Open Access Article
Open Access ArticleNitrogen-doped RuS2 nanoparticles containing in situ reduced Ru as an efficient electrocatalyst for hydrogen evolution†
Yan Xua,
Xiaoping Gao*b,
Jingyan Zhangc and
Daqiang Gao c
c
aPhysics and Electronic Engineering Department, Xinxiang University, Xinxiang 453003, P. R. China
bKey Laboratory of Sensor and Sensing Technology, Gansu Academy of Sciences, Lanzhou 730000, Gansu, China. E-mail: gao_xp02@163.com
cKey Laboratory for Magnetism and Magnetic Materials of MOE, Key Laboratory of Special Function Materials and Structure Design of MOE, Lanzhou University, Lanzhou 730000, P. R. China
First published on 7th May 2020
Abstract
The production of hydrogen via water electrolysis brings hope for the realization of hydrogen economy, but there is still a lack of highly efficient and appropriate electrocatalysts for the generation of hydrogen in practical applications. In particular, reasonable construction and feasible preparation strategies are the essential requirements for excellent electrocatalysts. Herein, the heterostructures of N-RuS2/Ru nanoparticles were designed by annealing the RuS2 nanoparticles in ammonia. By introducing a nitrogen dopant and single-phase Ru metal simultaneously, high-efficiency electrocatalytic performance for hydrogen evolution reaction (HER) was implemented, where the electrocatalyst of N-RuS2/Ru exhibited a low onset overpotential of 76 mV and small overpotential of 120 mV at 10 mA cm−2 in an acidic electrolyte. Besides, it displayed a low Tafel slope of 53 mV dec−1, a small interface charge transfer resistance, and long-time stability and durability, suggesting its remarkable properties as a promising HER electrocatalyst candidate.
1. Introduction
Sustainable energy development and increased environmental concerns have triggered imperative requirements for renewable and pollution-free new-generation energy sources.1,2 As an energy carrier with entirely zero carbon composition and high energy density, hydrogen is regarded as one of the ideal clean alternatives to substitute traditional fossil fuels in the future.3–5 The hydrogen evolution reaction (HER), a half-reaction occurring at the cathode during water electrolysis, can produce high purity hydrogen on a large scale in the industry. Owing to its renewability, theoretical feasibility and environmental benignity, HER has attracted increasing attention in recent decades. During the procedure of water electrolysis, the required theoretical voltage is 0 V for HER at 298 K.6,7 However, extra electricity is demanded to overcome the overpotential caused by the activation energy barrier to kick start the initial reaction. Hence, high-efficient electrocatalysts are necessary to reduce the overpotential and accelerate reaction kinetics. At present, Pt-based materials with their onset overpotentials close to zero are recognized as the state-of-the-art electrocatalysts for HER.8–10 Unfortunately, the rocketing cost, scarce reserve and mediocre stability of Pt-based materials seriously hinder their large-scale applications. Therefore, developing Pt-like, sustainable and cost-effective electrocatalysts remains a great challenge and urgent issue.In the past years, a considerable number of Pt-free materials have emerged as HER electrocatalysts, including transition-metal sulfides, oxides, selenides, nitrides, phosphides, carbon-based materials and their compounds.11–17 Among them, Ru-based materials are applied in various catalytic fields, such as hydrodesulfurization, electrocatalysis, photocatalysis, and supercapacitors.18–24 Deriving from their rich redox chemistry and multiple valence states, Ru-based catalysts usually present exceptional electrocatalytic activities. RuS2, one of the typical Ru-based materials, is usually used in hydrodesulfurization as an efficient catalyst.25 Besides, subsequent explorations have proved that RuS2 exhibits excellent HER activities.26–28 In addition, the cost of Ru is less than 5% of metallic Pt, which is even more encouraging.29,30 However, the previously reported electrocatalytic performance of RuS2 for HER is still insufficient to meet the industrial needs. Therefore, extra efforts should be made to further improve the electrocatalytic efficiency of RuS2.
