Open Access Article
Open Access ArticleWork function modification of PEDOT:PSS by mixing with barium acetylacetonate†
K. L. Woon *a,
W. S. Wonga,
N. Chanlekb,
H. Nakajimab,
S. Tunmeeb,
V. S. Leec,
A. Ariffinc and
P. Songsiriritthiguld
*a,
W. S. Wonga,
N. Chanlekb,
H. Nakajimab,
S. Tunmeeb,
V. S. Leec,
A. Ariffinc and
P. Songsiriritthiguld
aLow Dimensional Material Research Center, Department of Physics, University Malaya, 50603, Kuala Lumpur, Malaysia. E-mail: ph7klw76@um.edu.my
bSynchrotron Light Research Institute, Nakhon Ratchasima, 30000, Thailand
cDepartment of Chemistry, Faculty of Science, University Malaya, 50603, Kuala Lumpur, Malaysia
dResearch Network NANOTECH-SUT on Advanced Nanomaterials and Characterization, School of Physics, Suranaree University of Technology, Nakhon Ratchasima, 30000, Thailand
First published on 6th May 2020
Abstract
Poly(3,4-ethylenedioxythiophene)polystyrene sulfonate (PEDOT:PSS) is often used as a hole injection and extractor for various organic electronic devices. This study investigated whether it is possible to n-dope PEDOT:PSS with barium acetylacetonate (Ba(acac)2) to change its work function so that to be more suitable for electron injection and extraction. Molecular dynamics simulations suggested that barium cations can interact with the aromatic rings of PEDOT and the negatively charged sulfonate in PSS. At high doping concentration, we found that PEDOT became dedoped and precipitated resulting in a clear solution after filtration. The absence of the absorption peak of PEDOT at 263 nm indicates the removal of PEDOT after filtration. The shift in O 1s to a lower binding energy as seen in X-ray photoelectron spectroscopy suggested that the polystyrene sulfonic acids are being ionized to form barium polystyrene sulfonate (Ba–PSS). By spin-coating the solution on top of indium tin oxide, the work function can be adjusted to as low as 3.6 eV. The ability of such a mixture to inject and extract electrons is demonstrated using 2,7-bis(diphenylphosphoryl)-9,9′-spirobifluorene as an electron transporting layer. We attributed the lowering of the work function as the result of the formation of an interfacial dipole as large as 1.37 eV at the ITO/Ba–PSS interface.
Introduction
Poly(3,4-ethylenedioxythiophene)polystyrene sulfonate (PEDOT:PSS) is a polymer ionomer widely used in various electronic devices such as organic light emitting diodes,1,2 supercapacitors,3 field effect transistors,4 photovoltaic cells5,6 and many more due to its superior electronic and ionic conductivities, stability and solution processability. PEDOT:PSS consists of the insulating negatively charged poly(styrenesulfonate) (PSS) counter-balanced by the positively charged poly(3,4-ethylenedioxythiophene) (PEDOT). The maximum oxidation of PEDOT is one charge per three monomer units, with the hole polaron delocalized over the three monomers forming a quinoid structure7 counter-balanced by the electron located at the sulfonate side group. The weakly interacting ionomers result in an expanded coil conformation forming a colloidal dispersion in water.In recent years, much effort has been concentrated in deepening the work function of the PEDOT:PSS. Several attempts have been carried out such as the use of metal oxide as dopants in PEDOT:PSS,8 surface treatment with alkyl alcohol9 and addition of Nafion10,11 are found to deepen the work function up to 6 eV. Lowering the work function of PEDOT:PSS seems to be less successful. PEDOT:PSS modified with polyethylenimine can only reduce the work function to 4.0 eV.12,13 Hence, efforts have been concentrated on solution processable sodium bicarbonate,14 polymer zwitterion,15 lithium phenolate complexes16 and ruthenium acetylacetonate17 which are deposited below the aluminum electrode. The increase in the electron injection ability is found to be the result of formation of interfacial dipole between the conductive electrode which lowers the vacuum level of the semiconducting layer.18,19 Recent efforts such as the use of self-compensated multivalent anions electron donors20 is promising as the effective work function of silver can be reduced to as low as 2.4 eV.
The objective of this research is to investigate the possibility of n-doping the PEDOT:PSS and to study the mechanism of such effect. This will enable the use of solution processable low work function cathode opening up the potential of fabricating organic electronic devices without the use of high vacuum system hence reducing the cost of production significantly. In this research, we mixed Clevios™ PEDOT:PSS Al4083 with barium acetylacetonate (Ba(acac)2) in the hope of n-doping the PEDOT:PSS. We found that the work function can be reduced as low as 3.6 eV. X-ray photoelectron spectroscopy (XPS) and molecular dynamics simulations suggested that the polystyrene sulfonic acids (PSSH) is being deprotonated lowering the binding energy of O 1s resulting in the formation of Ba–PSS. We attributed the lowering of work function as a result of formation of interfacial dipole by Ba–PSS.
