Open Access Article
Open Access ArticleSelective cytotoxic effect against the MDA-MB-468 breast cancer cell line of the antibacterial palindromic peptide derived from bovine lactoferricin†
Andrea Barragán-Cárdenas a,
Maribel Urrea-Pelayoa,
Víctor Alfonso Niño-Ramírez
a,
Maribel Urrea-Pelayoa,
Víctor Alfonso Niño-Ramírez a,
Adriana Umaña-Pérez
a,
Adriana Umaña-Pérez a,
Jean Paul Vernotb,
Claudia Marcela Parra-Giraldoc,
Ricardo Fierro-Medinaa,
Zuly Rivera-Monroy
a,
Jean Paul Vernotb,
Claudia Marcela Parra-Giraldoc,
Ricardo Fierro-Medinaa,
Zuly Rivera-Monroy a and
Javier García-Castañeda
a and
Javier García-Castañeda *a
*a
aFacultad de Ciencias, Universidad Nacional de Colombia, Carrera 45 No 26-85, Building 451, Office 334, 11321 Bogotá, Colombia. E-mail: jaegarciac@unal.edu.co; Tel: +57-1-316-5000 (ext. 14436)
bFacultad de Medicina, Departamento de Ciencias fisiológicas, Universidad Nacional de Colombia, Carrera 45 No 26-85, 11321 Bogotá, Colombia
cPontificia Universidad Javeriana, Carrera 7 No. 43-82, Bogotá, Colombia
First published on 6th May 2020
Abstract
The cytotoxic effect against the breast cancer cell line MDA-MB-468 of the palindromic peptide LfcinB (21–25)Pal: 1RWQWRWQWR9 and its analogous peptides, obtained via alanine scanning, was evaluated. The results indicate that the palindromic peptide exhibited a concentration-dependent cytotoxic effect against this cell line. The cytotoxic effect of the palindromic peptide was fast and selective and was sustained for up to 48 h of treatment. MDA-MB-468 cells treated with the palindromic peptide exhibited severe cellular damage, acquiring rounded forms and shrinkage, a behavior typical of apoptotic events. The analogous peptides exhibited fewer cytotoxic effects than the original palindromic peptide, suggesting that the substitution of any amino acid with alanine diminishes the cytotoxic effect. The Arg and Trp residues proved to be the most relevant for the cytotoxic effect; the analogous peptides with substitutions of Trp with Ala did not induce a change in cellular morphology, while analogous peptides with substitutions of Arg or Gln with Ala induced cellular damage. Also, neither the palindromic peptide nor its analogues exerted a significant cytotoxic effect on normal fibroblasts, indicating that the peptides had a selective cytotoxic effect on cancerous cells. The peptide LfcinB (21–25)Pal, and its analogues exhibited antibacterial activity against E. coli and S. aureus strains and a selective cytotoxic effect against the breast cancer cell line MDA-MB-468.
Introduction
Breast cancer is one of the leading types of cancer worldwide in terms of incidence and cause of death, with approximately 2.1 million new diagnoses and about 627![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in 2018.1 Various local and systemic treatments have been developed to treat this disease, which include chemotherapy, surgery, hormone therapy, and radiotherapy, among others. These treatments have increased the survival rate and reduced recurrence; however, the incident rate of this cancer increases up to 5% per year in developing countries.2–4 In order to improve treatment, new therapeutic approaches based on antimicrobial peptides (AMPs) have been studied. Bovine lactoferricin (LfcinB): 17FKCRRWQWRMKKLGAPSITCVRRAF41 is an AMP generated from bovine lactoferrin (BLF) hydrolysis caused by the gastric pepsin. LfcinB has exhibited antibacterial, antifungal, and antiviral activity, as well anticancer activity against several types of cancer.5–8 LfcinB exhibited higher antibacterial activity than the native protein, suggesting that LfcinB is the bactericidal domain of BLF.9 The mechanism involved in the anticancer activity of LfcinB is not entirely clear. It is believed that the selectivity for cancer cells over normal cells is in part due to electrostatic interactions between the positively-charged peptide and the negatively-charged membrane components10,11 such as phosphatidylserine, glycosylated mucins, sialylated gangliosides, sialic acid, and heparan sulphate in cancer cells.12
000 deaths in 2018.1 Various local and systemic treatments have been developed to treat this disease, which include chemotherapy, surgery, hormone therapy, and radiotherapy, among others. These treatments have increased the survival rate and reduced recurrence; however, the incident rate of this cancer increases up to 5% per year in developing countries.2–4 In order to improve treatment, new therapeutic approaches based on antimicrobial peptides (AMPs) have been studied. Bovine lactoferricin (LfcinB): 17FKCRRWQWRMKKLGAPSITCVRRAF41 is an AMP generated from bovine lactoferrin (BLF) hydrolysis caused by the gastric pepsin. LfcinB has exhibited antibacterial, antifungal, and antiviral activity, as well anticancer activity against several types of cancer.5–8 LfcinB exhibited higher antibacterial activity than the native protein, suggesting that LfcinB is the bactericidal domain of BLF.9 The mechanism involved in the anticancer activity of LfcinB is not entirely clear. It is believed that the selectivity for cancer cells over normal cells is in part due to electrostatic interactions between the positively-charged peptide and the negatively-charged membrane components10,11 such as phosphatidylserine, glycosylated mucins, sialylated gangliosides, sialic acid, and heparan sulphate in cancer cells.12
The minimal motif with antibacterial and anticancer activity has been identified as LfcinB (20–25): RRWQWR. This sequence is amphipathic and positively charged.13 In previous studies, it has been reported that short synthetic peptides derived from the minimal motif exhibited higher antibacterial and anticancer activity than LfcinB and BLF.14 Furthermore, it has been observed that palindromic peptides derived from LfcinB have exhibited significant antibacterial activity against Gram-positive and Gram-negative bacteria.15 The peptide LfcinB (21–25)Pal (1RWQWRWQWR9) is derived from the minimal motif RRWQWR,16 in which the WQW sequence is flanked by Arg residues in a palindromic arrangement. This peptide has exhibited higher antibacterial activity than LfcinB and its minimal motif on E. coli ATCC 25922, E. faecalis ATCC 29212, S. enteritidis ATCC 13076 and P. aeruginosa ATCC 27853.17,18 Furthermore, this palindromic peptide exhibited a synergistic antibacterial effect with vancomycin19 and a cytotoxic effect against oral squamous cell carcinoma (OSCC) CAL-27 and SCC-17.20
In the present paper, the palindromic peptide LfcinB (21–25)Pal and its analogues (alanine scan) were synthesized via solid-phase peptide synthesis (SPPS) and purified and characterized using RP-HPLC and MALDI-TOF MS. The peptide's antibacterial activity against E. coli ATCC 25922 and S. aureus ATCC 25923 and cytotoxic effect against MDA-MB-468 breast cancer cell line and young fibroblasts primary cell culture were assessed. Our results allowed us to establish that the peptide LfcinB (21–25)Pal exhibits a significant and selective cytotoxic effect against MDA-MB-468 cells. It was possible to identify the fundamental amino acids responsible for its antibacterial activity against Gram-positive and Gram-negative bacteria. Also, for cytotoxic activity against breast cancer cell line MDA-MB-468 it was established that there is not critical amino acid, instead is the alternance of charged and hydrophobic residues which gives the palindromic peptide an effect. These results allow us to demonstrate the effect of the peptide sequence on the biological activity presented and therefore serve as a starting point for the design of new and improved peptides that present enhance activity.