Doping can modulate the electronic structure and narrow the bandgap, which is a commonly effective strategy to enhance the electrocatalytic performance of materials. A nitrogen (N) atom itself has a lone pair of electrons, strong electronegativity and small atomic radius. Therefore, N-doping can capture the electron around the metal atoms with weak electronegativity and affect the electronic density of the state of the material or cause the distortion of the material phase structure by the atomic substitution.31–33 Numerous experimental analysis as well as theoretical calculations revealed that the electronic conductivity and hydrogen adsorption capability of electrocatalysts are possible for notable improvements through N doping, enabling their implementation as an advanced cathode for HER.34,35 Besides, designing multiphase structures can also improve the electrocatalytic efficiency owing to the usual formation of defects as well as exposed active sites at the atomic-scale interfaces.36–38 A single atom of Ru has hydrogen adsorption properties similar to those of Pt since the bond strength of Ru–H is close to that of Pt–H, resulting in an outstanding electrocatalytic performance for the Ru catalyst.39,40 Consequently, it is anticipated that RuS2 embedded with a single atom of metallic Ru will exhibit a desirable HER activity.
Herein, we constructed a novel hybrid material, nitrogen-doped RuS2 nanoparticles with in situ reduced Ru (N-RuS2/Ru) as an electrocatalyst for HER. In order to introduce the nitrogen dopant, a pure RuS2 was annealed at a low temperature in ammonia. Surprisingly, the obtained product is not only doped with nitrogen, but also has the appearance of an in situ reduced Ru metal phase, leading to a significantly improved electrocatalytic activity. Benefiting from the N dopant and reasonable designing of the interface between Ru and RuS2, the electrocatalyst N-RuS2/Ru displays optimal performance for HER in acid conditions far better than those of RuS2 and RuS2/Ru. In terms of experimental results, the as-synthesized N-RuS2/Ru nanoparticles possess the lowest onset overpotential of 76 mV and a small Tafel slope of 53 mV dec−1. The required overpotential is only 120 mV at a current density of 10 mA cm−2 in 0.5 M H2SO4. Moreover, it has long-term cycling stability.
2. Results and discussions
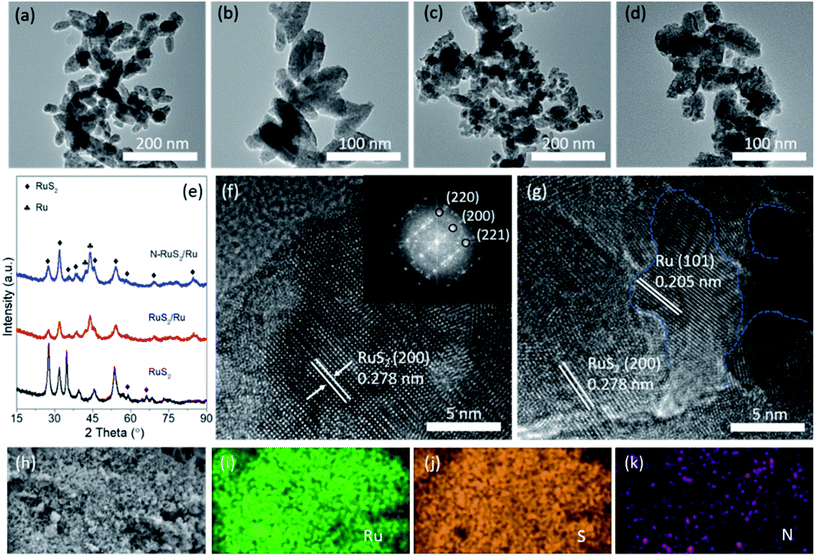
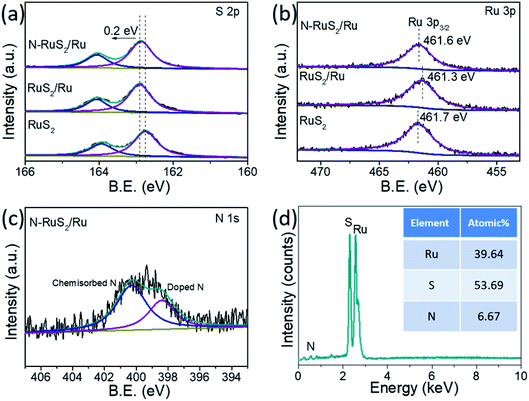
As shown in Fig. S1†, the pure RuS2 was formed via subsequent sulfidation of Ru-based RuO2 precursors (Fig. S2†) using a tube furnace. Next, the as-prepared RuS2 was annealed in using ammonia to form the N-RuS2/Ru product. For comparison, RuS2/Ru was also prepared. The details of the experiments are described in the ESI.† The morphology and microstructure of the electrocatalysts were characterized via transmission electron microscopy (TEM) and scanning electron microscope (SEM). The SEM images of the samples shown in Fig. S3† indicate that the morphology of RuS2, RuS2/Ru and N-RuS2/Ru is an overlapped-nanoparticle type. Besides, we can see that RuS2 is composed of scattered “wheat-like” nanoparticles with a diameter of about 50 nm (Fig. 1a and b). N-RuS2/Ru shows the same morphology as that of pure RuS2. It is worth mentioning that the particle size of N-RuS2/Ru decreases slightly, and the surface becomes rougher (Fig. 1c and d), which is very gratifying as rough surfaces may expose more active sites for electrocatalytic reactions. Fig. 1e shows the