Methodology
Materials
Fig. 1 shows the chemical structure of the materials used. PEDOT:PSS solution (Clevios™ PVP AI4083) was purchased from Heraeus. 2,7-Bis(diphenylphosphoryl)-9,9′-spirobifluorene (SPPO13) was purchased from Luminescence Technology (Taiwan). Ba(acac)2 and 2,2,3,3-tetrafluoro-1-propanol 98% were bought from Sigma-Aldrich. All materials were purchased and used as received without further purification.Device fabrications
Different blending ratios of PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) with X = 0.2, 0.5, 1 and 5 were prepared by adding 3 mg, 7.5 mg, 15 mg and 75 mg of Ba(acac)2 to 1 g of PEDOT:PSS solution respectively. Pristine Ba(acac)2 solution was prepared by adding 15 mg of Ba(acac)2 to 1 g of deionized water. SPPO13 solution was prepared by dissolving 25 mg of SPPO13 to 1 ml of 2,2,3,3-tetrafluoro-1-propanol. All the solutions were filtered by 0.45 μm poly(tetrafluoroethylene) filters before use unless otherwise stated. Electron only device has a device structure of ITO/PEDOT:PSS
X) with X = 0.2, 0.5, 1 and 5 were prepared by adding 3 mg, 7.5 mg, 15 mg and 75 mg of Ba(acac)2 to 1 g of PEDOT:PSS solution respectively. Pristine Ba(acac)2 solution was prepared by adding 15 mg of Ba(acac)2 to 1 g of deionized water. SPPO13 solution was prepared by dissolving 25 mg of SPPO13 to 1 ml of 2,2,3,3-tetrafluoro-1-propanol. All the solutions were filtered by 0.45 μm poly(tetrafluoroethylene) filters before use unless otherwise stated. Electron only device has a device structure of ITO/PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X)/SPPO13 (100 nm)/CsF (1 nm)/Al (100 nm). All the Indium Tin Oxide (ITO) coated glass substrates were ultrasonically cleaned using deionized (DI) water, acetone, isopropyl alcohol and DI water again for 10 min, followed by oxygen plasma treatment for 5 min. Pristine PEDOT:PSS was spin-coated on pre-patterned ITO with spin speed of 500 rpm, 1000 rpm, 1500 rpm and 2000 rpm each 10 s. PEDOT:PSS
X)/SPPO13 (100 nm)/CsF (1 nm)/Al (100 nm). All the Indium Tin Oxide (ITO) coated glass substrates were ultrasonically cleaned using deionized (DI) water, acetone, isopropyl alcohol and DI water again for 10 min, followed by oxygen plasma treatment for 5 min. Pristine PEDOT:PSS was spin-coated on pre-patterned ITO with spin speed of 500 rpm, 1000 rpm, 1500 rpm and 2000 rpm each 10 s. PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) samples were prepared by firstly spin coating PEDOT:PSS as buffer layer with similar recipe as pristine, before dispensing PEDOT:PSS
X) samples were prepared by firstly spin coating PEDOT:PSS as buffer layer with similar recipe as pristine, before dispensing PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) solutions on top of first layer at 30 s at 2000 rpm. The fabricated devices were then annealed in glovebox with 120 °C for 10 min. For electron transporting device, the SPPO13 solution was subsequently spin-coated on top of PEDOT:PSS
X) solutions on top of first layer at 30 s at 2000 rpm. The fabricated devices were then annealed in glovebox with 120 °C for 10 min. For electron transporting device, the SPPO13 solution was subsequently spin-coated on top of PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) and annealed with 100 °C for 15 min. Finally, CsF (1 nm) and Al (100 nm) were thermally evaporated through shadow mask at base pressure of 4.2 × 10−4 Pa. The current density (J), voltage (V) of the devices were characterized with Keithley 2612B System Sourcemeter. The thickness of all solution-processed films was measured using a profilometer (P-6 KLA-Tencor).
X) and annealed with 100 °C for 15 min. Finally, CsF (1 nm) and Al (100 nm) were thermally evaporated through shadow mask at base pressure of 4.2 × 10−4 Pa. The current density (J), voltage (V) of the devices were characterized with Keithley 2612B System Sourcemeter. The thickness of all solution-processed films was measured using a profilometer (P-6 KLA-Tencor).
Characterizations
The work functions of the PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) were probed using ultraviolet photoelectron spectroscopy (UPS) at the soft X-ray undulator beamline BL3.2Ua in the Synchrotron Light Research Institute (SLRI) in Thailand. The samples were measured in a vacuum chamber under the base pressure of 1 × 10−9 mbar, and subject to UV radiation with a photon energy of 39.5 eV and a pass energy of 81.9 eV. The chemical compositions of the spin-coated films were investigated using the PHI5000 Versa Probe II X-ray photoelectron spectroscopy (XPS) system at the SUT-NANOTEC-SLRI joint research facility at SLRI in Thailand. The XPS spectra were collected using Al Kα (1486.6 eV) radiation, under the base pressure of 1 × 10−9 mbar. The surface morphology of the spin-coated films was measured using atomic force microscopy (AFM NT-MDT NTEG RA-Prima) operating at tapping mode. A PerkinElmer Lambda 750 UV-Vis-NIR was used to measure the material absorbance of the drop-casted and spin-coated PEDOT:PSS
X) were probed using ultraviolet photoelectron spectroscopy (UPS) at the soft X-ray undulator beamline BL3.2Ua in the Synchrotron Light Research Institute (SLRI) in Thailand. The samples were measured in a vacuum chamber under the base pressure of 1 × 10−9 mbar, and subject to UV radiation with a photon energy of 39.5 eV and a pass energy of 81.9 eV. The chemical compositions of the spin-coated films were investigated using the PHI5000 Versa Probe II X-ray photoelectron spectroscopy (XPS) system at the SUT-NANOTEC-SLRI joint research facility at SLRI in Thailand. The XPS spectra were collected using Al Kα (1486.6 eV) radiation, under the base pressure of 1 × 10−9 mbar. The surface morphology of the spin-coated films was measured using atomic force microscopy (AFM NT-MDT NTEG RA-Prima) operating at tapping mode. A PerkinElmer Lambda 750 UV-Vis-NIR was used to measure the material absorbance of the drop-casted and spin-coated PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) and Ba(acac)2 thin films on quartz substrates. The pH of the PEDOT:PSS
X) and Ba(acac)2 thin films on quartz substrates. The pH of the PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 (1
Ba(acac)2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X) solution was measured using Mettler Toledo™ FiveEasy™ F20 pH mV−1 under atmospheric pressure and ambient temperature. Calibration was performed with buffer solution of pH 4.01, pH 7 and pH 9.21 before pH measurement.
X) solution was measured using Mettler Toledo™ FiveEasy™ F20 pH mV−1 under atmospheric pressure and ambient temperature. Calibration was performed with buffer solution of pH 4.01, pH 7 and pH 9.21 before pH measurement.
