific surface area also increases, which further increases the number of active sites on the sample surface.
The specific surface areas of pyrolysis products produced in four different atmospheres are ranked in the order of AT–H2-550-5 > AT–Ar-550-5 > AT–CO-550-5 > AT–CH4-550-5. This should be attributed to the difference in pyrolysis characteristics of ammonium thiocyanate under four atmospheres, including the generation of gas-phase products and differences in pyrolysis reaction rate.
The yields of g-C3N4 prepared from four precursors such as ammonium thiocyanate, thiourea, melamine, and dicyandiamide were 9.21%, 7.25%, 40.5%, and 31.1%, respectively. The types and yields of pyrolysis products of AT and T are significantly more than those of D and M, resulting in AT and T products exhibiting specific surface areas of 65.66 m2 g−1 and 54.74 m2 g−1 which are significantly greater than 23.94 m2 g−1 and 24.50 m2 g−1 of D and M products, which fits well with the results of the photodegradation experiment (Fig. 37). It can be seen that there is a positive correlation between the specific surface area of the prepared sample and the photocatalytic degradation rate,48 which can be interpreted as a larger specific surface area can provide more reaction sites for the reactants.
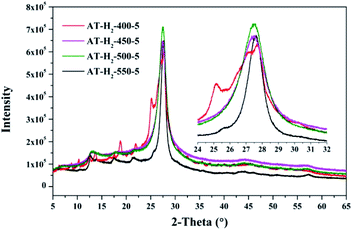
As shown in Fig. 12, with increasing of the heating temperature from 400 °C to 550 °C, the diffraction peak assigned to (002) shifted from 27.3° to 27.62°, indicating that increased heating temperature led to the damage of dense stacking between the conjugated aromatic systems. Note that g-C3N4 prepared at 400 °C contains an amount of impurity phase originating from incomplete decomposition of the ammonium thiocyanate.44
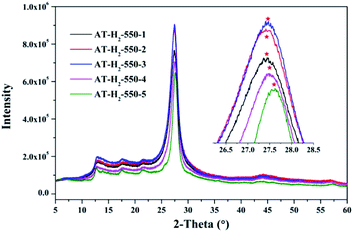
As can be seen in Fig. 13, when the constant temperature time is 1, 2, 3, 4, 5 h, the corresponding (002) peak positions of g-C3N4 prepared by ammonium thiocyanate are 27.44°, 27.45°, 27.47°, 27.50°, 27.62°. According to the Bragg equation, with the increase of the pyrolysis constant temperature time, the spacing of the graphite phase carbon nitride layer obtained continues to shrink, and the crystallinity of the obtained sample increases.
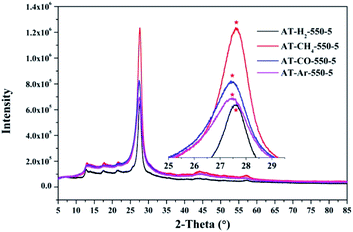
Similarly, the pyrolysis atmosphere also has an effect on the crystallinity of the product. As shown in Fig. 14, the XRD peaks (002) of the products obtained by ammonium thiocyanate in the Ar, CO, CH4 and H2 atmospheres at 550 °C for 5 h are located at 27.45°, 27.46°, 27.63° and 27.63°, respectively. This indicates that the interlayer spacing of the products obtained in the H2 or CH4 atmosphere is smaller than that of the products obtained in the Ar or CO atmosphere, which may be the result of the hydrogen bonding in the interlayer structure of the material.
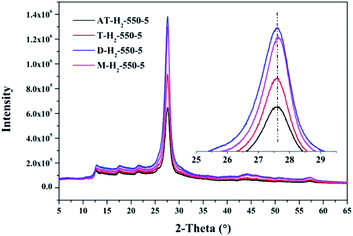
The XRD patterns (Fig. 15) of the pyrolysis products obtained by different precursors at 550 °C for 5 h under H2 atmosphere showed that there were no significant differences in the g-C3N4 characteristic diffraction peak positions prepared by the four precursors. The ranked intensities of the diffraction peaks were AT–H2-550-5 < T–H2-550-5 < M–H2-550-5 < D–H2-550-5.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N heterocycles, and the wider absorption signals in the range of 3000–3700 cm−1 are attributable to the stretching vibrations of N–H and O–H groups, exhibiting partially uncondensed amino groups and physically adsorbed water.50
N heterocycles, and the wider absorption signals in the range of 3000–3700 cm−1 are attributable to the stretching vibrations of N–H and O–H groups, exhibiting partially uncondensed amino groups and physically adsorbed water.50
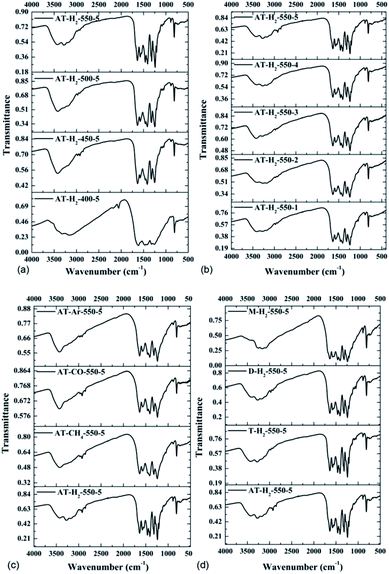 | ||
| Fig. 16 FTIR spectra of synthesized g-C3N4 prepared (a) at different temperaure (b) in different temperature maintenance time (c) in different atmospheres (d) by different precursors. | ||
It is worth noting that for samples obtained at low temperatures of 400 °C and 450 °C (Fig. 16(a)), incomplete polymerization of NH4SCN results in weak vibrational modes of heptazine units. The characteristic band at 2165 cm−1 corresponded to the C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching mode.50 As the pyrolysis temperature increases, the FTIR characteristic peak of the obtained product in the range of 1100–1600 cm−1 gradually becomes sharper and the peak intensity increases, indicating that the product polymerization degree is enhanced. It can be seen from Fig. 16(b, c and d) that the constant temperature time, pyrolysis atmosphere and pyrolysis precursor have no distinct influence on the FTIR characteristic peak position or intensity of the obtained product.