X-ray diffraction (XRD) patterns of RuS2, RuS2/Ru and N-RuS2/Ru. The pure RuS2 shows a cubic structure with a crystal parameter of 0.561 nm, wherein three strongest peaks locate at 27.5°, 31.9° and 35.8° are indexed to the (111), (200) and (220) crystal planes, respectively. For RuS2/Ru and N-RuS2/Ru, in addition to the original diffraction peaks of RuS2, different peaks of single metal Ru with the hexagonal structure were observed. The lattice fringes of RuS2 and N-RuS2/Ru were observed clearly in the high-resolution TEM images. The measured spacing of the lattice fringes for RuS2 is 0.278 nm, corresponding to the (200) crystal face. In the fast Fourier transform (FFT) of RuS2, the crystal planes corresponding to the diffraction points can be assigned to the (200), (220) and (221) planes, which is in good agreement with the XRD results. In the high-resolution TEM images of N-RuS2/Ru, we also found the (101) crystal face of Ru metal with an interplanar distance of 0.205 nm besides the lattice fringes of RuS2. Noting that the obvious crystal boundary can be observed between RuS2 and Ru, which is likely in favor of the improvement of the catalytic activity. The energy-dispersive X-ray spectrum (EDS) mapping images (Fig. 1h–k) indicate that Ru, S, N are evenly and uniformly distributed in the whole selected region, confirming that the N element was incorporated into the samples.The chemical states of RuS2, RuS2/Ru, and N-RuS2/Ru were systematically explored via X-ray photoelectron spectroscopy (XPS), where the signals of S, Ru, and N in N-RuS2/Ru were clearly observed. The S 2p spectrum of RuS2 (Fig. 2a) shows two peaks centered at 162.7 eV and 163.9 eV, corresponding to the 2p3/2 and 2p1/2, respectively, which is consistent with the previous reports.41 Note that the peak position of S 2p in RuS2 and RuS2/Ru shifts 0.3 eV to the higher binding energy. The possible reason is that the valence state of the surrounding sulfur atom is raised during the in situ Ru reduction process, while nitrogen doping has a trivial effect on the host anion. In Fig. 2b, the high-resolution Ru 3p3/2 spectra were magnified to check such subtle changes. The peak of Ru 3p3/2 in pure RuS2 is located at 461.4 eV and is assigned to Ru4+.26 Due to the appearance of metallic Ru, Ru 3p3/2 of RuS2/Ru naturally tends to the lower energy binding. After N-doping, some electrons of Ru around the dopant N are attracted by nitrogen with strong electronegativity, which enables the peak of Ru 3p3/2 in N-RuS2/Ru shift back to the high binding energy. The high-resolution of the N 1s spectrum is presented in Fig. 2c. Two representative peaks located at 398.4 eV and 400.3 eV can be detected, which are contributed to chemisorbed N and doped N, respectively.42 Furthermore, as shown in Fig. 2d, the results of the EDS spectrum illustrate that N-RuS2/Ru contains Ru, S, N elements, and we can roughly estimate the nitrogen content as 6.67 at% (approximately equal to the result of XPS: 7.21 at%), and the ratio of Ru to S to N is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.34
1.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.17. As a contrast, the EDS spectrum of RuS2/Ru is provided in Fig. S4.† Comprehensively, the contribution of N in the EDS spectrum and N 1s in the high-resolution XPS spectrum as well as in the EDS mapping of N element in Fig. 1g consistently demonstrated that in addition to the single-phase Ru metal, the N element has also been successfully doped into RuS2.