Computational calculations
For molecular dynamics simulations, the initial structures of PEDOT and PSS consisting five 3,4-ethylenedioxythiophene units and five styrene units respectively were minimized under semiempirical AM1 method to get the appropriate charge before subjecting for the molecular dynamics simulations for PEDOT:PSS. Four of each PEDOT and PSS were randomly distributed in the 100 × 100 × 100 Å3 box and sampled at 300 K under NVT periodic boundary condition for 500 ps with the time step of 1 fs using CHARMM force field in HyperChem package. Geometry optimizations were calculated using density functional theory (DFT) as implemented in the Terachem 1.9 software using unrestricted wB97XD functional at def2SVP basis level.21 The calculations were performed using a GPU server that had 64 GB RAM installed to support eight Tesla K10 graphic cards. Milliken charge obtained from optimized structure of Ba(acac)2 at wB97XD functional using def2-QZVP basis is used for molecular dynamics simulations. 10 of such molecules were added randomly in the system to be run for another 500 ps. The final structures were minimized using the steepest descent method until the convergence of 0.1 kcal (Å mol)−1 was obtained. Structural dynamics, potential energy, interaction energy between PEDOT and PSS of the systems with/without Ba(acac)2 were compared and analyzed.Results and discussion
When we mixed Ba(acac)2 into PEDOT:PSS, we observed a certain degree of precipitation. The pH of PEDOT:PSS is 2.15, while the pH of the resulting solutions of PEDOT:PSS to Ba(acac)2 ratios for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 are 2.24 and 2.45 respectively. At higher concentration, the pH becomes 5.42 and 8.13 for 1
0.5 are 2.24 and 2.45 respectively. At higher concentration, the pH becomes 5.42 and 8.13 for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios respectively. The change of pH indicates that chemical reactions must have taken place between Ba(acac)2 and PEDOT:PSS. We noticed that filtered mixtures at the ratios of 1
5 ratios respectively. The change of pH indicates that chemical reactions must have taken place between Ba(acac)2 and PEDOT:PSS. We noticed that filtered mixtures at the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 solutions are clear. The Ba(acac)2 solution (15 mg ml−1) has a pH 7.11. Fig. 2(a) and (b) show the ultraviolet absorption spectroscopic data of drop-casted and spin-coated filtered solutions respectively. The drop-casted films are used since the absorbance of PEDOT in the UV is very weak. The absorbance in Fig. 2(a) was vertically translated to provide better clarity of the peak at 265 nm. We can see that the absorbance peak at 265 nm (which is very weak if the thickness of the film is too thin) decreases with increasing concentration of Ba(acac)2 as seen in Fig. 2(a). The absorbance peak at 265 nm corresponds to the aromatic transition of single ethylenedioxythiophene monomer in PEDOT.22 Fig. 2(b) shows the extinction coefficient as defined in complex refractive index of the spin-coated films. The peaks at 195 nm and 225 nm correspond to transitions from the PSS aromatic rings23,24 remain clearly seen. In order to gain insights on how Ba(acac)2 and PEDOT:PSS interact, molecular dynamics simulations are carried out.
5 solutions are clear. The Ba(acac)2 solution (15 mg ml−1) has a pH 7.11. Fig. 2(a) and (b) show the ultraviolet absorption spectroscopic data of drop-casted and spin-coated filtered solutions respectively. The drop-casted films are used since the absorbance of PEDOT in the UV is very weak. The absorbance in Fig. 2(a) was vertically translated to provide better clarity of the peak at 265 nm. We can see that the absorbance peak at 265 nm (which is very weak if the thickness of the film is too thin) decreases with increasing concentration of Ba(acac)2 as seen in Fig. 2(a). The absorbance peak at 265 nm corresponds to the aromatic transition of single ethylenedioxythiophene monomer in PEDOT.22 Fig. 2(b) shows the extinction coefficient as defined in complex refractive index of the spin-coated films. The peaks at 195 nm and 225 nm correspond to transitions from the PSS aromatic rings23,24 remain clearly seen. In order to gain insights on how Ba(acac)2 and PEDOT:PSS interact, molecular dynamics simulations are carried out.
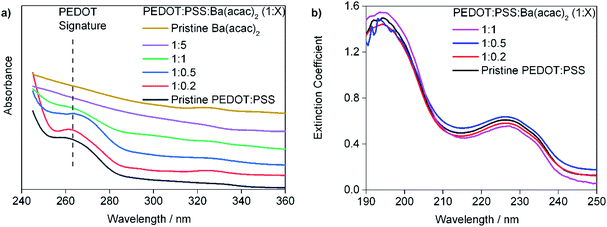 | ||
| Fig. 2 The ultraviolet absorption spectroscopic data of (a) drop-casted films (b) films formed from spin-coated filtered solution. | ||
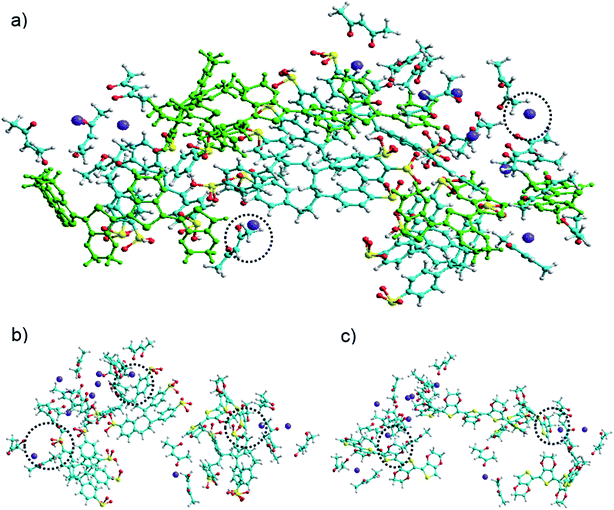
Fig. 3(a) shows the assembly of PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 without water with dot circle highlighting the Ba(acac)2 molecules remained intact. However, some Ba(acac)2 molecules have disassociated as seen in the molecular dynamics simulations. Careful inspection of the assembly of the molecules, we can identify two types of interactions: coulombic attractions between Ba2+ and the sulfonate of PSS as seen in Fig. 3(b) and cation–π interactions between Ba2+ with aromatic π fragments in PEDOT as depicted in Fig. 3(c). Based on CHARMM force field, the interaction energy between PEDOT and PSS is 80.3 kcal mol−1 while its interaction with
Ba(acac)2 without water with dot circle highlighting the Ba(acac)2 molecules remained intact. However, some Ba(acac)2 molecules have disassociated as seen in the molecular dynamics simulations. Careful inspection of the assembly of the molecules, we can identify two types of interactions: coulombic attractions between Ba2+ and the sulfonate of PSS as seen in Fig. 3(b) and cation–π interactions between Ba2+ with aromatic π fragments in PEDOT as depicted in Fig. 3(c). Based on CHARMM force field, the interaction energy between PEDOT and PSS is 80.3 kcal mol−1 while its interaction with![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 is 115 kcal mol−1. The higher interaction energy in the presence of Ba(acac)2 indicated interaction between PEDOT and PSS is weakened. In water, the poly(styrenesulfonate) acid (PSSH) can exist in equilibrium between PSS− and H+. We expect that as more Ba2+ are bound to sulfonate, more PSSH dissociates into PSS− and H+ ions in order to maintain the equilibrium while excess H+ ions recombine with acetylacetonate ions to form acetylacetone until all poly(styrenesulfonate) acids are being deprotonated. This could explain why the acidity of PEDOT:PSS is being reduced.