N stretching mode.50 As the pyrolysis temperature increases, the FTIR characteristic peak of the obtained product in the range of 1100–1600 cm−1 gradually becomes sharper and the peak intensity increases, indicating that the product polymerization degree is enhanced. It can be seen from Fig. 16(b, c and d) that the constant temperature time, pyrolysis atmosphere and pyrolysis precursor have no distinct influence on the FTIR characteristic peak position or intensity of the obtained product.
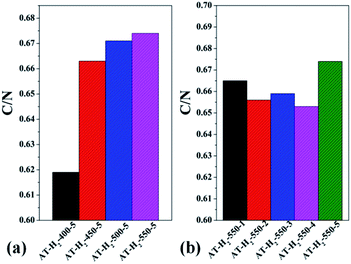 | ||
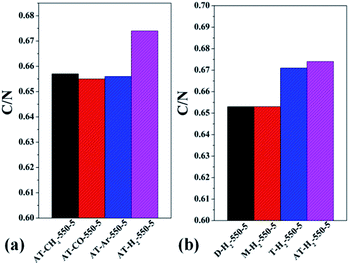
| Fig. 17 Molar ratios of C/N in the products obtained (a) at different temperaure (b) in different temperature maintenance time. | ||
 | ||
| Fig. 19 XPS survey spectrum for samples prepared (a) at different temperature (b) in different temperature maintenance time. | ||
 | ||
| Fig. 20 XPS survey spectrum for samples prepared (a) in different atmospheres (b) by different precursors. | ||
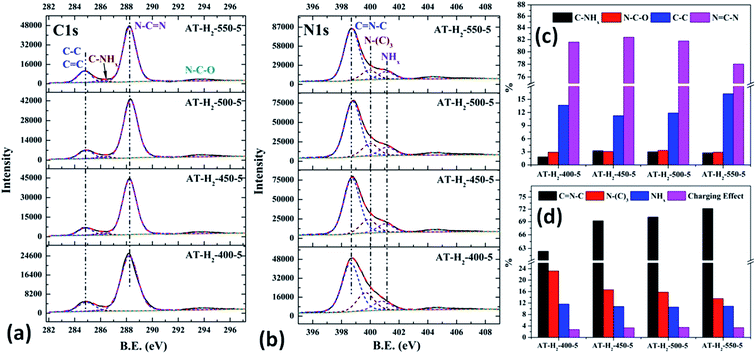 | ||
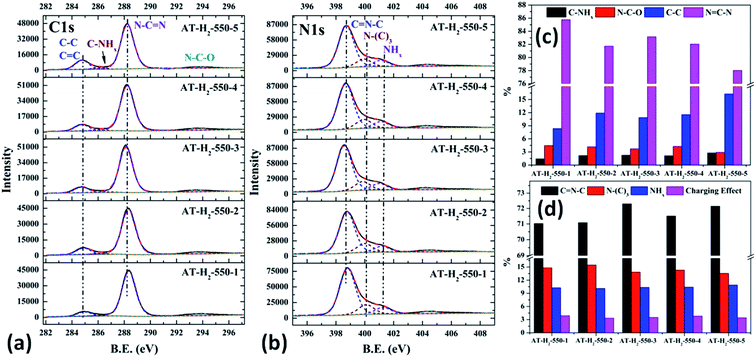
| Fig. 21 The high-resolution XPS spectra of C 1s (a), N 1s (b) and proportion of each state of C (c) and N (d) element for samples prepared at different temperature. | ||
 | ||
| Fig. 22 The high-resolution XPS spectra of C 1s (a), N 1s (b) and proportion of each state of C (c) and N (d) element for samples prepared in different temperature maintenance time. | ||
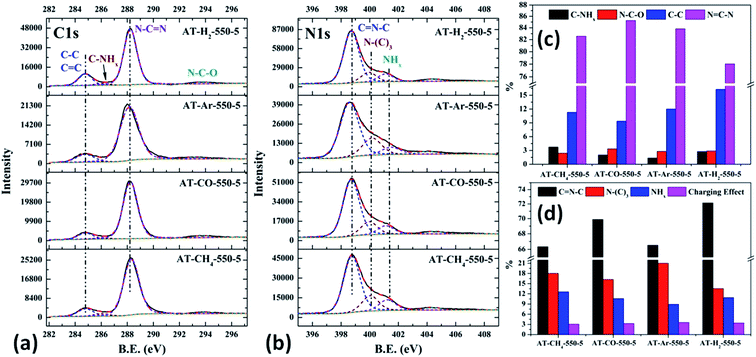 | ||
| Fig. 23 The high-resolution XPS spectra of C 1s (a), N 1s (b) and proportion of each state of C (c) and N (d) element for samples prepared in different atmospheres. | ||
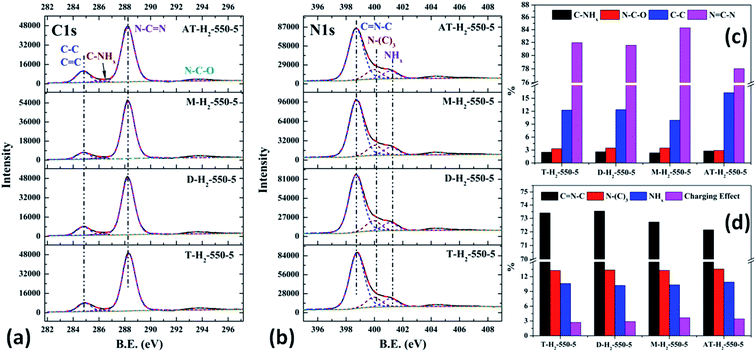 | ||
| Fig. 24 The high-resolution XPS spectra of C 1s (a), N 1s (b) and proportion of each state of C (c) and N (d) element for samples prepared by different precursors. | ||
As shown in Fig. 21(a), the high resolution spectrum of C 1s can be fitted into four peaks, indicating the presence of carbon elements in four different environments. The peak with binding energy of 284.8 eV is attributed to a special sp2 C–C bond in the CN from graphitic carbon or adsorbed amorphous carbon for peak position calibration. The peak at 286.2 eV belongs to the C atom on the aromatic ring connected to –NHx. The strong peak at 288.3 eV can be assigned to the sp2 hybrid C atom connected to N in the triazine ring N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. The peak near 293.3 eV is derived from possible N–C–O.52–54 The backbone of g-C3N4 is bound by a heptazine ring with a terminal NH/NH2, which indicates that there are three types of N atoms in g-C3N4. The peak fitting results of the N 1s high-resolution map (such as Fig. 21(b)) show that the N atom binding energy of 398.6 eV can be ascribed to the sp2 hybrid nitrogen orbital in the triazine ring (C
N. The peak near 293.3 eV is derived from possible N–C–O.52–54 The backbone of g-C3N4 is bound by a heptazine ring with a terminal NH/NH2, which indicates that there are three types of N atoms in g-C3N4. The peak fitting results of the N 1s high-resolution map (such as Fig. 21(b)) show that the N atom binding energy of 398.6 eV can be ascribed to the sp2 hybrid nitrogen orbital in the triazine ring (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C). Nitrogen with binding energy of 399.1 eV corresponds to bridging nitrogen atoms, that is, the sp2 hybrid nitrogen binds with three C atoms (N–(C)3).25,55 The peak observed at 401 eV is assigned to residual amino groups (N–H2) or sp3 hybrid nitrogens of the amino groups (C–N–H). In addition, the weak peak of N1s in the vicinity of 404.5 eV can be attributed to the localization of positive charges or the effect of charges on π excitation in heterocycles.56,57
N–C). Nitrogen with binding energy of 399.1 eV corresponds to bridging nitrogen atoms, that is, the sp2 hybrid nitrogen binds with three C atoms (N–(C)3).25,55 The peak observed at 401 eV is assigned to residual amino groups (N–H2) or sp3 hybrid nitrogens of the amino groups (C–N–H). In addition, the weak peak of N1s in the vicinity of 404.5 eV can be attributed to the localization of positive charges or the effect of charges on π excitation in heterocycles.56,57