0.17. As a contrast, the EDS spectrum of RuS2/Ru is provided in Fig. S4.† Comprehensively, the contribution of N in the EDS spectrum and N 1s in the high-resolution XPS spectrum as well as in the EDS mapping of N element in Fig. 1g consistently demonstrated that in addition to the single-phase Ru metal, the N element has also been successfully doped into RuS2.
 | ||
| Fig. 2 High-resolution XPS spectra of (a) S 2p, (b) Ru 3p, and (c) N 1s. (d) EDS spectrum of N-RuS2/Ru. | ||
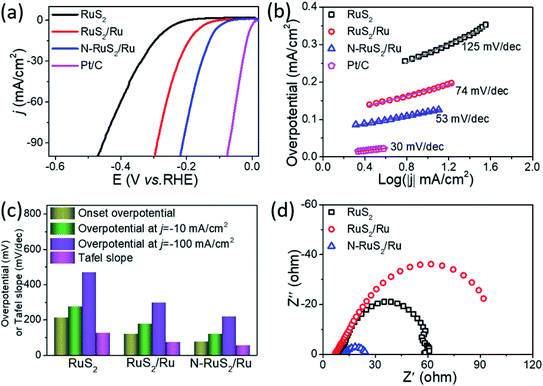
The electrocatalytic activities for HER of all the electrocatalysts were measured in an acidic medium (0.5 M H2SO4) using a typical three-electrode system. Commercial Pt/C was used as a benchmark sample for comparison under the same conditions. As shown in Fig. 3a, the polarization curves were first measured to analyze the HER activities of RuS2, RuS2/Ru and N-RuS2/Ru nanoparticles electrocatalysts via the linear sweep voltammogram (LSV) methods. The onset overpotential, an important parameter of HER performance, can be known from the polarization curves. However, there are various methods to determine the value of the onset overpotential. For the sake of fairness, we chose the overpotential at the current density of 1 mA cm−2 as the onset overpotential. The results display that the N-RuS2/Ru nanoparticles need a much lower onset potential of 76 mV to overcome the energy barrier of the initial reaction than do RuS2 (∼212 mV) and RuS2/Ru (∼121 mV) (Fig. 3a), which indicates that N-RuS2/Ru has more reasonable hydrogen adsorption capacity and more efficient electrocatalytic active sites for HER. The satisfactory promotion is tremendously attributed to the interface effect of embedded-Ru with RuS2, which leads to the formation of a large number of highly active reaction sites. Moreover, the defects and synergistic effect derived N-doping plays a vital role. To reach a current density of 10 mA cm−2, a required overpotential is only 120 mV for the N-RuS2/Ru nanoparticles, smaller than that of RuS2/Ru (177 mV) and less than half of RuS2 (276 mV). If we intend to continue to increase the current density to 100 mA cm−2, the overpotential demand for N-RuS2/Ru is 219 mV, while it is 297 mV for RuS2/Ru, and 470 mV for RuS2. To evaluate the HER kinetics of these electrocatalysts, their corresponding Tafel plots were further calculated and fitted based on the polarization curves, as shown in Fig. 3b. Obviously, compared with the pristine RuS2 (125 mV dec−1) and RuS2/Ru (74 mV dec−1), the N-RuS2/Ru shows a much smaller Tafel slope of 53 mV dec−1. Fig. 3c shows the overpotentials at different current densities for three samples and corresponding Tafel slopes, their electrocatalytic activities can be seen and compared at a glance. These results are comparable or superior to those recently reported on representative HER electrocatalysts (Table S1, ESI†). Electrochemical impedance spectra (EIS) of the three electrocatalysts were obtained by the AC electrochemistry impendence test. A smaller impedance arc radius relates a lower interface charge transfer resistance (Rct), i.e., a faster reaction rate.7,17 It can be seen in Fig. 3d that N-RuS2/Ru has a minimum impedance arc radius among three samples, indicating its outstanding interface transfer conductivity. The ahead analysis results confirm that N-doping has modulated the electron density of RuS2, and the in situ reduced Ru also affected the electronic state of catalysts. Changes in Rct demonstrate that N-doping and embedded-Ru have successfully promoted the interface electron transfer rate of RuS2 in the reaction process.