Ba(acac)2 is 115 kcal mol−1. The higher interaction energy in the presence of Ba(acac)2 indicated interaction between PEDOT and PSS is weakened. In water, the poly(styrenesulfonate) acid (PSSH) can exist in equilibrium between PSS− and H+. We expect that as more Ba2+ are bound to sulfonate, more PSSH dissociates into PSS− and H+ ions in order to maintain the equilibrium while excess H+ ions recombine with acetylacetonate ions to form acetylacetone until all poly(styrenesulfonate) acids are being deprotonated. This could explain why the acidity of PEDOT:PSS is being reduced.
Since precipitates can be detrimental to devices, we filtered the solutions just before spin-coating. Fig. 4(a) shows the work functions of PEDOT:PSS with different concentrations of Ba(acac)2. The PEDOT:PSS has a work function of 4.90 eV for Clevios P VP Al4083. Increasing the amount of Ba(acac)2 into PEDOT:PSS lowers the work functions to 4.08 eV, 3.64 eV and 3.56 eV for the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, 1
0.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 respectively. 1
5 respectively. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 are not shown since the work function is very similar to 1
0.5 are not shown since the work function is very similar to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2. Note that, Ba(acac)2 on top of the ITO has a work function of 4.06 eV while the 1
0.2. Note that, Ba(acac)2 on top of the ITO has a work function of 4.06 eV while the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios have lower work functions than Ba(acac)2. Fig. 4(b) shows the current–voltage behavior of electron transporting SPPO13 with a device structure of ITO/PEDOT:PSS
5 ratios have lower work functions than Ba(acac)2. Fig. 4(b) shows the current–voltage behavior of electron transporting SPPO13 with a device structure of ITO/PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2/SPPO13/CsF/Al. CsF/Al is an excellent injection layer and electrons are expected to be injected with ease from the CsF/Al.25,26 As pristine PEDOT:PSS is not a perfect electron blocker, injected electron can be recombined in the hole polaron of PEDOT giving rise to current at high forward bias voltage i.e. ∼3 mA cm−2 at 10 V. However, at reverse bias, little current is detected implying that PEDOT:PSS is a poor electron injector as expected. In short, the forward bias indicates the ability for the interface to extract electrons while the reverse-bias indicates its ability to inject electrons. Addition of Ba(acac)2 drastically enhances such behavior in particular at a higher concentration of Ba(acac)2. The lowest unoccupied molecular orbital (LUMO) of SPPO13 is 2.91 eV and the interfacial dipole between PEDOT:PSS
Ba(acac)2/SPPO13/CsF/Al. CsF/Al is an excellent injection layer and electrons are expected to be injected with ease from the CsF/Al.25,26 As pristine PEDOT:PSS is not a perfect electron blocker, injected electron can be recombined in the hole polaron of PEDOT giving rise to current at high forward bias voltage i.e. ∼3 mA cm−2 at 10 V. However, at reverse bias, little current is detected implying that PEDOT:PSS is a poor electron injector as expected. In short, the forward bias indicates the ability for the interface to extract electrons while the reverse-bias indicates its ability to inject electrons. Addition of Ba(acac)2 drastically enhances such behavior in particular at a higher concentration of Ba(acac)2. The lowest unoccupied molecular orbital (LUMO) of SPPO13 is 2.91 eV and the interfacial dipole between PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at 1
Ba(acac)2 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio is negligible as seen in Fig. S1.† This indicates an electron injection barrier of 0.6–0.7 eV even at high concentration of Ba(acac)2. For the ratio of 1
1 ratio is negligible as seen in Fig. S1.† This indicates an electron injection barrier of 0.6–0.7 eV even at high concentration of Ba(acac)2. For the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the forward bias electron current density is very similar to the electron only device published elsewhere.27
1, the forward bias electron current density is very similar to the electron only device published elsewhere.27
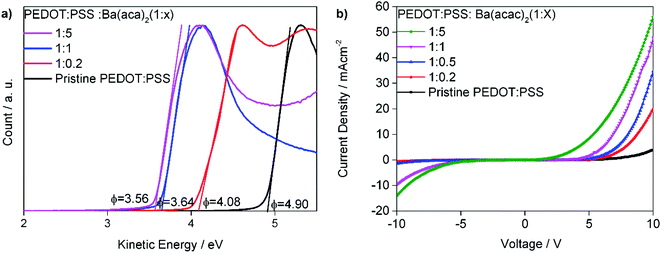 | ||
Fig. 4 (a) Work function of PEDOT:PSS with different concentrations of Ba(acac)2. (b) The current–voltage behavior of device structure of ITO/PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2/SPPO13/CsF/Al. Ba(acac)2/SPPO13/CsF/Al. | ||
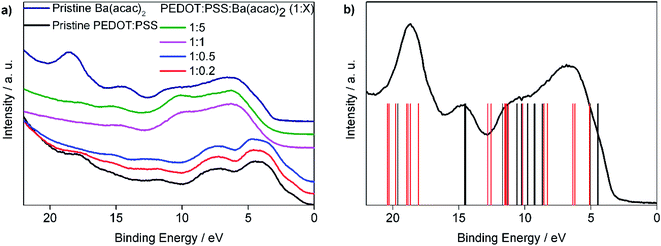
We noticed that UPS spectra can be approximately divided into two groups as shown in Fig. 5(a). We shifted the UPS spectra so that the first peak of each curve coincided with the rest of the other samples. For the ratios at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, the low energy UPS spectra are very similar to the pristine PEDOT:PSS while at higher ratios (1
0.5, the low energy UPS spectra are very similar to the pristine PEDOT:PSS while at higher ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) the differences are obvious compared with pristine Ba(acac)2. This is in agreement so the fact that the 1
5) the differences are obvious compared with pristine Ba(acac)2. This is in agreement so the fact that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 filtered solutions are clear while the 1
5 filtered solutions are clear while the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 are not. However, 1
0.5 are not. However, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 are not Ba(acac)2 solutions, in particular the peak at 18.6 eV for Ba(acac)2 is absent in all other ratios. The energy levels are obtained from the DFT optimized Ba(acac)2 structure and these energies are compared with the UPS spectra which are subtracted from the secondary electron background as shown in Fig. 5(b). The red vertical bars correspond to excitation of electron clouds involving both barium and acetylacetonate ions. It is clear that the peak at 18.6 eV composed mainly from the excitations that involve the barium ions with the list of electronic orbitals can be found at Fig. S2.† This indicates that Ba(acac)2 is likely to have been disassociated which is consistent with the molecular dynamics simulations. The reduction of acidity of PEDOT:PSS resulted in the dedoping of PEDOT:PSS. In previous studies, mixing sodium poly(styrene sulfonate) (Na–PSS) with barium chloride resulted in an exothermic reaction forming poly(styrene sulfonate) (Ba–PSS).28 Here, we suspected that Ba–PSS might have formed. The dedoping of PEDOT:PSS can inevitably lead to the separation of PEDOT and PSS that could result in precipitation.