Results and discussion
Peptide synthesis
Peptide LfcinB (21–25)Pal (1RWQWRWQWR9) and its analogues were synthesized through the SPPS-Fmoc/tBu strategy, purified by means of RP-SPE, and characterized using RP-HPLC and MALDI-TOF MS. The peptide synthesis was fast and efficient, and neither amino acid coupling nor Fmoc group removal reactions proved to be difficult. All purified peptides showed one main peak, with chromatographic purity between 85% and 95%. MALDI-TOF MS analysis showed that in all cases the purified peptide corresponded to the ratio m/z of the expected [M + H]+ (Table 1 and ESI, S1†).| Peptide code | Sequence | RP-HPLC | MALDI-TOF MS m/z [M + H]+ | E. coli ATCC 25922 | S. aureus ATCC 25923 | ||
|---|---|---|---|---|---|---|---|
| tR (min) | Purity (%) | Theor. | Exp. | MIC/MBC (μM) | |||
| LfcinB (21-25)Pal | 1R W Q W R W Q W R9 | 6.4 | 91 | 1485.8 | 1486.8 | 34/67 | 135/135 |
| A1 | A W Q W R W Q W R | 6.6 | 85 | 1400.7 | 1400.5 | 71/143 | 71/71 |
| A2 | R A Q W R W Q W R | 5.8 | 93 | 1370.7 | 1371.2 | 146/>146 | 146/>146 |
| A3 | R W A W R W Q W R | 6.4 | 88 | 1428.7 | 1428.8 | 140/140 | 140/ND |
| A4 | R W Q A R W Q W R | 5.6 | 95 | 1370.7 | 1369.6 | 146/>146 | 146/>146 |
| A5 | R W Q W A W Q W R | 6.7 | 92 | 1400.7 | 1401.9 | 71/143 | 143/>143 |
| A6 | R W Q W R A Q W R | 5.6 | 85 | 1370.7 | 1371.7 | 37/73 | 146/<146 |
| A7 | R W Q W R W A W R | 6.5 | 89 | 1428.7 | 1429.5 | 18/35 | 140/ND |
| A8 | R W Q W R W Q A R | 5.7 | 90 | 1370.7 | 1371.3 | 37/73 | 146/>146 |
| A9 | R W Q W R W Q W A | 6.7 | 90 | 1400.7 | 1401.5 | 143/>143 | 143/143 |
In the chromatographic profile of peptide LfcinB (21–25)Pal there is a principal signal at retention time (tR) of 6.4 min with a purity of 91%; when Arg (side chain charged positively) was replaced by Ala (side chain aliphatic), the tR of analogous peptides A1, A5, and A9 was higher than the tR of LfcinB (21–25)Pal, indicating that these peptides are more hydrophobic than the original peptide (Table 1). The analogous peptides A2, A4, A6, and A8 (substitution of Trp with Ala) exhibited a tR lower than the original peptide, suggesting that the Trp side chain is more hydrophobic than the Ala side chain. When Gln was replaced with Ala in peptides A3 and A7, the tR was similar to the tR of the original peptide, indicating that the change does not affect the polarity of the peptide (Table 1).
The differences observed in the tR of analogous peptides reflex differences in their physicochemical properties (net charge, polarity, hydrophobicity, and amphipacity) associated with the substitution of Arg, Trp, or Gln with Ala in the LfcinB (21–25)Pal sequence. In addition, the functional groups of the N-terminal (amine group) and C-terminal (amide group) ends could be responsible for the differences between analogues with the same substitutions at a different position of the sequence.
Peptide antibacterial activity
The antibacterial activity of the palindromic peptide LfcinB (21–25)Pal and its analogues against a Gram-negative strain of E. coli ATCC 25922 and a Gram-positive strain of S. aureus ATCC 25923 was evaluated (Table 1). It was found that the palindromic peptide LfcinB (21–25)Pal exhibited significant antibacterial activity against E. coli strain (MIC = 34 μM); this result is similar to previous studies, which reported similar MIC values for this peptide in the same strain.18,23,30 The palindromic peptide exhibited similar antibacterial activity to those exhibited by the peptide containing the minimal motif against E. coli (JM-109) strain.31Peptides A1, A5, and A9 exhibited lower antibacterial activity against E. coli ATCC 25922 than the palindromic peptide, showing that the positive charge of Arg residue is critical for its antibacterial activity. The net charge of the palindromic peptide is +4, while when Arg was replaced by Ala, the net charge of these analogous peptides A1, A5, and A9 was +3, and their antibacterial activity decreased to the MIC values 71, 143, and 143 μM, respectively.
This behaviour suggests that the decrease in antibacterial activity could be related to the loss of one positive charge, which mainly affects the net charge, polarity, and amphipathicity of the sequence. Furthermore, the substitution of any amino acid of the 2WQW4 motif with Ala (A2, A3, and A4 peptides) causes complete loss of antibacterial activity against the E. coli strain, indicating that these amino acids are fundamental for the antibacterial activity against this Gram-negative strain. On the other hand, it was found that the substitution of any amino acid of the 6WQW8 motif with Ala in the C-terminal region (A6, A7, and A8 peptides) did not significantly affect the antibacterial activity. Moreover, the replacement of the Gln8 by Ala (peptide A8) located in the C-terminal region led to increased activity, with a MIC of 17.5 μM. This shows that changes in the two WQW motifs of the sequence affect the antibacterial activity against the E. coli strain in different ways, any change in the motif located near the N-terminal region being critical.