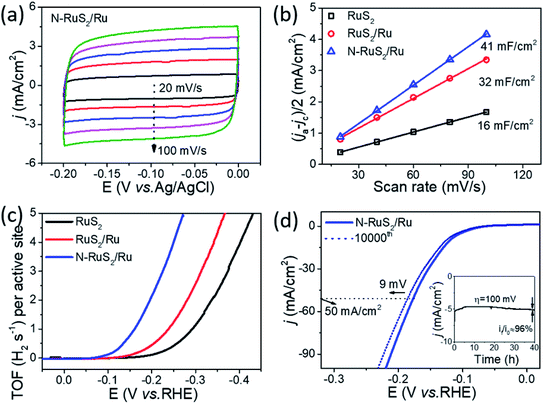
Fig. 4a shows the CV curves of N-RuS2/Ru, which were collected at −0.2 to 0 V (vs. Ag/AgCl) under a series of scan rates ranged from 20 to 100 mV s−1 via cyclic voltammetry. The CV curves of RuS2 and RuS2/Ru have been provided in Fig. S5 in ESI.† Next, half of the differences in current density variation (Δj/2= (ja − jc)/2) at a certain potential of −0.1 V (vs. Ag/AgCl) were plotted against the scan rate, as shown in Fig. 4b. The electrochemical double-layer capacitance (Cdl) can be estimated by the linear fitting. N-RuS2/Ru exhibits the largest Cdl of 41 mF cm−2, more than twice that of RuS2 (16 mF cm−2). The largest value of Cdl indicates that N-RuS2/Ru has the largest electrochemically active surface area (ECSA) because Cdl is considered to be directly related to ECSA.43,44 In addition, N-RuS2/Ru also displays the best turn over frequency (TOF) (2.115 s−1 at an overpotential of 200 mV) among the three electrocatalysts (Fig. 4c). Under the equal overpotential, the TOF values of RuS2 and RuS2/Ru are 0.107 s−1 and 0.438 s−1, respectively, which are far lower than that of N-RuS2/Ru. To reach 0.725 s−1 of TOF as Pt,45 N-RuS2/Ru requires an overpotential of 147 mV, much smaller than those of RuS2 (276 mV) and RuS2/Ru (221 mV). This data further confirms the superior HER performances of N-RuS2/Ru. Both the results of ECSA and TOF suggest that N-RuS2/Ru has higher electrocatalytic activity toward HER. The electrocatalytic parameters of the three electrocatalysts are listed in Table S2.† Except for the electrocatalytic activity, the durability of N-RuS2/Ru is also evaluated under a long-term cycling test. As shown in Fig. 4d, the overpotential at a current density of 50 mA cm−2 for N-RuS2/Ru nanoparticles has a slight increase of only 9 mV, better than those of RuS2 (+26 mV) and RuS2/Ru (+16 mV) (Fig. S6†). Besides, the current density is reduced by only 4% after a constant chronoamperometry (overpotential: 100 mV) test for 40 h, further illustrating the excellent stability of N-RuS2/Ru nanoparticles. It is worth mentioning that the micromorphology of N-RuS2/Ru nanoparticles is basically retained after the cycling test (Fig. S7†).