5 are not Ba(acac)2 solutions, in particular the peak at 18.6 eV for Ba(acac)2 is absent in all other ratios. The energy levels are obtained from the DFT optimized Ba(acac)2 structure and these energies are compared with the UPS spectra which are subtracted from the secondary electron background as shown in Fig. 5(b). The red vertical bars correspond to excitation of electron clouds involving both barium and acetylacetonate ions. It is clear that the peak at 18.6 eV composed mainly from the excitations that involve the barium ions with the list of electronic orbitals can be found at Fig. S2.† This indicates that Ba(acac)2 is likely to have been disassociated which is consistent with the molecular dynamics simulations. The reduction of acidity of PEDOT:PSS resulted in the dedoping of PEDOT:PSS. In previous studies, mixing sodium poly(styrene sulfonate) (Na–PSS) with barium chloride resulted in an exothermic reaction forming poly(styrene sulfonate) (Ba–PSS).28 Here, we suspected that Ba–PSS might have formed. The dedoping of PEDOT:PSS can inevitably lead to the separation of PEDOT and PSS that could result in precipitation.
Fig. 6(a)–(c) are the surface morphology for PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at different concentrations. The root means square roughness (Ra) are 1.35 nm, 1.29 nm and 10.9 nm for pristine PEDOT:PSS, 1
Ba(acac)2 at different concentrations. The root means square roughness (Ra) are 1.35 nm, 1.29 nm and 10.9 nm for pristine PEDOT:PSS, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios respectively. It is clear that the morphology has changed from the fiber-like network structure at low concentration of Ba(acac)2 to compacted elongated sphere and pearl necklace-like appearance. The compactification could be due to the coulombic attraction between Ba2+ with the negatively charged sulfonate within the same chain or different chains that collapsed the chains (coil-globule transition).29 In terms of phase image of PEDOT:PSS, the bright color is often referred to as PEDOT-rich domains while PSS-rich domains often appear darker (Fig. 6(d)).30 When 20% loading is added into PEDOT:PSS, the surface becomes noticeably darker, possibly an indication of PSS enriched surface (Fig. 6(e)). Fig. 6(f) shows the morphology of PEDOT:PSS
1 ratios respectively. It is clear that the morphology has changed from the fiber-like network structure at low concentration of Ba(acac)2 to compacted elongated sphere and pearl necklace-like appearance. The compactification could be due to the coulombic attraction between Ba2+ with the negatively charged sulfonate within the same chain or different chains that collapsed the chains (coil-globule transition).29 In terms of phase image of PEDOT:PSS, the bright color is often referred to as PEDOT-rich domains while PSS-rich domains often appear darker (Fig. 6(d)).30 When 20% loading is added into PEDOT:PSS, the surface becomes noticeably darker, possibly an indication of PSS enriched surface (Fig. 6(e)). Fig. 6(f) shows the morphology of PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at 1
Ba(acac)2 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with the uniform distributed fibrils nature as a background juxtaposed by the appearance of elongated sphere and pearl necklace-like states. The thickness of the resulting thin films are (84 ± 9) nm, (38 ± 3) nm, (43 ± 2) nm and (7 ± 1) nm for PEDOT:PSS, at ratios of 1
1 ratio with the uniform distributed fibrils nature as a background juxtaposed by the appearance of elongated sphere and pearl necklace-like states. The thickness of the resulting thin films are (84 ± 9) nm, (38 ± 3) nm, (43 ± 2) nm and (7 ± 1) nm for PEDOT:PSS, at ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2, 1
0.2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 and 1
0.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 respectively. For 1
1 respectively. For 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratio, the thickness of the film is too thin to be measured reliably. If a solution of 15 mg ml−1 Ba(acac)2 are spin-coated, we found the resulting thickness will be less than 5 nm (see Fig. S3†).
5 ratio, the thickness of the film is too thin to be measured reliably. If a solution of 15 mg ml−1 Ba(acac)2 are spin-coated, we found the resulting thickness will be less than 5 nm (see Fig. S3†).