The histogram of the proportions of different forms of C and N in the products obtained at different final pyrolysis temperatures were shown as Fig. 21(c) and (d). As AT–H2-400-5 contains a large amount of impurities, so it is not compared with other samples here. In the order of AT–H2-450-5, AT–H2-500-5 and AT–H2-550-5, the proportion of C existing as N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN gradually decreases, which are 82.39%, 81.77%, and 78.02%, respectively; the proportion as C–NHx is 3.26%, 3.01%, and 2.79%; the proportion as C–C attributable to graphitic carbon or amorphous carbon is 11.26%, 11.88%, and 16.29%. Among different forms of N, the proportion of N–(C)3 gradually decreases, which are 16.64%, 15.80%, and 13.54%, respectively; the proportion of C
CN gradually decreases, which are 82.39%, 81.77%, and 78.02%, respectively; the proportion as C–NHx is 3.26%, 3.01%, and 2.79%; the proportion as C–C attributable to graphitic carbon or amorphous carbon is 11.26%, 11.88%, and 16.29%. Among different forms of N, the proportion of N–(C)3 gradually decreases, which are 16.64%, 15.80%, and 13.54%, respectively; the proportion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C is 69.21%, 70.10%, and 72.14%, respectively. As the temperature increases, the material may undergo graphitization under the influence of H2 and high temperature. At the same time, the bridge N atom transformed to C
N–C is 69.21%, 70.10%, and 72.14%, respectively. As the temperature increases, the material may undergo graphitization under the influence of H2 and high temperature. At the same time, the bridge N atom transformed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C, which makes the degree of polymerization of the material increase. In addition, the decrease of the proportion of N
N–C, which makes the degree of polymerization of the material increase. In addition, the decrease of the proportion of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N and the increase of the proportion of C
C–N and the increase of the proportion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C in triazine indicate the generation of C defects in the material.
N–C in triazine indicate the generation of C defects in the material.
As shown in the Fig. 22(c) and (d), the proportion of C–NHx slightly increases with the extension of the constant temperature time, and the proportion of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N generally decreases. The proportion of NH2 of the N element in the obtained sample is almost the same, while the proportions of C
C–N generally decreases. The proportion of NH2 of the N element in the obtained sample is almost the same, while the proportions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C and N–(C)3 are complementary. According to the analysis, due to the extension of the constant temperature time, the corrosion effect of H2 on the sample becomes more and more obvious. This is also a possible cause of the C defect, which is mainly reflected by the increase of the amorphous C content and the decrease of the N
N–C and N–(C)3 are complementary. According to the analysis, due to the extension of the constant temperature time, the corrosion effect of H2 on the sample becomes more and more obvious. This is also a possible cause of the C defect, which is mainly reflected by the increase of the amorphous C content and the decrease of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N content.
C–N content.
As shown in Fig. 23(c) and 12(d), the distribution ratios of C and N elements in the products obtained under the atmospheres of CH4, CO, Ar, and H2 are as follows: N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN accounts for 82.57%, 85.27%, 83.83%, and 78.02%; C
CN accounts for 82.57%, 85.27%, 83.83%, and 78.02%; C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC accounts for 66.33%, 69.89%, 66.51%, and 72.14%; N–(C)3 accounts for 18.03%, 16.24%, 21.01% and 13.54%, respectively. Among the products prepared under four different atmospheres, AT–H2-550-5 has the lowest ratio of N
NC accounts for 66.33%, 69.89%, 66.51%, and 72.14%; N–(C)3 accounts for 18.03%, 16.24%, 21.01% and 13.54%, respectively. Among the products prepared under four different atmospheres, AT–H2-550-5 has the lowest ratio of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN and N–(C)3 and the highest ratio of C
CN and N–(C)3 and the highest ratio of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC, which indicate that the H2 atmosphere is more conducive to the transition from N–(C)3 to C
NC, which indicate that the H2 atmosphere is more conducive to the transition from N–(C)3 to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC and the formation of C defects in the ring.
NC and the formation of C defects in the ring.