The enhancement of the catalytic activity of N-RuS2/Ru is mainly due to the combination of nitrogen doping and the in situ reduction Ru metal as well as the synergistic effect. Doping nitrogen can adjust the electronic structure, narrow the bandgap of the semiconductor catalysts RuS2, accelerate the surface electronic transfer and activate the surrounding atoms (such as Ru or S) as the efficient active sites, which will lead to an increase in active sites.46,47 Moreover, the introduction of nitrogen may result in some defects in the structures, which will be of great benefit to the improvement of catalytic efficiency. In addition, Ru, as a precious metal, has similar hydrogen adsorption capacity to Pt, indicating the presence of extremely efficient active sites.39,40 Meanwhile, it forms an atomic-scale interface structure with RuS2. We know that interface engineering is a critical and effective route to expand the catalytic activity.48–50 The electronic states near the interface will be optimized, and the chemical states of atoms near the interface will also be affected, which will eventually promote a rapid electron transfer and appropriate hydrogen adsorption in HER.
3. Conclusions
In summary, we have successfully prepared N-RuS2/Ru nanoparticles used as an efficient electrocatalyst for HER. Reasonable design and simplified synthesis strategy produced a cost-effective and high-performance electrocatalyst. N-doping can optimize the electronic structure to enhance the conductivity of N-RuS2/Ru. The in situ reduced Ru metal phase increases the atomic-scale interface in the materials, which is particularly conducive to the promotion of the electrocatalytic activity. Thus, the as-synthesized N-RuS2/Ru exhibits a remarkable performance for HER in 0.5 M H2SO4. It presents the lowest onset overpotential of 76 mV, a required overpotential of 120 mV and 219 mV at the current density of 10 mA cm−2 and 100 mA cm−2, respectively. The N-RuS2/Ru electrocatalyst also has a small Tafel slope of 53 mV dec−1 and a large electrochemically active surface area. The turnover frequency of N-RuS2/Ru is high up to 2.115H2 s−1 at an overpotential of 200 mV. Moreover, it has long-term stability over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. This work paves a new avenue to design robust Pt-free electrocatalysts for hydrogen production toward commercial water electrolysis.
000 cycles. This work paves a new avenue to design robust Pt-free electrocatalysts for hydrogen production toward commercial water electrolysis.
Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This work is supported by the Science Fund of Educational Department of Henan Province of China and the Ninth Group of Key Disciplines in Henan Province (grant no. 2018119). It is also supported by the Application Research and Development Plan of Gansu Academy of Sciences (2018JK-16), Key Research and Development Plan of Gansu Province (No. 18YF1GA088) and Innovative Team Construction Project of Gansu Academy of Science (2020CX005-01).References
- A. J. Bard and M. A. Fox, Acc. Chem. Res., 1995, 28, 141 CrossRef CAS.
- J. Chow, R. J. Kopp and P. R. Portney, Science, 2003, 302, 1528 CrossRef PubMed.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148 RSC.
- N. Armaroli and V. Balzani, Angew. Chem., Int. Ed., 2007, 46, 52–66 CrossRef CAS PubMed.
- H. W. Liang, S. Brüller, R. Dong, J. Zhang, X. Feng and K. Müllen, Nat. Commun., 2015, 6, 7992 CrossRef CAS PubMed.
- S. P. S. Badwal, S. S. Giddey, C. Munnings, A. I. Bhatt and A. F. Hollenkamp, Front. Chem., 2014, 2, 79 Search PubMed.
- J. Wang, F. Xu, H. Jin, Y. Chen and Y. Wang, Adv. Mater., 2017, 1605838 CrossRef PubMed.
- R. Subbaraman, D. Tripkovic, D. Strmcnik, K. C. Chang, M. Uchimura, A. P. Paulikas, V. tamenkovic and N. M. Markovic, Science, 2011, 334, 1256 CrossRef CAS PubMed.
- D. V. Esposito, S. T. Hunt, Y. C. Kimmel and J. G. G. Chen, J. Am. Chem. Soc., 2012, 134, 3025 CrossRef CAS PubMed.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. B. Chorkendorff and J. K. Norskov, Nat. Mater., 2006, 5, 909 CrossRef CAS PubMed.
- Y. G. Li, H. L. Wang, L. M. Xie, Y. Y. Liang, G. S. Hong and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 7296 CrossRef CAS PubMed.