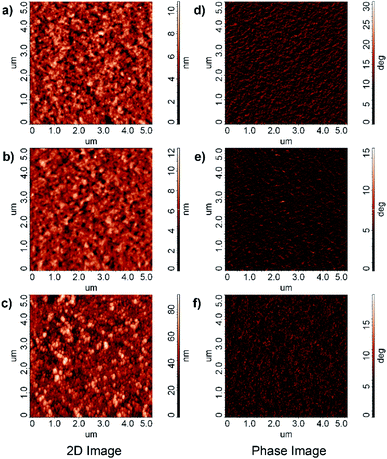 | ||
Fig. 6 Surface morphology for (a) PEDOT:PSS (b) PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at 1 Ba(acac)2 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (c) at 1 0.2 (c) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios. Phase image for (d) PEDOT:PSS (e) PEDOT:PSS 1 ratios. Phase image for (d) PEDOT:PSS (e) PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at 1 Ba(acac)2 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (f) at 1 0.2 (f) at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratios. 1 ratios. | ||
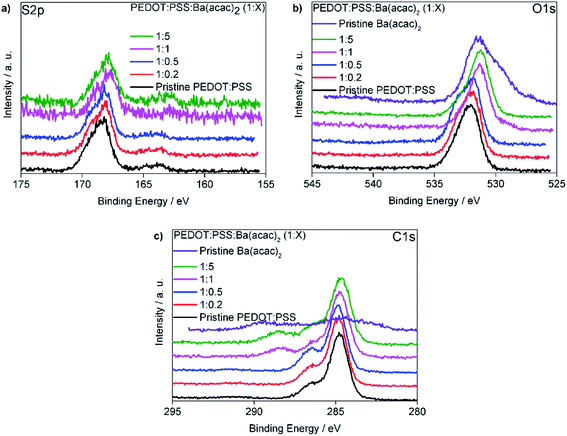
In order to elucidate the interactions between PEDOT:PSS and Ba(acac)2, we carried out XPS measurements. Fig. 7(a) is the S 2p core level displaying two signature peaks. The higher binding energy (∼168 eV) belongs to PSS because of the electronegative oxygen attached to the sulphur atoms in the sulfonate fragment of the PSS. The S 2p signature from PEDOT is far weaker with a composition ratio of ∼ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8.9 (the ratio of PEDOT to PSS) indicating a richer PSS at the top surface in comparison with homogeneous mixed Al4083 which has a ratio of 1
8.9 (the ratio of PEDOT to PSS) indicating a richer PSS at the top surface in comparison with homogeneous mixed Al4083 which has a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.5. At 20% loading, the ratio of PEDOT to PSS does not change while at 50% loading the ratio has changed to 1
7.5. At 20% loading, the ratio of PEDOT to PSS does not change while at 50% loading the ratio has changed to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11. At even higher loading of Ba(acac)2, the S 2p peak for PEDOT has reduced significantly with high noise and hence reliable PEDOT to PSS ratio cannot be obtained (see Fig. S4†). The composition of the surface are measured at a ratio of sulphur S 2p to barium Ba 4p corrected by photoionization cross-section giving the elemental ratio of 25
11. At even higher loading of Ba(acac)2, the S 2p peak for PEDOT has reduced significantly with high noise and hence reliable PEDOT to PSS ratio cannot be obtained (see Fig. S4†). The composition of the surface are measured at a ratio of sulphur S 2p to barium Ba 4p corrected by photoionization cross-section giving the elemental ratio of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 14
1, 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2.6
1, 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1.8
1 and 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for PEDOT:PSS
1 for PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at the ratios of 1
Ba(acac)2 at the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 1
0.2 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 respectively as seen in Fig. S5.† This might suggest that at higher ratios Ba–PSS may have formed. Fig. 7(b) shows the binding energy of O 1s for the mixtures and the pristine Ba(acac)2. The two peaks in Ba(acac)2 are contributed by the negatively charged O–C (lower binding energy at ∼ 529.3 eV) and neutral O
5 respectively as seen in Fig. S5.† This might suggest that at higher ratios Ba–PSS may have formed. Fig. 7(b) shows the binding energy of O 1s for the mixtures and the pristine Ba(acac)2. The two peaks in Ba(acac)2 are contributed by the negatively charged O–C (lower binding energy at ∼ 529.3 eV) and neutral O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (∼531.5 eV) in acetylacetonate ions. Usually metal acetylacetonates bind to metal through the central carbon atom in a coordination complex. However, our XPS suggested that the barium only bond through one oxygen-bonded acac ligand. The optimized structure obtained through uwB97X/def2SVP level and even at a higher basis level at def2QZVP confirmed this as seen in Fig. S6.† Attempts to create the initial complex by bonding through the central carbon-bonded acac ligand resulted in bad geometry upon optimization. We attributed the shift of the binding energy of O 1s to a lower binding energy due to the ionization of S(
C (∼531.5 eV) in acetylacetonate ions. Usually metal acetylacetonates bind to metal through the central carbon atom in a coordination complex. However, our XPS suggested that the barium only bond through one oxygen-bonded acac ligand. The optimized structure obtained through uwB97X/def2SVP level and even at a higher basis level at def2QZVP confirmed this as seen in Fig. S6.† Attempts to create the initial complex by bonding through the central carbon-bonded acac ligand resulted in bad geometry upon optimization. We attributed the shift of the binding energy of O 1s to a lower binding energy due to the ionization of S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2–OH fragment from PSSH consistent with our understanding that Ba–PSS has formed. Fig. 7(c) shows the binding energy of C 1s for the mixture and the pristine Ba(acac)2.The peak at 284.8 eV corresponds to the C atoms in the PSS and the C atoms in the PEDOT which are not bonded to the oxygen. The peak at a higher binding energy 286.5 eV corresponds to those C atoms in the PEDOT which are bonded to oxygen atoms. A new peak at 288.3 eV appeared at a higher concentration of Ba(acac)2. We assigned this to the C
O)2–OH fragment from PSSH consistent with our understanding that Ba–PSS has formed. Fig. 7(c) shows the binding energy of C 1s for the mixture and the pristine Ba(acac)2.The peak at 284.8 eV corresponds to the C atoms in the PSS and the C atoms in the PEDOT which are not bonded to the oxygen. The peak at a higher binding energy 286.5 eV corresponds to those C atoms in the PEDOT which are bonded to oxygen atoms. A new peak at 288.3 eV appeared at a higher concentration of Ba(acac)2. We assigned this to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the acetylacetone residuals that are still embedded inside the thin films. This peak is located very close to the peak at ∼289 eV which we assigned to the C
O bonds in the acetylacetone residuals that are still embedded inside the thin films. This peak is located very close to the peak at ∼289 eV which we assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O− of the acetylacetone in Ba(acac)2. Since UPS spectra indicated that upon mixing of Ba(acac)2 and PEDOT:PSS, most Ba2+ cations dissociated from the acetylacetonate counter ions and since, acetylacetone is a volatile liquid with a boiling point of 140 °C, one would assume that any remaining acetylacetone will be evaporated in high vacuum (UPS and XPS chambers) and the residual of acetylacetone unlikely to remain yet the detection of its signature at XPS (288.3 eV) indicates not all Ba(acac)2 are dissociated consistent with the molecular dynamics simulations.
O and C–O− of the acetylacetone in Ba(acac)2. Since UPS spectra indicated that upon mixing of Ba(acac)2 and PEDOT:PSS, most Ba2+ cations dissociated from the acetylacetonate counter ions and since, acetylacetone is a volatile liquid with a boiling point of 140 °C, one would assume that any remaining acetylacetone will be evaporated in high vacuum (UPS and XPS chambers) and the residual of acetylacetone unlikely to remain yet the detection of its signature at XPS (288.3 eV) indicates not all Ba(acac)2 are dissociated consistent with the molecular dynamics simulations.