Among the various forms of C and N in AT–Ar-550-5, N–(C)3 and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN account for a relatively high proportion, while C
CN account for a relatively high proportion, while C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC account for a relatively low proportion, and both the proportion of C–NHx and NHx were the lowest in the samples obtained in four different atmospheres, which indicate that the sheet structure of AT–Ar-550-5 was relatively large and there were obvious intra-ring nitrogen vacancies. In comparison with AT–Ar-550-5, the proportions of C
NC account for a relatively low proportion, and both the proportion of C–NHx and NHx were the lowest in the samples obtained in four different atmospheres, which indicate that the sheet structure of AT–Ar-550-5 was relatively large and there were obvious intra-ring nitrogen vacancies. In comparison with AT–Ar-550-5, the proportions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C in AT–CH4-550-5 has not changed significantly; N
N–C in AT–CH4-550-5 has not changed significantly; N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN and N–(C)3 ratios have decreased significantly, while the ratio of C–NHx and NHx increased, which indicates that CH4 will convert more N–(C)3 into NHx, and then produce more terminal carbon and terminal amino groups, causing fragmentation of the sheet structure. This is consistent with the shift of the (100) peak of AT–CH4-550-5 to a large diffraction angle in XRD patterns described above. Compared with the products made in the other three atmospheres (CH4, Ar, and H2), the largest N
CN and N–(C)3 ratios have decreased significantly, while the ratio of C–NHx and NHx increased, which indicates that CH4 will convert more N–(C)3 into NHx, and then produce more terminal carbon and terminal amino groups, causing fragmentation of the sheet structure. This is consistent with the shift of the (100) peak of AT–CH4-550-5 to a large diffraction angle in XRD patterns described above. Compared with the products made in the other three atmospheres (CH4, Ar, and H2), the largest N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN ratio, the second highest C
CN ratio, the second highest C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC ratio, and the smaller N–(C)3 ratio indicate that AT–CO-550-5 has a relatively complete ring structure and a large sheet structure.
NC ratio, and the smaller N–(C)3 ratio indicate that AT–CO-550-5 has a relatively complete ring structure and a large sheet structure.
As shown in the Fig. 24(c) and (d), the proportion of element C in the form of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CN for the products prepared by using thiourea, dicyandiamide, melamine, and ammonium thiocyanate as precursors is 81.95%, 81.56%, 84.28%, and 78.02%, respectively; the proportion of C–NHx is 2.50%, 2.60%, 2.34%, and 2.79%; the proportion of C–C is 12.25%, 12.36%, 9.91%, and 16.29%, respectively. In the N element composition of the above samples, the proportion of C
CN for the products prepared by using thiourea, dicyandiamide, melamine, and ammonium thiocyanate as precursors is 81.95%, 81.56%, 84.28%, and 78.02%, respectively; the proportion of C–NHx is 2.50%, 2.60%, 2.34%, and 2.79%; the proportion of C–C is 12.25%, 12.36%, 9.91%, and 16.29%, respectively. In the N element composition of the above samples, the proportion of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC reaches 73.40%, 73.54%, 72.72%, and 72.14%; the proportion of N–(C)3 is 13.26%, 13.35%, 13.28%, and 13.54%; NHx accounted for 10.62%, 10.22%, 10.33%, and 10.90%. The main differences are reflected in the relative content of N
NC reaches 73.40%, 73.54%, 72.72%, and 72.14%; the proportion of N–(C)3 is 13.26%, 13.35%, 13.28%, and 13.54%; NHx accounted for 10.62%, 10.22%, 10.33%, and 10.90%. The main differences are reflected in the relative content of N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N, C–C and C
C–N, C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C, which can be attributed to the differences in the paths of the precursors thermally polymerized to g-C3N4.
N–C, which can be attributed to the differences in the paths of the precursors thermally polymerized to g-C3N4.
 | ||
| Fig. 26 DSR UV-Vis diffuse reflectance spectra of the samples prepared in different temperature maintenance time. | ||
The band gap (ECB) and valence band (EVB) positions of the composite material can be calculated by the following formulas:58–60
| ECB = X − EC − 1/2Eg | (2) |
| EVB = ECB + Eg | (3) |
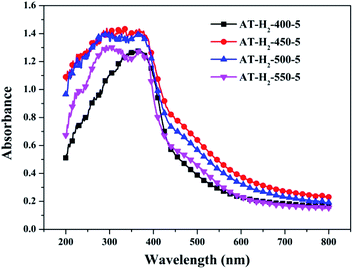
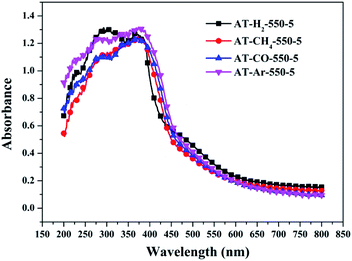
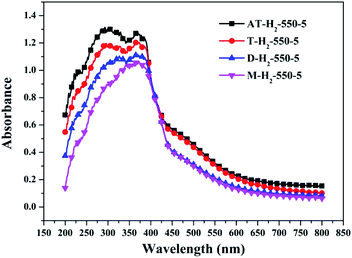
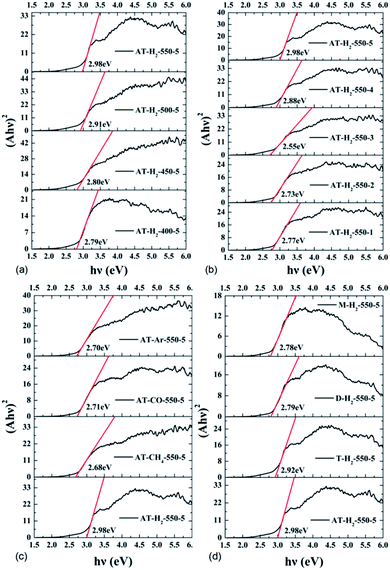
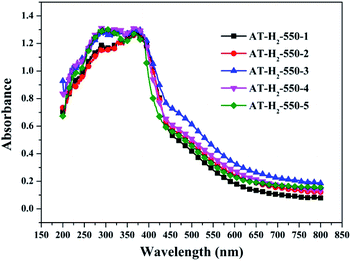
Fig. 25 shows that g-C3N4 prepared under different conditions can respond to visible light. As seen from Fig. 25, as the pyrolysis temperature increases from 400 °C to 550 °C, the band gap of the product obtained by pyrolysis of ammonium thiocyanate in H2 atmosphere gradually increases, and the absorption spectrum of the sample shows a blueshift phenomenon, which is attributed to the quantum confinement effect of nanomaterials.51 As the constant temperature time increases from 1 h to 5 h, the band gap of the resulting products exhibits a decrease and then an increase. The band gap of the product obtained at a constant temperature time of 3 h was the smallest (2.55 eV), and the forbidden band width of the product at a constant temperature of 5 h was 2.98 eV. The band gaps of the products obtained under Ar, CO and CH4 atmospheres are basically the same, and all of them are approximately 2.70 eV, which is much smaller than the band gap of the product obtained in the H2 atmosphere, which matches the experimental results of photocatalytic evaluation (Fig. 35). In addition, the absorption properties of the products obtained by the thermal reaction of dicyandiamide with melamine are essentially the same; the products obtained by pyrolysis of thiourea and ammonium thiocyanate have similar band gaps; that is, their light absorption properties are also essentially consistent. The schematic illustration of the band gap structure of the prepared samples is shown as Fig. 30.