Otherwise, the analogous peptides (except A1) and the original peptide exhibited similar antibacterial activity against S. aureus ATCC 25923, suggesting that there were no critical residues in the antibacterial activity against this Gram-positive strain. Interestingly, the A1 peptide exhibited higher antibacterial activity against S. aureus ATCC 25923 than the original peptide, suggesting that the replacement of the Arg1 with Ala (A1: ARWQWRWQWR) increased the antibacterial activity against this Gram-positive strain. These results suggest that the action mechanism of the palindromic peptide in Gram-positive strains is different from that for the Gram-negative strain.
Our results are in agreement with previous reports, which proposed that the antibacterial activity of the peptide LfcinB (21–25)Pal is due to the amino acid sequence,17 it has a net charge of +4, and the alternation of Arg and Trp residues confers amphipathic properties.16,24 Furthermore, it has been shown that AMPs with symmetric sequences exhibit higher antibacterial activity than similar AMPs with asymmetric sequences.25
Additionally, a severe loss of antibacterial activity against E. coli 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 922 also was observed when Trp22 or Trp24 residues were changed to Ala in the peptide LfcinB (18–32): 18KCRRWQWRMKKLGAP32.24 In a way similar to our results, all alanine-modified analogues of peptide LfcinB (18–32) exhibited reduced antibacterial activity against S. aureus 25
922 also was observed when Trp22 or Trp24 residues were changed to Ala in the peptide LfcinB (18–32): 18KCRRWQWRMKKLGAP32.24 In a way similar to our results, all alanine-modified analogues of peptide LfcinB (18–32) exhibited reduced antibacterial activity against S. aureus 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923.24 Our results and those of other authors suggest that residues Trp22 and Trp24 are essential for the antibacterial activity against certain strains.12,26 It has been suggested that the Trp side chain is involved in the interaction of the peptide with the lipid membrane, causing membrane disruption that leads to cellular lysis. Also, it has been suggested that Trp is involved in the internalization of the peptide for reaching intracellular targets.31,32 As well it is interesting that amphiphilic peptides containing charged and hydrophobic residues like our peptides not only can be used as a potent antimicrobial, but they are also great molecules which can be used in other delivery materials such as hydrogels46 which maintain its polycationicity, generate membrane disruption and makes it difficult to gain resistance.47
923.24 Our results and those of other authors suggest that residues Trp22 and Trp24 are essential for the antibacterial activity against certain strains.12,26 It has been suggested that the Trp side chain is involved in the interaction of the peptide with the lipid membrane, causing membrane disruption that leads to cellular lysis. Also, it has been suggested that Trp is involved in the internalization of the peptide for reaching intracellular targets.31,32 As well it is interesting that amphiphilic peptides containing charged and hydrophobic residues like our peptides not only can be used as a potent antimicrobial, but they are also great molecules which can be used in other delivery materials such as hydrogels46 which maintain its polycationicity, generate membrane disruption and makes it difficult to gain resistance.47
In similar way to previous results the palindromic peptide and their analogues exhibited higher antibacterial activity against E. coli strain compared with those observed in S. aureus strain.19 In this work was possible to identify the critical amino acids in the antibacterial activity against E. coli and S. aureus strains, interestingly as we study the primary structure of LfcinB (21–25)Pal we were able to find peptides with higher antibacterial activity as A1 against the Gram-positive strain and A7 against the Gram negative strain.
Cytotoxic assays
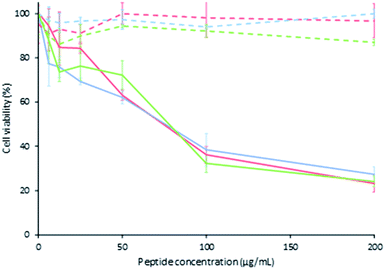
The cytotoxic effect of peptide LfcinB (21–25)Pal and its analogues against the breast cancer cell line MDA-MB-468 and young human fibroblast primary cell culture was evaluated. The cytotoxic effect of the palindromic peptide LfcinB (21–25)Pal against MDA-MB-468 cells was significant (cell viability of 23%) for 2, 24, and 48 h of treatment. The cytotoxic effect of the palindromic peptide was fast and concentration dependent and was sustained for up to 48 h of treatment, IC50 = 75 μg mL−1 (49 μM). When young human fibroblast primary cells were treated with the palindromic peptide LfcinB (21–25)Pal, there was no cytotoxic effect at the peptide concentrations evaluated for the three treatment times, IC50 > 75 μg mL−1 (135 μM). This suggests that the cytotoxic effect of the palindromic peptide was selective for human breast cancer cells in all the evaluated concentrations for all treatment times. The selective cytotoxic effect observed indicate that palindromic peptide doesn't affect the cell viability of the fibroblasts which are a cell type found in breast and which has even been shown to have a close relationship with the cells within the tumour microenvironment of various types of cancer, including breast cancer.48–50The cytotoxic effect of the palindromic peptide against breast and oral cancer cell lines is according with other reports that showed that BLF, LfcinB and other peptides containing the minimal motif exhibited cytotoxic effect in vitro and/or in vivo assays against different cancer types included breast cancer.9,18,20,22,33–40
In similar way to palindromic peptide, synthetic short peptides derived of hLfcin containing Trp and Arg residues reported to be active against E. coli and bacterial lipid model systems, also exhibited selective cytotoxic effect against primary melanoma cells (SBcl-2) and metastatic lesions (WM164), a melanoma cell line (A375), a rhabdomyosarcoma cell line (TE671), a glioblastoma cell line (U-87 mg) and did not show to be toxic for non-cancer cells as melanocytes and normal human dermal fibroblasts. The selective cytotoxic effect of these peptides was associated to PI-uptake indicating loss of cell membrane integrity and cell death.41
This behaviour suggests that the decrease in antibacterial activity could be related to the loss of one positive charge, which mainly affects the net charge, polarity, and amphipathicity of the sequence.