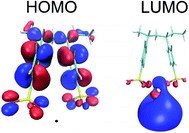
According to Stephan van Reenen et al. who studied the magnitude of the work functions modification by X+PSS− with X = H+, Li+ Na+, a trend is observed in terms of ion size.31 Caesium stearate, an ionic electrolyte with a very large counter ion has been shown to improve the electron injection of the aluminum.32 Based on the trend of X+PSS−, Stephan van Reenen et al. suggested that the relatively large size of the Cs+ ion might make image charge screening more favorable. Nevertheless, n-type doping by Cs+ may also explain the observed results. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) of Ba–PSS are located at PSS and Ba respectively as shown in Fig. 8. It is important to note that the LUMO of Ba–PSS is 0.53 eV, a value greater than zero indicating that any added electrons are unbounded, ie the electrons are free to move. Motivated by this finding, we studied Y2+PSS2− with Y = Be2+, Mg2+, Ca2+, Sr2+ and Ba2+ and X−PSS+ with X= Li+, Na+,K+, Rb+ and Cs+ systems. We found that all the systems studied, the LUMO energies are always positive with the LUMOs are located at Ba2+, Sr2+ and Cs+ metal counter ions while for other alkali counter ions, the LUMOs are located at the PSS which can be seen in Fig. S7.†
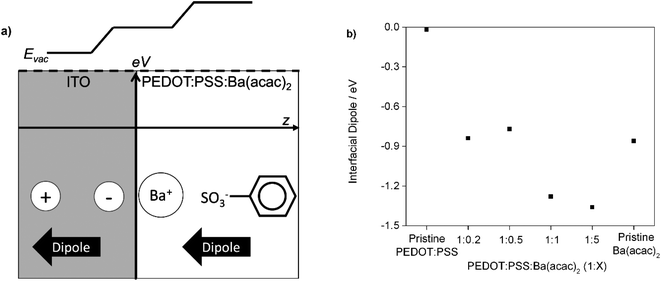
We know that the wettability of the ITO surface is enhanced after oxygen plasma treatment. Because PSS has a flexible backbone and the Ba2+ cations have high degree of freedom in water solution compared with PSS, the Ba2+ cations can preferentially reorientate on the ITO surface during the film formation. The barium ions would polarize the ITO to form an electric field (image charges) that opposes the desorption of positive charged ions. Since the ITO is a highly doped n-type semiconductor, the static dielectric constant should be large enough to stabilize the image charges. The collective of positive charge and the negative charge of sulfonates create a dipole near the ITO surface. Since the dipole of the image charges is the same direction as the dipole of Ba–PSS, the total dipole is enhanced as shown in Fig. 9(a). Hence, we attributed the change of work function as a result of formation of interfacial dipole. This is particularly true at the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The vacuum levels between ITO and PEDOT:PSS
5. The vacuum levels between ITO and PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at different ratios can be obtained by minus the high energy cut-off onset from the UPS spectra onset (Fig. S8†) from the photon energy (39.5 eV). The vacuum levels of ITO and PEDOT:PSS
Ba(acac)2 at different ratios can be obtained by minus the high energy cut-off onset from the UPS spectra onset (Fig. S8†) from the photon energy (39.5 eV). The vacuum levels of ITO and PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 at different ratios are not aligned at same height indicated the presence of interfacial dipole. Fig. 9(b) shows the interfacial dipole formed by the various ratios of PEDOT:PSS
Ba(acac)2 at different ratios are not aligned at same height indicated the presence of interfacial dipole. Fig. 9(b) shows the interfacial dipole formed by the various ratios of PEDOT:PSS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ba(acac)2 on the ITO. The interfacial dipole at the ratios of 1
Ba(acac)2 on the ITO. The interfacial dipole at the ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 are 1.26 eV and 1.37 eV respectively indicating an upward vacuum shift that lowers the effective work function. This is larger than the vacuum shift of pristine Ba(acac)2 (∼0.93 eV) possibly indicating the flexibility of counter ions might be important for reorientation. Since the thickness is less than 10 nm (1
5 are 1.26 eV and 1.37 eV respectively indicating an upward vacuum shift that lowers the effective work function. This is larger than the vacuum shift of pristine Ba(acac)2 (∼0.93 eV) possibly indicating the flexibility of counter ions might be important for reorientation. Since the thickness is less than 10 nm (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 ratios), the charge injection/extraction can be dominated by quantum tunneling. The vacuum shift for 1
5 ratios), the charge injection/extraction can be dominated by quantum tunneling. The vacuum shift for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 and 1
0.2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 ratios are ∼0.85 eV and 0.77 eV respectively and the thickness of these films are larger than 10 nm. Electron conduction for thicker films can occur through combination of ionic motion, hopping through the barium ions within these layer under applied bias.33
0.5 ratios are ∼0.85 eV and 0.77 eV respectively and the thickness of these films are larger than 10 nm. Electron conduction for thicker films can occur through combination of ionic motion, hopping through the barium ions within these layer under applied bias.33
Conclusions
We have demonstrated that by mixing high concentration of Ba(acac)2 into PEDOT:PSS, formation of PEDOT precipitates and Ba–PSS ionomer salts has occurred. At high concentration of Ba(acac)2, more PSSH is being deprotonated as evident from the XPS. Filtering out the PEDOT precipitates before spin-coating lowered the work function of ITO to as low as 3.6 eV. We attributed the lowering of work function as a result of the formation of interfacial dipole from the reorientation of Ba cations on top of the ITO surface. This finding implies that ionic polymer with barium or caesium as counter cation can be used to lower the effective work function of a surface. Since PSSH is an insulating polymer, we anticipate that future works will involve the use of highly conjugated polymer as an ionomer.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by University Malaya Postgraduate Research Grant (PG043-2015B), MyBrainScholarship and University Malaya Impact Orientated Interdisciplinary Research Grant (IIRG005A-19FNW). The Research Network NANOTEC program of the National Nanotechnology Center of Thailand is also acknowledged for partial financial support.References
- K. L. Woon, W. S. Wong, N. Chanlek, H. Nakajima, S. Tunmee, C. Songsiriritthigul, V. S. Lee and P. Songsiriritthigul, Org. Electron., 2019, 74, 1–6 CrossRef CAS.