 | ||
| Fig. 32 Photoluminescence spectra of all samples prepared in different atmospheres (a) and prepared by different precursors (b). | ||
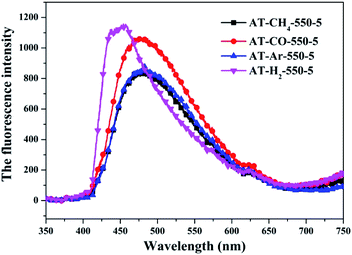
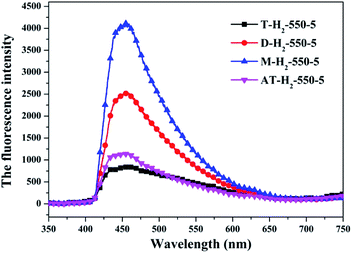
The PL spectrum of the product obtained by thermal polymerization of four precursors in an H2 atmosphere is shown in Fig. 31. The PL spectrum of AT–H2-550-5 and T–H2-550-5 displayed a platform emission peak between 437–456 nm due to the band-to-band recombination of electron–hole. The PL peak intensities are sequentially increased in order of T–H2-550-5 < AT–H2-550-5 < D–H2-550-5 < M–H2-550-5, demonstrating that the recombination of photo-generated electron–hole pairs was more suppressed for AT–H2-550-5 and T–H2-550-5 than that for D–H2-550-5 and M–H2-550-5, which is advantageous for explaining the relationship between the performance of each catalyst shown in Fig. 36 for the photocatalytic degradation of RhB. In addition, compared to M and D, the PL spectra of AT and T are slightly blue-shifted, which can also correspond well to the DRS results. In general, this happens in the presence of a suitable π–π conjugated electronic system, which was found to be beneficial for the migration of photo-generated electrons, thereupon restricting the recombination of photo-produced electron–hole.53
It should be noted that T–H2-550-5 exhibits the lowest PL intensity, but its photocatalytic RhB performance is still inferior to AT–H2-550-5. It can be seen that the recombination of photogenerated charges is not the determinant of the photocatalyst's catalytic activity here. The reasons that influence the activity of the materials in this paper may be attributed more to the increased reactive sites of the large specific surface area and the enhanced redox capacity due to the wide band gap.
3.2 Photocatalytic activities of samples
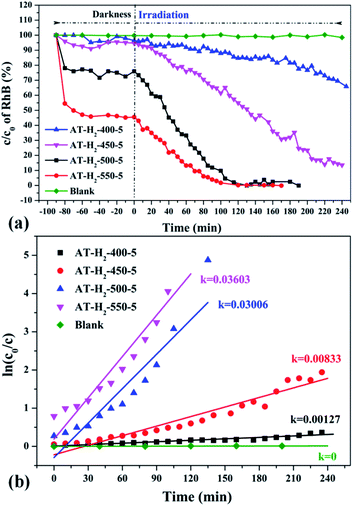 | ||
| Fig. 33 (a) Photo degradation of RhB over the synthesized g-C3N4 at different temperature (b) influence of photocatalyst preparation temperature on degradation kinetics. | ||
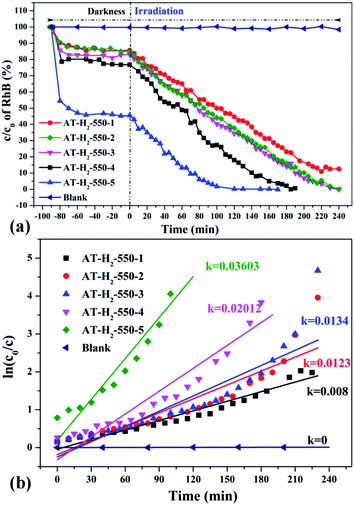 | ||
| Fig. 34 The degradation curve of RhB (a) photocatalyzed by g-C3N4 prepared in different temperature maintenance time and the first-order kinetic fitting curve(b). | ||
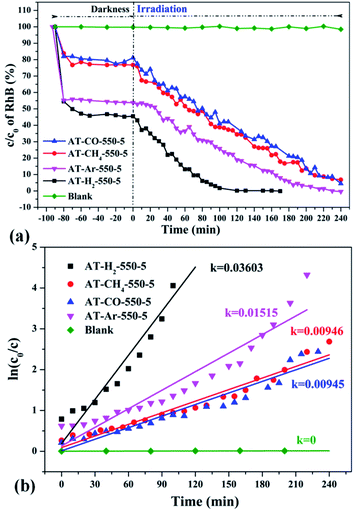 | ||
| Fig. 35 The degradation curve of RhB (a) photocatalyzed by g-C3N4 synthesized in different atmospheres and their first-order kinetic fitting curve (b). | ||
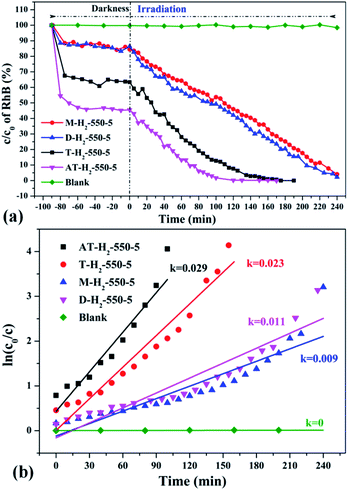 | ||
| Fig. 36 The degradation curve of RhB (a) photocatalyzed by g-C3N4 synthesized by different precursors and the first-order kinetic fitting curve (b). | ||
It can be seen from Fig. 34(a) that as the constant temperature time increases from 1 h to 5 h at 550 °C, the adsorption capacity of the obtained catalyst also gradually increases. When the dark adsorption equilibrium was reached, the adsorption rates of the respective catalysts for the RhB solution were 16%, 16%, 17%, 24%, and 56%. This is attributed to the fact that the polymerization degree of the material is gradually increased with the extension of the constant temperature time and that the specific surface area, pore volume and pore structure of the sample are increased, which is favorable for the adsorption of the degraded material. After 4 hours of light irradiation, the degradation rate of AT–H2-550-1-catalyzed RhB was 88%, and the degradation rates of RhB by AT–H2-550-2 and AT–H2-550-3 were almost 100%.