It was found that all the analogous peptides exhibited a lower cytotoxic effect against MDA-MB-468 cells than the palindromic peptide (cellular viability 23%), the cellular viability being between 40% and 71% (Table 2). The cytotoxic effect of palindromic peptide and all the analogous peptides was similar when the cells were treated for 2 h, 24 h, or 48 h, and the cytotoxic effect was concentration-dependent in all cases (Table 2, Fig. 1, see ESI, S2†). These results allow us to infer that all the residues played an important role in the peptide's cytotoxic effect. This agrees with previous reports, which indicated that the RRWQWRMKKLG sequence is critical for the biological activity of LfcinB.27
| Peptide code | Cellular Viability (%) | |||||
|---|---|---|---|---|---|---|
| MDA-MB-468 cells | Fibroblast cells | |||||
| 2 h | 24 h | 48 h | 2 h | 24 h | 48 h | |
| a Statistic significant differences with regard to the effect on MDA-MB-468 cells at same incubation time p < 0.0001.b Statistic significant differences with regard to the effect on MDA-MB-468 cells at same incubation time p < 0.001.c Statistic significant differences with regard to the effect on MDA-MB-468 cells at same incubation time p < 0.05. | ||||||
| LfcinB(21–25)Pal | 23 ± 4 | 27 ± 4 | 24 ± 2 | 97 ± 5a | 100 ± 2a | 92 ± 2a |
| A1 | 53 ± 4 | 48 ± 5 | 51 ± 2 | 99 ± 2a | 100 ± 1a | 92 ± 2a |
| A2 | 62 ± 4 | 63 ± 4 | 58 ± 6 | 100 ± 2a | 94 ± 2a | 87 ± 2a |
| A3 | 45 ± 7 | 40 ± 6 | 43 ± 6 | 100 ± 3a | 91 ± 5a | 86 ± 4a |
| A4 | 59 ± 9 | 52 ± 1 | 51 ± 9 | 100 ± 4a | 79 ± 4a | 66 ± 2c |
| A5 | 71 ± 8 | 62 ± 7 | 64 ± 4 | 99 ± 1a | 81 ± 1b | 82 ± 1a |
| A6 | 55 ± 6 | 49 ± 5 | 52 ± 5 | 100 ± 2a | 88 ± 2a | 79 ± 4a |
| A7 | 59 ± 6 | 57 ± 3 | 56 ± 1 | 96 ± 5a | 100 ± 1a | 79 ± 5a |
| A8 | 69 ± 5 | 62 ± 5 | 61 ± 6 | 100 ± 1a | 94 ± 1a | 85 ± 4a |
| A9 | 64 ± 7 | 55 ± 6 | 65 ± 7 | 100 ± 4a | 98 ± 3a | 100 ± 3a |
The importance of Arg, Trp, and Gln for the cytotoxic effect against breast cancer cells lies in the fact that these amino acids are what give the peptide the amphipathic properties and allow it to establish the electrostatic interaction with the cell membrane. It has also been proposed that Trp residues are the key for inducing peptide internalization.26,28,29 These results are in accordance with the fact that the LfcinB interacts with the cell surface through electrostatic attraction between the positive charges of the peptide and the negative charges on the cell surface, and the substitution of any amino acid of peptide LfcinB (21–25)Pal affects both the amphipaticity and the net charge of the sequence.
The morphologic changes in MDA-MB-468 and fibroblast cells induced by the peptide LfcinB (21–25)Pal and its analogues were evaluated. The MDA-MB-468 cells treated with the peptide LfcinB (21–25)Pal exhibited dramatic morphological changes, rounded forms and shrinkage (Fig. 2), which has been related to apoptotic events. While untreated cells showed the morphological characteristics of typical MDA-MB-468 cells in a stellate shape, the morphological changes in breast cancer cells induced by the palindromic peptide could be associated with the cytotoxic effect observed in MTT assays. In a similar way, when the MDA-MB-468 cells were treated with the peptides A1, A3, A5, and A7 (substitution of Arg or Gln with Ala), morphological changes were observed, acquiring rounded forms. In a previous report, we showed that the tetrameric peptide LfcinB (20–25)4, which contains the minimal motif RRWQWR, exhibited a selective and fast cytotoxic effect against MDA-MB-468 cells, which is associated with the apoptotic process.22
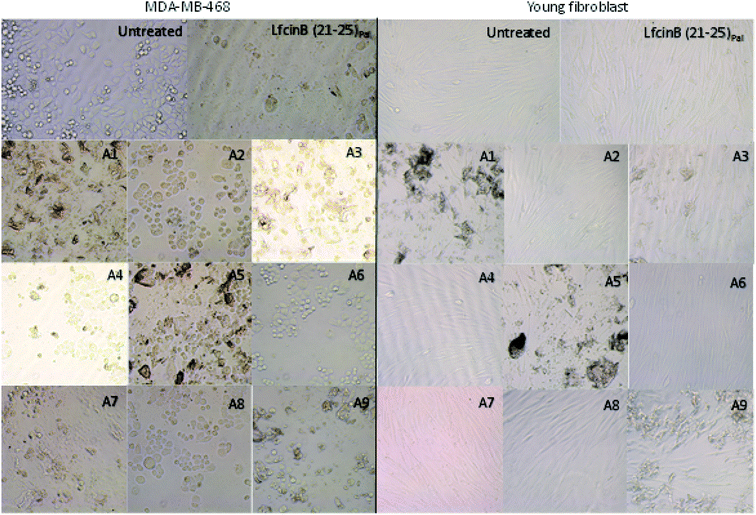 | ||
| Fig. 2 Microphotographs by contrast microscopy of the MDA-MB-468 and young fibroblast cells untreated, treated with LfcinB (21–25)Pal and analogous peptides at 134,61 μM by 24 h. | ||
It has been suggested that BLF, cyclic LfcinB, and linear LfcinB exerted antitumor activities through activating diverse signalling pathways, including p53, apoptosis, and angiopoietin signalling, which could induce cellular morphological changes.35 Furthermore, LfcinB causes severe damage in cytoplasm and mitochondria membranes of neuroblastoma cells.29
When the breast cancer cells were treated with peptides A2, A4, A6, and A8 (substitution of Trp with Ala), significant morphological changes associated with the cytotoxic effect were not observed. On the other hand, young human fibroblasts treated with the peptide LfcinB (21–25)Pal or its analogous peptides did not exhibit morphological changes (Fig. 2). The morphology of treated and untreated young human fibroblasts was similar, which is in accord with the results of the MTT assays, confirming that the cytotoxic effect of the evaluated peptides is selective for breast cancer cells. These results indicate that the cytotoxic effect of the peptides A2, A4, A6, and A8 is lesser than original peptide and is not associated with significantly morphological changes suggesting that the change of one Trp residue affects the activity of the peptide. These results are agreed with reports that showed that Trp residues in LfcinB has been associated with membrane disruption and/or internalization which could induces cellular severe damage.31,32
The selective cytotoxic effect of palindromic peptide and their analogous peptides is related with the cellular damage observed in breast cancer cells MDA-MB-468. Fundamental differences exist between the cell membranes of malignant cells and normal cells that likely account for the ability of certain AMPs to kill cancer cells. The electrostatic interactions between cationic peptides and anionic cell membrane components as phospholipids and O-glycosylated mucin can be the major factor in the selective killing of cancer cells. The selectivity of palindromic peptide by cancer cells has been attributed to the membrane fluidity of cancer cells because is greater than that of untransformed cells, being their amphipathic nature the main responsible of the enhanced lytic activity through membrane destabilization.42
Interesting, that the all peptides exhibited significantly selective cytotoxic effect against breast cell cancer the MDA-MB-468, suggesting that punctual changes in the sequence diminished the cytotoxic effect but it not was suppressed completely. By other hand, it can be observed that the results obtained by analogous peptides in antibacterial assays was unlike of those registered for cytotoxic assays. This behaviour is according with the fact that cytotoxic effect of palindromic peptide could be related with an independent mechanism to the one established in bacterial strains. In order to determine if the antibacterial and anticancerogenic activities are related with changes in secondary elements structural, CD Spectra were recorded for each peptide.