- W. B. Lu, T. T. Liu, L. S. Xie, C. Tang, D. N. Liu, S. Hao, F. L. Qu, G. Du, Y. J. Ma, A. M. Asiri and X. P. Sun, Small, 2017, 13, 1700805 CrossRef PubMed.
- S. J. Deng, F. Yang, Q. H. Zhang, Y. Zhong, Y. X. Zeng, S. W. Lin, X. L. Wang, X. H. Lu, C. Z. Wang, L. Gu, X. H. Xia and J. P. Tu, Adv. Mater., 2018, 30, 1802223 CrossRef PubMed.
- Z. Y. Chen, Y. Song, J. Y. Cai, X. S. Zheng, D. D. Han, Y. S. Wu, Y. P. Zang, S. W. Niu, Y. Liu, J. F. Zhu, X. J. Liu and G. M. Wang, Angew. Chem., Int. Ed., 2018, 57, 5076 CrossRef CAS PubMed.
- Q. Liu, J. Q. Tian, W. Cui, P. Jiang, N. Y. Cheng, A. M. Asiri and X. P. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710 CrossRef CAS PubMed.
- Y. P. Liu, G. T. Yu, G. D. Li, Y. H. Sun, T. Asefa, W. Chen and X. X. Zou, Angew. Chem., Int. Ed., 2015, 54, 10752 CrossRef CAS PubMed.
- I. K. Mishra, H. Zhou, J. Sun, K. Dahal, S. Chen and Z. Ren, Energy Environ. Sci., 2018, 1 CAS.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267 CrossRef CAS PubMed.
- E. J. Popczun, C. G. Read, C. W. Roske, N. S. Lewis and R. E. Schaak, Angew. Chem., Int. Ed., 2014, 53, 5427 CrossRef CAS PubMed.
- W. Cui, Q. Liu, Z. Xing, A. M. Asiri, K. A. Alamry and X. Sun, Appl. Catal., B, 2015, 164, 144 CrossRef CAS.
- Z. Pu, I. S. Amiinu, Z. Kou, W. Li and S. Mu, Angew. Chem., Int. Ed., 2017, 56, 11559 CrossRef CAS PubMed.
- A. P. Gaikwad, D. Tyagi, C. A. Betty and R. Sasikala, Appl. Catal., A, 2016, 517, 91 CrossRef CAS.
- B. S. Lou, P. Veerakumar, S. M. Chen, V. Veeramani, R. Madhu and S. B. Liu, Sci. Rep., 2016, 6, 19949 CrossRef CAS PubMed.
- P. R. Deshmukh, S. N. Pusawale, A. D. Jagadale and C. D. Lokhande, J. Mater. Sci., 2012, 47, 1546 CrossRef CAS.
- M. Lacroix, N. Boutarfa, C. Guillard, M. Vrinat and M. Breysse, J. Catal., 1989, 120, 473 CrossRef CAS.
- J. Yu, Y. Guo, S. Miao, M. Ni, W. Zhou and Z. Shao, ACS Appl. Mater. Interfaces, 2018, 10, 34098 CrossRef CAS PubMed.
- M. Cheng, H. Geng, Y. Yang, Y. Zhang and C. C. Li, Chem.–Eur. J., 2019, 25, 8579 CrossRef CAS PubMed.
- J. Q. Chi, X. J. Zeng, X. Shang, B. Dong, Y. M. Chai, C. G. Liu, M. Marin and Y. Yin, Adv. Funct. Mater., 2019, 1901790 CrossRef.
- Y. Zheng, Y. Jiao, Y. Zhu, L. Li, Y. Han, Y. Chen, M. Jaroniec and S. Qiao, J. Am. Chem. Soc., 2016, 138, 16174 CrossRef CAS PubMed.
- J. Mahmood, F. Li, S. Jung, M. S. Okyay, I. Ahmad, S. Kim, N. Park, H. Y. Jeong and J. B. Baek, Nat. Nanotechnol., 2017, 12, 441 CrossRef CAS PubMed.
- S. Chen and S. Z. Qiao, ACS Nano, 2013, 7, 10190 CrossRef CAS PubMed.