Fig. 35 shows the activity evaluation curve of the photocatalytic degradation of RhB by pyrolysis products of NH4SCN as precursor in H2, CH4, CO and Ar atmospheres. It can be seen that CO and CH4 have similar effects on the photocatalytic activity of NH4SCN pyrolysis products. The adsorption capacity of RhB in dark treatment was 22%, and the percentage of degradation of RhB after irradiation for 4 hours reached 96%. The adsorption capacity of AT–Ar-550-5 (adsorption rate of 47%) and photocatalytic activity (RhB degradation rate of almost 100% after 4 hours of illumination) are superior to those of AT–CO-550-5 and AT–CH4-550-5, but not as good as in the case of AT–H2-550-5. This can be more intuitively reflected by the pyrolysis kinetic constants in Fig. 35(b).
Fig. 36 exhibits evaluation curves of the photocatalytic degradation of RhB by g-C3N4 prepared by different kind of precursors in H2 atmosphere at 550 °C for 5 h. It is obvious that the products prepared by using dicyandiamide and melamine as precursors have similar adsorption characteristics (14%) and photocatalytic degradation of RhB (98% after 4 hours of illumination). The adsorption performance (37%) and photocatalytic performance (approximately 100% of RhB degradation ability after 3 hours of illumination) of the product obtained with thiourea as the precursor are superior to those of the above two precursors. The kinetic constant of the above reaction shows that the AT–H2-550-5 exhibits nearly three times the photocatalytic capacity of M–H2-550-5.
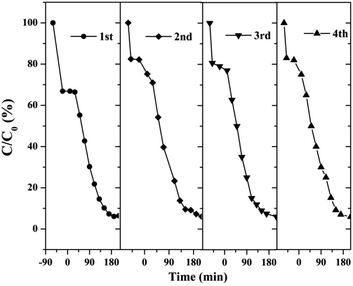
Fig. 37 shows that the as-prepared material has good recyclability. In addition to the decrease in adsorption performance compared to the initial sample, there is no significant deactivation of the catalyst after 4 cycles in RhB degradation. The cycling experiments determined the stability and reusability of the samples.
3.3 Free radical capture experiment results
Hydroxyl radicals (˙OH), holes (h+) and superoxide radicals (˙O2−) are considered to be common active substances in the degradation of photocatalytic organic compounds. Therefore, in this study, different sacrificial agents were added to the reaction system to determine which radicals act in the photocatalytic reaction in order to determine the reaction mechanism. Next, 1 mM TEOA, BQ, IPA and AgNO3 were added to the reaction system as hole (h+), superoxide radical (˙O2−), hydroxyl radical (˙OH) and electron (e−) trapping agents, respectively. The RhB degradation curves (Fig. 38) obtained show that after TEOA and BQ were added into the reaction system, RhB almost did not degrade after dark adsorption. When IPA and AgNO3 were added into the reaction system, the catalytic effect of g-C3N4 on RhB degradation was enhanced compared with that of g-C3N4 alone. When electron trapping agent is added to the reaction system, the recombination of photogenerated carriers is weakened and the reaction process is accelerated. It can be concluded that the main active components of photocatalytic RhB degradation should be holes (h+) and superoxide radicals (˙O2−).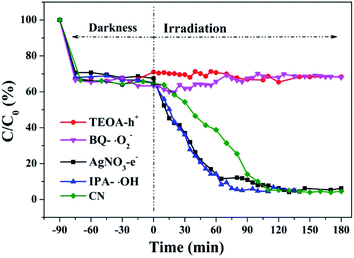 | ||
| Fig. 38 Experimental results of free radical capture during photo catalytic degradation of RhB by the prepared samples. | ||
Based on the above results and discussion, the reaction mechanism of the photocatalytic degradation of RhB by g-C3N4 under blue light irradiation was proposed.
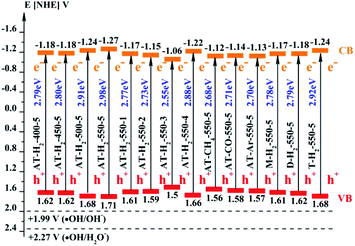
As the CB position of as prepared g-C3N4 (−1.06 ∼ −1.27 eV) are more negative than that of O2/˙O2− (−0.33 eV vs. NHE),64 the photogenerated electrons of samples could easily reduce the oxygen molecules absorbed on the samples or dissolved in solution to produce the superoxide radical (˙O2−) and hydrogen peroxide (H2O2),65–67 which can further create the hydroxyl radical (˙OH). The resulting oxygen-active species will oxidize the RhB into CO2 and H2O. In addition, the VB positions of samples (+1.5 ∼ +1.71 eV) are more negative than those of ˙OH/OH− (+1.99 eV vs. NHE)68,69 and ˙OH/H2O (+2.27 eV vs. NHE), which means the photo excited holes on the VB of samples had no ability to oxidize the H2O molecules or hydroxyl (OH−) to hydroxyl radical (˙OH),64 but can directly oxidize RhB to decompose. The above results are consistent with the results of the free radical trapping experiment: that is, OH is not the main active substance in the photodegradation of RhB. The photodegradation of RhB is achieved by the synergistic catalysis of ˙O2− and h+.