The CD spectrum of peptide LfcinB (21–25)Pal exhibits a pattern with a positive band at 220–230 nm and a negative band at 195–210 nm, which suggests a random coil structure. The same pattern is observed for all analogous peptides, suggesting that the antibacterial activity and the selective cytotoxic effect against breast cancer cell line MDA-MB-468 isn't related to secondary structural elements (ESI, S3†).
The CD results are according with previous reports that showed that CD spectra of motif RRWQWR at 10 μM in 10 mM Tris pH 7.4 is characteristic of random coil pattern.43 The random coil structure observed in CD spectra of palindromic peptide is coherent with the CD spectra of BLF, LfcinB and synthetic peptides LfcinB (17–31), LfcinB (20–25)4 and LfcinB (20–25)Cyc, suggesting that native protein, the LfcinB and other short peptides containing the RRWQWR motif do not have secondary structural elements of Beta sheet and/or Helix conformation.44 Our results agree with previous reports of CD spectra which indicate that the secondary structure of LfcinB is independent from the environment conditions and the helical population is lower than 10%, the remaining being almost equally distributed between the unstructured and B-sheet structure.45
The evaluated cytotoxic effect against breast cancer cell line MDA-MB-468 was selective, fast, concentration dependent, and stable up to 48 h, and could be associated with the apoptotic pathway. Our results confirm that the antibacterial and anticancer activity of a palindromic peptide mainly depends on its amino acid and symmetry sequence, net charge, and amphipaticity. The peptide LfcinB (21–25)Pal could be considered to be a promising candidate for developing new therapeutic agents against breast cancer.
Material and methods
Solid-phase peptide synthesis
The peptides were synthesized using manual SPPS-Fmoc/tBu in accordance with Vargas et al.19 Briefly, 100 mg of Rink amide resin (0.46 meq g−1) was treated with DCM for 2 h at room temperature (RT). (i) Fmoc group removal was carried out through treatment with 2.5% 4-methylpiperidine in DMF for 10 min at RT with constant stirring (2×). (ii) For the coupling reaction, Fmoc-amino acids (0.21 mmol) were pre-activated with DCC/6-Cl–HOBt (0.20/0.21 mmol) in DMF for 15 min at RT with stirring. Afterward, the pre-activated amino acid was added to the resin or resin-peptide and the reaction mixture was stirred for 2 h at RT. The Fmoc group removal and the coupling reactions were monitored with the Kaiser test. (iii) Side-chain deprotection reactions and peptide separation from the resin were carried out by means of treatment of resin-peptide with a solution containing TFA/water/TIPS/EDT (93/2/2.5/2.5 v/v/v) for 8 h with stirring at RT. (iv) Crude peptides were precipitated by treatment with cool ethyl ether and were washed five times with cool ethyl ether, and then the solid was dried at RT.Reverse-phase HPLC RP-HPLC
The peptides were characterized by means of RP-HPLC chromatography in accordance with Insuasty et al.21 Briefly, the analysis was performed on a Merck Chromolith® C18 (50 mm × 4.6 mm) column using an Agilent 1200 liquid chromatograph (Omaha, NE, USA) with UV-Vis detector (210 nm). 10 μL of peptide solution (1 mg mL−1) was injected, and a linear gradient was applied from 5% to 50% solvent B (0.05% TFA in ACN) in solvent A (0.05% TFA in water) for 8 min at a flow rate of 2.0 mL min−1 at RT.Peptide purification
The peptides were purified using solid-phase extraction columns following the methodology reported by Insuasty et al.21 Briefly, SPE columns (SUPELCO LC-18 with 2.0 g resin) were activated with 30 mL of solvent B and equilibrated with 30 mL of solvent A according to the manufacturer's recommendations. Crude peptides were passed through the column, and a gradient of solvent B (5–50%) was used for their elution. Collected fractions were analyzed using RP-HPLC (as described above), and fractions that contained the pure peptide were collected and lyophilized.MALDI-TOF MS
The peptide (1 mg mL−1) was mixed with 2,5-dihydroxybenzoic acid or sinapinic acid (1 mg mL−1) at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 (v/v), and 1 μL was seeded on the steel target. The experiment was performed on an Microflex TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) in reflectron mode, using an polished steel target (Bruker Daltonics, Bremen, Germany) laser: 500 shots and 25–30% power.
18 (v/v), and 1 μL was seeded on the steel target. The experiment was performed on an Microflex TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) in reflectron mode, using an polished steel target (Bruker Daltonics, Bremen, Germany) laser: 500 shots and 25–30% power.