- A. Aijaz, J. Masa, C. Rosler, W. Xia, P. Weide, A. J. Botz, R. A. Fischer, W. Schuhmann and M. Muhler, Angew. Chem., Int. Ed., 2016, 55, 4087 CrossRef CAS PubMed.
- J. Zhang, T. Wang, D. Xue, C. Guan, P. Xi, D. Gao and W. Huang, Energy Storage Mater., 2020, 25, 202 CrossRef.
- M. Yu, Z. Wang, C. Hou, Z. Wang, C. Liang, C. Zhao, Y. Tong, X. Lu and S. Yang, Adv. Mater., 2017, 1602868 CrossRef PubMed.
- D. Hou, W. Zhou, K. Zhou, Y. Zhou, J. Zhong, L. Yang, J. Lu, G. Li and S. Chen, J. Mater. Chem. A, 2015, 3, 15962 RSC.
- Y. Liu, C. Ma, Q. Zhang, W. Wang, P. Pan, L. Gu, D. Xu, J. Bao and Z. Dai, Adv. Mater., 2019, 31, 1900062 CrossRef PubMed.
- Q. Liang, L. Zhong, C. Du, Y. Luo, J. Zhao, Y. Zheng, J. Xu, J. Ma, C. Liu, S. Li and Q. Yan, ACS Nano, 2019, 13, 7975 CrossRef CAS PubMed.
- P. Liu, J. Ran, B. Xia, S. Xi, D. Gao and J. Wang, Nano-Micro Lett., 2020, 12, 68 CrossRef.
- Y. Zheng, Y. Jiao, Y. H. Zhu, L. H. Li, Y. Han, Y. Chen, M. Jaroniec and S. Z. Qiao, J. Am. Chem. Soc., 2016, 138, 16174 CrossRef CAS PubMed.
- J. Wang, Z. Z. Wei, S. J. Mao, H. R. Li and Y. Wang, Energy Environ. Sci., 2018, 11, 800 RSC.
- K. Krishnamoorthy, P. Pazhamalai and S. J. Kim, Electrochim. Acta, 2017, 227, 85 CrossRef CAS.
- M. Yu, Z. Wang, C. Hou, Z. Wang, C. Liang, C. Zhao, Y. Tong, X. Lu and S. Yang, Adv. Mater., 2017, 29, 1602868 CrossRef PubMed.
- I. K. Mishra, H. Q. Zhou, J. Y. Sun, K. Dahal, Z. S. Ren, R. He, S. Chen and Z. F. Ren, Mater. Today Phys., 2018, 4, 1 CrossRef.
- H. Q. Zhou, F. Yu, Y. F. Huang, J. Y. Sun, Z. Zhu, R. J. Nielsen, R. He, J. M. Bao, W. A. Goddard III, S. Chen and Z. F. Ren, Nat. Commun., 2016, 7, 12765 CrossRef CAS PubMed.
- T. F. Jaramillo, K. P. Jørgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100 CrossRef CAS PubMed.
- J. Jiang, F. Sun, S. Zhou, W. Hu, H. Zhang, J. Dong, Z. Jiang, J. Zhao, J. Li, W. Yan and M. Wang, Nat. Commun., 2018, 9, 2885 CrossRef PubMed.
- S. H. Ye, Z. X. Shi, J. X. Feng, Y. X. Tong and G. R. Li, Angew. Chem., Int. Ed., 2018, 57, 2672 CrossRef CAS PubMed.
- Y. Wu, F. Li, W. Chen, Q. Xiang, Y. Ma, H. Zhu, P. Tao, C. Song, W. Shang, T. Deng and J. Wu, Adv. Mater., 2018, 30, 1803151 CrossRef PubMed.
- J. Yin, Y. Li, F. Lv, Q. Fan, Y.-Q. Zhao, Q. Zhang, W. Wang, F. Cheng, P. Xi and S. Guo, ACS Nano, 2017, 11, 2275 CrossRef CAS PubMed.
- J. Yin, Y. Li, F. Lv, M. Lu, K. Sun, W. Wang, L. Wang, F. Cheng, Y. Li, P. Xi and S. Guo, Adv. Mater., 2017, 29, 1704681 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02530e |
| This journal is © The Royal Society of Chemistry 2020 |