| g-C3N4 + hν → eCB− + hVB+ | (4) |
| O2 + e− → ˙O2−/H2O2 → ˙OH | (5) |
| ˙O2−/˙OH/h+ + RhB → CO2 + H2O | (6) |
4 Conclusions
When NH4SCN is used as a precursor to prepare g-C3N4, the increase of thermal polymerization temperature (400–550 °C) and the extension of constant temperature time (1–5 h) are beneficial to increase the specific surface area of the obtained sample, reduce the interlayer spacing, and enhance the crystallinity of the sample. At the same time, the amount of RhB adsorbed by the material and the photocatalytic degradation ability gradually increased. The effect of hydrogen bonding in the material structure makes the interlayer spacing of products obtained in H2 and CH4 atmospheres smaller than those prepared in Ar and CO. The band gaps of the products obtained in Ar, CO, and CH4 are basically the same, all about 2.70 eV, which are much smaller than that obtained in H2. The H2 atmosphere is more conducive to the transition of bridging N atoms to N in the triazine ring and the formation of C defects in the ring. The Ar atmosphere product has more obvious nitrogen vacancy in the ring. CH4 can promote the conversion of more bridged nitrogen to NHx to produce more terminal carbons and terminal amino groups, which in turn causes the layered structure to break. The adsorption capacity and photocatalytic activity of the products obtained in Ar and H2 atmospheres are better than those produced in CO and CH4, which can be attributed to the combined effect of large specific surface area and structural defects of the materials. The g-C3N4 prepared with AT, T, D and M as precursors has a similar sheet-like stacking structure and a clear UV-visible light absorption capability. However, the sample's large specific surface area, wide band gap, and excellent photogenerated carrier separation and transfer capabilities make the adsorption performance and photocatalytic performance of the products obtained with AT and T as precursors better than those prepared with D and M as precursors.This study has important theoretical significance and application background for the treatment of desulfurization waste liquid with waste heat of reducing waste gas and the preparation of g-C3N4 catalyst using the main salt of the desulfurization waste liquid as raw materials, which opens up a new direction for the treatment of the main salt of the desulfurization waste liquid.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by the Coal Based Key Program of the Scientific and Technological Project of Shanxi Province, China (2014) and the Technology Development and Engineering Demonstration of Coke Oven Gas Desulfurization Wastewater Resource Utilization (MJH2014-09).References
- M. Xiao, B. Luo, S. Wang and L. Wang, J. Energy Chem., 2018, 27, 1111–1123 CrossRef.
- T. Jiang, Y. Du, M. Dong and Q. Zhao, New J. Chem., 2019, 43, 10915–10925 RSC.
- G. Liu, P. Niu, C. H. Sun, S. C. Smith, Z. G. Chen, G. Q. Lu and H. M. Cheng, J. Am. Chem. Soc., 2010, 132, 11642–11648 CrossRef CAS PubMed.
- Y. Kang, Y. Yang, L.-C. Yin, X. Kang, L. Wang, G. Liu and H.-M. Cheng, Adv. Mater., 2016, 28, 6471–6477 CrossRef CAS PubMed.
- Z. Zhou, Y. Zhang, Y. Shen, S. Liu and Y. Zhang, Chem. Soc. Rev., 2018, 47, 2298–2321 RSC.
- G. Liao, Y. Gong, L. Zhang, H. Gao, G.-J. Yang and B. Fang, Energy Environ. Sci., 2019, 12, 2080–2147 RSC.
- A. Y. Liu and M. L. Cohen, Science, 1989, 245, 841–842 CrossRef CAS PubMed.
- A. Y. Liu and R. M. Wentzcovitch, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 10362–10365 CrossRef CAS.
- J. E. Lowther, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 11683–11686 CrossRef CAS.
- J. Ortega and O. F. Sankey, Phys. Rev. B: Condens. Matter Mater. Phys., 1995, 51, 2624–2627 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- C. Sheng, C. Wang, J. Feng, W. Ying and Z. Zou, Int. J. Hydrogen Energy, 2014, 39, 13519–13526 CrossRef.
- W. Yong, W. Xinchen and A. Markus, Angew. Chem., 2012, 51, 68–89 CrossRef PubMed.
- S. Katharina, M. B. Mesch, D. Viola, Z. Christian, S. Jürgen and B. V. Lotsch, J. Am. Chem. Soc., 2014, 136, 1730–1733 CrossRef PubMed.
- X. Yuan, PhD thesis, YanShan University, 2018 Search PubMed.
- T. Komatsu, J. Mater. Chem., 2001, 11, 799–801 RSC.
- B. V. Lotsch and S. Wolfgang, Chem.–Eur. J., 2010, 13, 4956–4968 CrossRef PubMed.
- Y. Zhang, Q. Pan, G. Chai, M. Liang, G. Dong, Q. Zhang and J. Qiu, Sci. Rep., 2013, 3, 1943 CrossRef PubMed.
- H. Fang, C. Gang, Y. Yaoguang, Z. Yansong, Z. Yi and H. Sue, Chem. Commun., 2015, 51, 425–427 RSC.
- V. L. Solozhenko, E. G. Solozhenko, P. V. Zinin, C. M. Li, J. Chen and J. B. Parise, J. Phys. Chem. Solids, 2003, 64, 1265–1270 CrossRef CAS.
- Y. C. Zhao, D. L. Yu, H. W. Zhou, Y. J. Tian and O. Yanagisawa, J. Mater. Sci., 2005, 40, 2645–2647 CrossRef CAS.
- B. Juncao, L. Jianfu, K. Sergii, W. Yu, L. Qian, L. Tsz Chun, T. A. Niehaus, A. L. Rogach and Z. Rui-Qin, ChemPhysChem, 2015, 16, 954–959 CrossRef PubMed.
- Z. Zhao, J. Fan, Y. Xue, H. Chang, Y. Masubuchi and S. Yin, J. Alloys Compd., 2018, 735, 1297–1305 CrossRef CAS.
- A. S. Gerardo, S. Nikolai, S. Y. Chong, B. R. Torbj?Rn, R. G. Palgrave, L. Andrea, A. Markus, Y. Z. Khimyak, A. V. Krasheninnikov and J. P. Rabe, Angew. Chem., 2014, 126, 7580–7585 CrossRef.
- A. Torres-Pinto, M. J. Sampaio, C. G. Silva, J. L. Faria and A. M. T. Silva, Appl. Catal., B, 2019, 252, 128–137 CrossRef CAS.
- M. J. Lima, A. M. T. Silva, C. G. Silva and J. L. Faria, J. Catal., 2017, 353, 44–53 CrossRef CAS.
- Y. Cui, J. Zhang, G. Zhang, J. Huang, P. Liu, M. Antonietti and X. Wang, J. Mater. Chem., 2011, 21, 13032 RSC.
- B. V. Lotsch, M. Döblinger, J. Sehnert, L. Seyfarth, J. Senker, O. Oeckler and W. Schnick, Chem, 2010, 13, 4969–4980 CrossRef PubMed.
- F. Fina, S. K. Callear, G. M. Carins and J. T. S. Irvine, Chem. Mater., 2015, 27, 2612–2618 CrossRef CAS.
- E. G. Gillan, Chem. Mater., 2000, 12, 3906–3912 CrossRef CAS.
- L. H. Amorin, V. Y. Suzuki, N. H. de Paula, J. L. Duarte, M. A. Toledo da Silva, C. A. Taft and F. d. A. La Porta, New J. Chem., 2019, 43, 13647–13653 RSC.