Circular dichroism
The CD spectrum of the peptides was recorded following the methodology described by Huertas et al.17 Briefly, the peptide (0.2 mM) was dissolved in 2,2,2-trifluoroethanol (30%) aqueous solution and then analyzed in a spectropolarimeter Jasco J-810, between 190 and 260 nm at 25 °C in a quartz cuvette with a 1 cm path length. The result is the average of three scans taken at 20 nm min−1 with a spectral bandwidth of 1 nm.Antibacterial activity assays
The antibacterial activity of peptides against E. coli ATCC 25922 and S. aureus ATCC 25923 strains was evaluated in accordance with Vargas et al.18 Briefly, the minimum inhibitory concentration (MIC) was determined by a microdilution assay (CLSI) in a 96-well microplate. 90 μL of peptide (200, 100, 50, 25, 12.5 and 6.2 μg mL−1) and 10 μL of inoculum (5 × 106 CFU mL−1) were added and incubated for 24 h at 37 °C, and afterward the absorbance was measured at 620 nm. The minimum bactericidal concentration (MBC) was determined by taking samples from each well where there was no visible bacterial growth, which were spread on Mueller Hinton agar boxes (AMH) and incubated for 24 h. The MBC was chosen as the lowest concentration where no CFU was evidenced. Each analysis was performed in duplicate (n = 2).MTT assays
Cytotoxicity assays were performed as previously described in Rodriguez et al.22 Briefly, breast cancer cell line MDA-MB-468 (ATCC® HTB-132™) cells or young fibroblast primary cells (100 μL; 1 × 104 cells per well) were seeded in 96-well flat-bottom tissue culture treated plates and were incubated at 37 °C in a 5% CO2 humidified atmosphere for 14 h, allowing for cell adhesion. Then the media was removed and 50 μL of supplemented RPMI medium (10%) and 50 μL of peptide (200, 100, 50, 25, 12.5 and 6.25 μg mL−1) were added, and the final FBS concentration was 5%. The plates were incubated for 2, 24, and 48 h at 37 °C in a 5% CO2 humidified atmosphere (physiologic pH), and cell viability was determined using the MTT assay. For this, 10 μL of MTT solution (5 mg mL−1) was added to each well, and the plates were incubated for 4 h at 37 °C. Formazan crystals were dissolved in DMSO (100 μL), and the absorbance (570 nm) was measured using a Bio-Rad 680 microplate reader (n = 3).Microscopy assays
Prior to the addition of MTT to the cytotoxicity assays, photographic recording was performed via phase-contrast microscopy of the cells treated with the peptides and the untreated ones using a Leica DMi microscope at 20×.Conclusions
In the present work, 9 peptides derived from an Alanine scan of the LfcinB (21–25)Pal peptide were designed and synthesized in order to determine the critical amino acids related to their secondary structure and biological activity against both bacteria and cancer cells. It was evidenced that all the peptides presented a random coil type secondary structure so that the changes in their primary structure do not affect the secondary structure elements. Regarding antibacterial activity, for S. aureus 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 it was shown that changes in the sequence generated total loss of activity, determining that all amino acids are relevant to exert their bacteriostatic and bactericidal effect. The data obtained for E. coli ATCC 25922 showed that the change in the amino acids relating to the 2WQW4 motif generate total loss of their antibacterial activity, being these so-called critics. These results allow us to suggest that the mechanism of action exerted may differ on Gram-positive and Gram-negative bacteria.
923 it was shown that changes in the sequence generated total loss of activity, determining that all amino acids are relevant to exert their bacteriostatic and bactericidal effect. The data obtained for E. coli ATCC 25922 showed that the change in the amino acids relating to the 2WQW4 motif generate total loss of their antibacterial activity, being these so-called critics. These results allow us to suggest that the mechanism of action exerted may differ on Gram-positive and Gram-negative bacteria.
About the cytotoxicity exerted against the cell line derived from breast cancer MDA-MB-468, it was shown that the palindromic peptide has a concentration-dependent effect, fast and maintained for up to 48 h after a single administered dose. In addition, it was observed that under none of the concentrations and times evaluated was the cell viability of the primary culture of fibroblasts affected, which could indicate that the effect of the palindrome is selective for cancer cells. When evaluating the effect of the analogues, it could be established that the change in any of the amino acids generated a decrease in the cytotoxic effect compared to that exerted by the palindrome, which indicates that this cytotoxicity is not given by critical amino acids but is alternation of charged and hydrophobic residues given by the palindromic arrangement which generates this effect.
These results allow us to demonstrate that the antibacterial and cytotoxic effect is influenced to a greater extent by changes in the primary structure than in the secondary structure of the LfcinB (21–25)Pal peptide, in addition to evidencing that depending on the biological model the effect of the sequence is differential. Additionally, in an interesting way peptides A7 (change in Gln residue at the C-terminal) and A1 (change in Arg residue at the N-terminal) derived from the palindrome shown an improved activity for E. coli and S. aureus, respectively. In this way, our results not only generate a greater understanding of the LfcinB (21–25)Pal peptide, but also represent a starting point for the design of novel and improve peptides that can enhance its activity. The palindromic peptide could be considered as promissory candidate to develop new therapeutically options based in synthetic peptides.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was conducted with the financial support of COLCIENCIAS 807-2018, Project: “Desarrollo de un medicamento contra el cáncer de mama basado en un péptido polivalente derivado de la LfcinB: Estudio de la fase preclínica (fase cero), caracterización fisicoquímica de un lote del fármaco para estudios preclínicos. Code 110180762973”, contract RC No. 706-2018.Notes and references
- World Health Organization, Latest global cancer data: Cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018, IARC Communications, 2018 Search PubMed.
- C. Shapiro and A. Recht, Side Effects of Adjuvant Treatment of Breast Cancer, N. Engl. J. Med., 2001, 26, 1997–2008 CrossRef PubMed.
- S. Coughlin and D. Ekwueme, Breast cancer as a global health concern, Cancer Epidemiol., 2009, 33, 315–318 CrossRef PubMed.
- R. Rodney and S. John, Breast Cancer: A Review of the Literature, J. Insur. Med., 2003, 35, 85–101 Search PubMed.
- H. Jenssen, Anti-herpes simplex virus activity of lactoferrin/lactoferricin: an example of antiviral activity of antimicrobial protein/peptide, Cell. Mol. Life Sci., 2005, 24, 3002–3013 CrossRef PubMed.
- W. Bellamy, K. Yamauchi, H. Wakabayashi, M. Takase, N. Takakura, S. Shimamura and M. Tomita, Antifungal properties of lactoferricin B, a peptide derived from the N-terminal region of bovine lactoferrin, Lett. Appl. Microbiol., 1994, 18, 230–233 CrossRef CAS.
- L. H. Vorland, H. Ulvatne, J. Andersen, H. H. Haukland, O. Rekdal, J. S. Svendsen and T. J. Gutteberg, Antibacterial effects of lactoferricin B, Scand. J. Infect. Dis., 1999, 31, 179–184 CrossRef CAS PubMed.