- S. Chen, Q. Zhang, W. Shang, L. Liu, H. Yu, S. Zhang, L. Deng, M. Wang, M. Wang, X. Li, B. Mi and W. Huang, Sci. Rep., 2018, 8, 8155 CrossRef PubMed.
- Y. Liu, B. Weng, J. M. Razal, Q. Xu, C. Zhao, Y. Hou, S. Seyedin, R. Jalili, G. G. Wallace and J. Chen, Sci. Rep., 2015, 5, 17045 CrossRef CAS PubMed.
- S. H. Kim, J. Kim, S. Nam, H. S. Lee, S. W. Lee and J. Jang, ACS Appl. Mater. Interfaces, 2017, 9, 12637–12646 CrossRef CAS PubMed.
- C. T. Howells, S. Saylan, H. Kim, K. Marbou, T. Aoyama, A. Nakao, M. Uchiyama, I. D. W. Samuel, D.-W. Kim, M. S. Dahlem and P. André, J. Mater. Chem. A, 2018, 6, 16012–16028 RSC.
- T. J. Whitcher, N. A. Talik, K. Woon, N. Chanlek, H. Nakajima, T. Saisopa and P. Songsiriritthigul, J. Phys. D: Appl. Phys., 2014, 47, 055109 CrossRef.
- D. Kim and I. Zozoulenko, J. Phys. Chem. B, 2019, 123, 5160–5167 CrossRef CAS PubMed.
- A. Kanwat and J. Jang, RSC Adv., 2016, 6, 114800–114807 RSC.
- M. R. Lenze, N. M. Kronenberg, F. Würthner and K. Meerholz, Org. Electron., 2015, 21, 171–176 CrossRef CAS.
- S. A. Mauger, J. Li, Ö. T. Özmen, A. Y. Yang, S. Friedrich, M. D. Rail, L. A. Berben and A. J. Moulé, J. Mater. Chem. C, 2014, 2, 115–123 RSC.
- K. H. Yeoh, N. A. Talik, T. J. Whitcher, C. Y. B. Ng and K. L. Woon, J. Phys. D: Appl. Phys., 2014, 47, 205103 CrossRef.
- Z. Li, F. Qin, T. Liu, R. Ge, W. Meng, J. Tong, S. Xiong and Y. Zhou, Org. Electron., 2015, 21, 144–148 CrossRef CAS.
- Y. Zhang, L. Chen, X. Hu, L. Zhang and Y. Chen, Sci. Rep., 2015, 5, 12839 CrossRef CAS PubMed.
- D. X. Long and Y.-Y. Noh, J. Mater. Chem. C, 2018, 6, 12871–12878 RSC.
- M. Ruscello, S. Stolz, D. L. Gonzalez Arellano, F. Ullrich, S. Hillebrandt, E. Mankel, A. Pucci, W. Kowalsky, T. Emrick, A. L. Briseno and G. Hernandez-Sosa, Org. Electron., 2017, 50, 384–388 CrossRef CAS.
- Z. Q. Zhao, S. You, J. Huang, L. Yuan, Z. Y. Xiao, Y. Cao, N. Cheng, L. Hu, J. F. Liu and B. H. Yu, J. Mater. Chem. C, 2019, 7, 9735–9742 RSC.
- W. Chen, S. Luo, Z. Wan, X. Feng, X. Liu and Z. He, Opt. Express, 2017, 25, A253–A263 CrossRef CAS PubMed.
- C.-H. Wu, K.-W. Tsai, W.-J. Huang, C.-Y. Wu, T.-Y. Chen, T.-F. Guo, Y.-J. Hsu and T.-C. Wen, Adv. Mater. Interfaces, 2016, 3, 1500621 CrossRef.
- W. Liu, T. Liang, Q. Chen, Z. Yu, Y. Zhang, Y. Liu, W. Fu, F. Tang, L. Chen and H. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 9254–9261 CrossRef CAS PubMed.
- C. G. Tang, M. N. Syafiqah, Q.-M. Koh, C. Zhao, J. Zaini, Q.-J. Seah, M. J. Cass, M. J. Humphries, I. Grizzi, J. H. Burroughes, R.-Q. Png, L.-L. Chua and P. K. H. Ho, Nature, 2019, 573, 519–525 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- A. Elshchner, S. Kirchmeyer, W. Lovenich, U. Merker and K. Reuter, PEDOT. Principles And Applications Of An Intrinsically Conductive Polymer, 2011 Search PubMed.
- Y. H. Kim, C. Sachse, M. L. Machala, C. May, L. Müller-Meskamp and K. Leo, Adv. Funct. Mater., 2011, 21, 1076–1081 CrossRef CAS.
- Y. Xia, K. Sun and J. Ouyang, Energy Environ. Sci., 2012, 5, 5325–5332 RSC.
- H.-X. Wei, Q.-D. Ou, Z. Zhang, J. Li, Y.-Q. Li, S.-T. Lee and J.-X. Tang, Org. Electron., 2013, 14, 839–844 CrossRef CAS.
- R. Zheng, W. Huang, W. Xu and Y. Cao, Synth. Met., 2012, 162, 1919–1922 CrossRef CAS.
- S. O. Jeon, K. S. Yook, C. W. Joo and J. Y. Lee, J. Mater. Chem., 2009, 19, 5940–5944 RSC.
- M. Hansch, H. P. Kaub, S. Deck, N. Carl and K. Huber, J. Chem. Phys., 2018, 148, 114906 CrossRef PubMed.
- M. Hansch, B. Hämisch, R. Schweins, S. Prévost and K. Huber, J. Chem. Phys., 2018, 148, 014901 CrossRef PubMed.
- B. Lu, H. Yuk, S. Lin, N. Jian, K. Qu, J. Xu and X. Zhao, Nat. Commun., 2019, 10, 1043 CrossRef PubMed.
- S. van Reenen, S. Kouijzer, R. A. J. Janssen, M. M. Wienk and M. Kemerink, Adv. Mater. Interfaces, 2014, 1, 1400189 CrossRef.
- G. A. H. Wetzelaer, A. Najafi, R. J. P. Kist, M. Kuik and P. W. M. Blom, Appl. Phys. Lett., 2013, 102, 053301 CrossRef.
- C.-C. Chueh, C.-Z. Li and A. K. Y. Jen, Energy Environ. Sci., 2015, 8, 1160–1189 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02575e |
| This journal is © The Royal Society of Chemistry 2020 |