- M. Ismael, Y. Wu, D. H. Taffa, P. Bottke and M. Wark, New J. Chem., 2019, 43, 6909–6920 RSC.
- K. Li, M. Sun and W.-D. Zhang, Carbon, 2018, 134, 134–144 CrossRef CAS.
- C. Hu, W.-Z. Hung, M.-S. Wang and P.-J. Lu, Carbon, 2018, 127, 374–383 CrossRef CAS.
- Y. Wu, L.-M. Liu, X. An and T. Wei, New J. Chem., 2019, 43, 4511–4517 RSC.
- H. Liu, X. Zhu, R. Han, Y. Dai, Y. Sun, Y. Lin, D. Gao, X. Wang and C. Luo, New J. Chem., 2020, 44, 1795–1805 RSC.
- Y. Z. Dongya Ni, Y. Shen, S. Liu and Y. Zhang, Chin. Chem. Lett., 2020, 31, 115–118 CrossRef.
- C. H. Ziyu Gan, Y. Shen, Q. Zhou, D. Han, J. Ma, S. Liu and Y. Zhang, Chin. Chem. Lett., 2020, 31, 513–516 CrossRef.
- T. Zhao, Q. Zhou, Y. Lv, D. Han, K. Wu, L. Zhao, Y. Shen, S. Liu and Y. Zhang, Angew. Chem., Int. Ed., 2020, 59, 1139–1143 CrossRef CAS PubMed.
- H. Zhao, S. Wang, F. He, J. Zhang, L. Chen, P. Dong, Z. Tai, Y. Wang, H. Gao and C. Zhao, Carbon, 2019, 150, 340–348 CrossRef CAS.
- M. Inagaki, T. Tsumura, T. Kinumoto and M. Toyoda, Carbon, 2019, 141, 580–607 CrossRef CAS.
- S. Zhang, G. Li, H. Wang, C. Li, T. Li and Y. Zhang, J. Anal. Appl. Pyrolysis, 2018, 134, 427–438 CrossRef CAS.
- Z. Qitao, PhD thesis, Yangzhou University, 2017 Search PubMed.
- P. J. Hong, Z. Xiwang, D. Alan Jianhong, D. D. Sun and J. O. Leckie, J. Am. Chem. Soc., 2008, 130, 11256–11257 CrossRef PubMed.
- L. Hexing, B. Zhenfeng, Z. Jian, H. Yuning, L. Hui and L. Yunfeng, J. Am. Chem. Soc., 2007, 129, 4538–4539 CrossRef PubMed.
- L. Tian, J. Li, F. Liang, J. Wang, S. Li, H. Zhang and S. Zhang, Appl. Catal., B, 2017, 225 Search PubMed.
- H. Dong, X. Guo, C. Yang and Z. Ouyang, Appl. Catal., B, 2018, 230, 65–76 CrossRef CAS.
- Y. Yu, C. Wang, L. Luo, J. Wang and J. Meng, Chem. Eng. J., 2018, 334, 1869–1877 CrossRef CAS.
- S. Cao, B. Fan, Y. Feng, H. Chen, F. Jiang and X. Wang, Chem. Eng. J., 2018, 353, 147–156 CrossRef CAS.
- Z. Yang, K. Hu, X. Meng, Q. Tao, J. Dong, B. Liu, Q. Lu, H. Zhang, B. Sundqvist and P. Zhu, Carbon, 2017, 130, 170–177 CrossRef.
- T. Song, P. Zhang, T. Wang, A. Ali and H. Zeng, Appl. Catal., B, 2018, 224, 877–885 CrossRef CAS.
- J. Wu, N. Li, H.-B. Fang, X. Li, Y.-Z. Zheng and X. Tao, Chem. Eng. J., 2019, 358, 20–29 CrossRef CAS.
- J. Zhuang, J. Zhang, J. Pang, A. Wang, X. Wang and W. Zhu, Dyes Pigm., 2019, 163, 634–640 CrossRef CAS.
- D. Zhang, Y. Guo and Z. Zhao, Appl. Catal., B, 2018, 226, 1–9 CrossRef CAS.
- H. Yu, R. Shi, Y. Zhao, T. Bian, Y. Zhao, C. Zhou, G. I. N. Waterhouse, L.-Z. Wu, C.-H. Tung and T. Zhang, Adv. Mater., 2017, 29, 1605148 CrossRef PubMed.
- D. Yaocheng, PhD thesis, Hunan University, 2018 Search PubMed.
- Y. Li, L. Fang, R. Jin, Y. Yang, X. Fang, Y. Xing and S. Song, Nanoscale, 2014, 7, 758–764 RSC.
- S. Meng, X. Ning, T. Zhang, S. F. Chen and X. Fu, Phys. Chem. Chem. Phys., 2015, 17, 11577 RSC.
- D. Xu, C. Bei, S. Cao and J. Yu, Appl. Catal., B, 2015, 164, 380–388 CrossRef CAS.
- L. Huang, X. U. Hui, R. Zhang, X. Cheng, J. Xia, X. U. Yuanguo and L. I. Huaming, Appl. Surf. Sci., 2013, 283, 25–32 CrossRef CAS.
- G. Mamba and A. K. Mishra, Appl. Catal., B, 2016, 198, 347–377 CrossRef CAS.
- W. Wanjun, J. C. Yu, X. Dehua, W. Po Keung and L. Yecheng, Environ. Sci. Technol., 2013, 47, 8724–8732 CrossRef PubMed.
- B. Lin, G. Yang, B. Yang and Y. Zhao, Appl. Catal., B, 2016, 198, 276–285 CrossRef CAS.
- C. Xue, T. Wang, G. Yang, B. Yang and S. Ding, J. Mater. Chem. A, 2014, 2, 7674–7679 RSC.
- S. Xiao, W. Zhu, P. Liu, F. Liu, W. Dai, D. Zhang, W. Chen and H. Li, Nanoscale, 2016, 8, 2899 RSC.
- Q. Liu, Y. Guo, Z. Chen, Z. Zhang and X. Fang, Appl. Catal., B, 2016, 183, 231–241 CrossRef CAS.
- Y. Cui, Z. Ding, P. Liu, M. Antonietti, X. Fu and X. Wang, Phys. Chem. Chem. Phys., 2012, 14, 1455–1462 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02454f |
| This journal is © The Royal Society of Chemistry 2020 |