- C. M. Yin, J. H. Wong, J. Xia and T. B. Ng, Studies on anticancer activities of lactoferrin and lactoferricin, Curr. Protein Pept. Sci., 2013, 14, 492–503 CrossRef CAS PubMed.
- W.-R. Pan, P.-W. Chen, Y.-L. S. Chen, H.-C. Hsu, C.-C. Lin and W.-J. Chen, Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage, J. Dairy Sci., 2013, 96, 7511–7520 CrossRef CAS PubMed.
- S. R. Dennison, M. Whittaker, F. Harris and D. A. Phoenix, Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes, Curr. Protein Pept. Sci., 2006, 7, 487–499 CrossRef CAS PubMed.
- M. R. Felício, O. N. Silva, S. Gonçalves, N. C. Santos and O. L. Franco, Peptides with Dual Antimicrobial and Anticancer Activities, Frontiers in Chemistry, 2017, 5, 1–9 CrossRef PubMed.
- S. Riedl, D. Zweytick and K. Lohner, Membrane-active host defense peptides – Challenges and perspectives for the development of novel anticancer drugs, Chem. Phys. Lipids, 2011, 8, 766–781 CrossRef PubMed.
- A. Richardson, R. de Antueno, R. Duncan and D. W. Hoskin, Intracellular delivery of bovine lactoferricin's antimicrobial core (RRWQWR) kills T-leukemia cells, Biochem. Biophys. Res. Commun., 2009, 388, 736–741 CrossRef CAS PubMed.
- B. Wayne, T. Mitsunori, Y. Koji, W. Hiroyuki, K. Kouzou and T. Mamoru, Identification of the bactericidal domain of lactoferrin, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 1992, 1121, 130–136 CrossRef.
- S. Changbao, L. Yingying, C. Songsong, W. Haimei, J. Chenggang, P. Shiyue, H. Muhammad Altaf and H. Juncai, Antibacterial Activity and Mechanism of Action of Bovine Lactoferricin Derivatives with Symmetrical Amino Acid Sequences, Int. J. Mol. Sci., 2018, 19, 1–20 Search PubMed.
- L. Migliolo, M. R. Felício, M. H. Cardoso, O. N. Silva, M.-A. E. Xavier, D. O. Nolasco, A. S. de Oliveira, I. Roca-Subira, J. V. Estape, L. D. Teixeira, S. M. Freitas, A. J. Otero-Gonzalez, S. Gonçalves, N. C. Santos and O. L. Franco, Structural and functional evaluation of the palindromic alanine-rich antimicrobial peptide Pa-MAP2, BBA, Biochim. Biophys. Acta, Gen. Subj., 2016, 1858, 1488–1498 CrossRef CAS PubMed.
- N. D. J. Huertas Méndez, Y. Vargas Casanova, A. K. Gómez Chimbi, E. Hernández, A. L. Leal Castro, J. M. Melo Diaz, Z. J. Rivera Monroy and J. E. García Castañeda, Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076, Molecules, 2017, 22, 1–10 CrossRef PubMed.
- Y. Vargas Casanova, J. A. Rodríguez Guerra, Y. A. Umaña Pérez, A. L. Leal Castro, G. Almanzar Reina, J. E. García Castañeda and Z. J. Rivera Monroy, Antibacterial Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Cytotoxic Effect against MDA-MB-468 and MDA-MB-231 Breast Cancer Cell Lines, Molecules, 2017, 22, 1–11 CrossRef PubMed.
- Y. Vargas Casanova, A. V. Rodriguez Mayor, K. J. Cárdenas, A. L Leal Castro, L. C. Molina, R. Fierro Medina, Z. J. Rivera Monroy and J. E. García Castañeda, Synergistic bactericide and antibiotic effects of dimeric, tetrameric, or palindromic peptides containing the RWQWR motif against Grampositive and Gram-negative strains, RSC Adv., 2019, 10, 7239–7245 RSC.
- V. A. Solarte, J. E. Rosas, Z. J. Rivera, M. L. Arango-Rodríguez, J. E. García and J.-P. Vernot, A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines, BioMed Res. Int., 2015, 12, 1–13 CrossRef PubMed.
- D. S. Insuasty, Z. J. Rivera, J. E. Garcia, H. M. Pineda, M. Maldonado, R. Fierro and A. V. Rodriguez, Synthetic Peptide Purification via Solid-Phase Extraction with Gradient Elution: A Simple, Economical, Fast, and Efficient Methodology, Molecules, 2019, 24, 1–9 Search PubMed.
- J. Rodríguez Guerra, A. Barragán Cárdenas, A. Ochoa-Zarzosa, J. López Meza, A. Umaña Pérez, R. Fierro-Medina, Z. J. Rivera Monroy and J. E. García Castañeda, The tetrameric peptide LfcinB (20–25)4 derived from bovine lactoferricin induces apoptosis in the MCF-7 breast cancer cell line, RSC Adv., 2019, 9, 20497–20504 RSC.
- J. T. Román, C. A. Fuenmayor, C. M. Zuluaga, D. Clavijo-Grimaldo, M. Acosta, J. E. García, R. Fierro and Z. J. Rivera, Pullulan nanofibers containing the antimicrobial palindromic peptide LfcinB (21–25)Pal obtained via electrospinning, RCS adv., 2019, 9, 20432–20438 Search PubMed.
- M. B. Strøm, J. S. Svendsen and O. Rekdal, Antibacterial activity of 15-residue lactoferricin derivatives, Int. J. Pept. Res. Ther., 2000, 5, 265–274 CrossRef PubMed.
- C. Sun, Y. Li, S. Cao, H. Wang and C. Jiang, Antibacterial Activity and Mechanism of Action of Bovine Lactoferricin Derivatives with Symmetrical Amino Acid Sequences, Int. J. Mol. Sci., 2018, 19, 1–20 CAS.
- L. H. Vorland, H. Ulvatne, O. Rekdal and J. S. Svendsen, Initial binding sites of antimicrobial peptides in Staphylococcus aureus and Escherichia coli, Scand. J. Infect. Dis., 1999, 31, 467–473 CrossRef CAS PubMed.
- J. H. Kang, M. K. Lee, K. L. Kim and K. S. Hahm, Structure-biological activity relationships of 11-residue highly basic peptide segment of bovine lactoferrin, Int. J. Pept. Protein Res., 1996, 48, 357–363 CrossRef CAS PubMed.
- S. Farnaud and R. Evans, Lactoferrin - a multifunctional protein with antimicrobial properties, Mol. Immunol., 2003, 40, 395–405 CrossRef CAS PubMed.
- L. T. Eliassen, G. Berge, A. Leknessund, M. Wikman, I. Lindin, C. Løkke, F. Ponthan, J. I. Johnsen, B. Sveinbjørnsson, P. Kogner, T. Flægstad and Ø. Rekdal, The antimicrobial peptide, Lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and exhibits xenographt growth in vivo, Int. J. Cancer, 2006, 119, 493–500 CrossRef CAS PubMed.
- N. d. J. Huertas, Z. J. Rivera Monroy, R. Fierro Medina and J. E. García Castañeda, Antimicrobial Activity of Truncated and Polyvalent Peptides Derived from the FKCRRQWQWRMKKGLA Sequence against Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923, Molecules, 2017, 22, 1–10 CrossRef PubMed.
- M. Moniruzzaman, Z. Islam, S. Sharmin, H. Dohra and M. Yamazaki, Entry of 6-Residue Antimicrobial Peptide Derived from Lactoferricin B into Single Vesicles and E. coli Cells without Damaging their Membranes, Biochemistry, 2017, 33, 4419–4431 CrossRef PubMed.
- D. I. Chan, E. J. Prenner and H. J. Vogel, Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action, Biochim. Biophys. Acta, 2006, 9, 1184–1202 CrossRef PubMed.
- I. Z. Sadiq, K. Babagana, D. Danlami, L. I. Abdullahi and A. R. Khan, Molecular Therapeutic Cancer Peptides: A Closer Look at Bovine Lactoferricin, Asian Journal of Biochemistry, Genetics and Molecular Biology, 2018, 2, 1–9 CrossRef.
- J. P. Guedes, C. S. Pereira, L. R. Rodrigues and M. Côrte-Real, Bovine Milk lactoferrin selectively Kills highly Metastatic Prostate cancer Pc-3 and Osteosarcoma Mg-63 cells In Vitro, Frontiers in Oncology, 2018, 8, 1–12 CrossRef PubMed.
- R. Jiang and B. Lönnerdal, Bovine lactoferrin and lactoferricin exert antitumor activities on human colorectal cancer cells (HT-29) by activating various signaling pathways, Biochem. Cell Biol., 2016, 95, 99–109 CrossRef PubMed.
- C. Freiburghaus, B. Janicke, H. Lindmark-Månsson, S. M. Oredsson and M. A. Paulsson, Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line, J. Dairy Sci., 2009, 92, 2477–2484 CrossRef CAS PubMed.
- F. Amiri, F. Moradian and A. Rafiei, Anticancer effect of lactoferrin on Gastric Cancer Cell Line AGS, Res. Mol. Med., 2015, 3, 11–16 CAS.
- A. Cutone, L. Rosa, G. Ianiro, M. S. Lepanto, M. C. Bonaccorsi di Patti, P. Valenti and G. Musci, Lactoferrin's Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action, Biomolecules, 2020, 10, 26 CrossRef PubMed.
- R. K. Kanwar and J. R. Kanwar, Immunomodulatory Lactoferrin in the Regulation of Apoptosis Modulatory Proteins in Cancer, Protein Pept. Lett., 2013, 20, 450–458 CAS.
- H. Tsuda, K. Fukamachi, J. Xu, K. Sekine, S. i. Ohkubo, N. Takasuka and M. Iigo, Prevention of carcinogenesis and cancer metastasis by bovine lactoferrin, Proc. Jpn. Acad., Ser. A, 2006, 82, 208–216 CAS.
- S. Riedl, R. Leber, B. Rinner, H. Schaider, K. Lohner and D. Zweytick, Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine, BBA, Biochim. Biophys. Acta, Gen. Subj., 2017, 1848, 2918–2931 CrossRef.
- D. W. Hoskin and A. Ramamoorthy, Studies on Anticancer Activities of Antimicrobial Peptides, BBA, Biochim. Biophys. Acta, Gen. Subj., 2008, 1778, 357–375 CrossRef CAS PubMed.
- D. J. Schibli, P. M. Hwang and H. J. Vogel, The structure of the antimicrobial active center of lactoferricin B bound to sodium dodecyl sulfate micelles, FEBS Lett., 1999, 446, 213–217 CrossRef CAS.
- W. D. Schwarcz, L. Carnelocce, J. L. Silva, A. C. Oliveira and R. B. Goncalves, Conformational changes in bovine lactoferrin induced by slow or fast temperature increases, Biol. Chem., 2008, 389, 1137–1142 CAS.
- I. Daidone, A. Magliano, A. Di Nola, G. Mignogna, M. M. Clarkson, A. R. Lizzi, A. Oratore and F. Mazza, Conformational study of bovine lactoferricin in membrane-micking conditions by molecular dynamics simulation and circular dichroism, BioMetals, 2011, 24, 259–268 CrossRef CAS.
- N. Nandi, K. Gayen, S. Ghosh, D. Bhunia, S. Kirkham, S. K. Sen, S. Ghosh, I. W. Hamley and A. Banerjee, Amphiphilic Peptide-Based Supramolecular, Noncytotoxic, Stimuli-Responsive Hydrogels with Antibacterial Activity, Biomacromolecules, 2017, 18, 3621–3629 CrossRef CAS PubMed.
- A. S. Veiga and J. P. Schneider, Antimicrobial hydrogels for the treatment of infection, Biopolymers, 2014, 100, 637–644 CrossRef PubMed.
- B. Wang, C. Xi, M. Lui, H. Sun, S. Liu, L. Song and H. Kang, Breast fibroblasts in both cancer and normal tissues induce phenotypic transformation of breast cancer stem cells: a preliminary study, PeerJ, 2018, 6, e4805 CrossRef PubMed.
- T. Marsh, K. Pietras and S. S. McAllister, Fibroblasts as architects of cancer pathogenesis, BBA, Mol. Basis Dis., 2011, 1832, 1070–1078 CrossRef.
- F. Zhang, C. Song, Y. Ma, Y. Xu and H. Wang, Effect of fibroblasts on breast cancer cell mammosphere formation and regulation of stem cell-related gene expression, Int. J. Mol. Med., 2011, 28, 365–371 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra02688c |
| This journal is © The Royal Society of Chemistry 2020 |