Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Graphene oxide/polydimethylsiloxane composite sponge for removing Pb(II) from water
Liao Liua,
Jiannan Chen *b,
Wuhuan Zhangc,
Meikun Fan*a,
Zhengjun Gong
*b,
Wuhuan Zhangc,
Meikun Fan*a,
Zhengjun Gong a and
Jianqiang Zhanga
a and
Jianqiang Zhanga
aSchool of Geosciences and Environmental Engineering, Southwest Jiaotong University, Chengdu 611756, Sichuan Province, China. E-mail: mkfan@swjtu.edu.cn; Tel: +86 18628194419
bDepartment of Civil, Environmental, and Construction Engineering, University of Central Florida, Orlando, FL 32816, USA. E-mail: jc6mu@virginia.edu; Tel: +1 6089605108
cDepartment of Engineering Systems and Environment, University of Virginia, Charlottesville, VA 22904, USA
First published on 11th June 2020
Abstract
An efficient adsorbent to remove Pb(II) from water was prepared by treating polydimethylsiloxane (PDMS) sponge with polyvinyl alcohol and then coating the sponge with graphene oxide (GO). The GO–PDMS sponge was highly hydrophilic, easily handled during and after use, and easily recycled. The kinetics and isotherms of Pb(II) sorption onto the GO–PDMS sponge were investigated by performing batch sorption tests. The kinetics of Pb(II) sorption onto the GO–PDMS sponge indicated that sorption equilibrium occurred rapidly (within 60 min) and that the sorption data could be described using a pseudo-second-order model. Maximum Pb(II) sorption onto the GO–PDMS sponge occurred at pH > 5. Increasing GO loading on the PDMS sponge increased the amount of Pb(II) that could be sorbed. The isotherm for Pb(II) sorption onto the GO–PDMS sponge was non-linear and was well described by the Langmuir isotherm model, indicating that Pb(II) sorption onto the GO–PDMS sponge was homogeneous and occurred through sorption of a monolayer of Pb(II). The GO–PDMS sponge, used as a filter, removed Pb(II) efficiently from water. The Pb(II) removal efficiencies were more than 50% and the maximum was 85%.
1. Introduction
Heavy metal pollution of water negatively affects human health and the environment.1–3 Lead (Pb) is one of the most common heavy metals found in wastewater. It is harmful to the human nervous system, blood circulation, and kidneys.4–6 Studies of techniques to remove Pb(II) from wastewater and drinking water have been performed. The most common techniques to remove Pb(II) from water are coagulation, ion exchange, membrane separation, precipitation, and sorption.6–12 Among these techniques, sorption is the most effective because it is efficient, selective, and cost-effective.7–9Graphene-based materials have been used widely as sorbents in water treatment plants, particularly to remove heavy metals.10,13,14 Graphene oxide (GO: C14OH42O20) is a derivative of graphene. GO has many oxygen-containing functional groups such as hydroxyl (–OH), carboxyl (–COOH), and epoxy groups. These groups make GO surfaces hydrophilic and negatively charged, meaning GO is an excellent sorbent of positively charged heavy metal ions.3,15–18 However, GO generally disperses in water thus it is difficult to collect and recycle.19 Three-dimensional (3D) porous sorbents (e.g., sponge-like 3D polydimethylsiloxane (PDMS; (C2H6OSi)n) sorbents) that have stable morphologies have been developed and used to treat contaminated water.20–23 Sponge-like 3D PDMS sorbents can be easily retrieved and recycled after being used to treat water. In addition, they are highly porous, have high surface areas, and are non-toxic.24,25 However, owing to its hydrophobic nature, PDMS can only be used to remove hydrophobic contaminants such as dyes, oil, and organic compounds.26–33
A novel approach to preparing a hydrophilic GO–PDMS sponge for removing Pb(II) ions from aqueous solutions is described in the present study. The GO–PDMS sponge was prepared by dip-coating a PDMS sponge in polyvinyl alcohol (PVA) to modify the surface, followed by loading of GO nanoparticles onto the coated PDMS sponge. The Pb(II) sorption kinetics and efficiency of the GO–PDMS sponge were evaluated by performing batch tests and the sorption isotherm and sorption mechanism were investigated.
2. Materials and methods
2.1 Sorbent materials
To modify the surface of the fabricated PDMS sponges, the latter were treated with PVA (molecular weight 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, >99% hydrolyzed, Mowiol 28-99; Polysciences, Warrington, PA, USA). The modified PDMS sponge surfaces were hydrophilic, whereas the unmodified PDMS sponge surfaces were hydrophobic. Specifically, the as-prepared PDMS sponge was cleaned with air plasma produced using a YES G1000P system (Alttek Company USA, Huntington Beach, CA, USA) for 15 min to remove hydrophobic functional groups. The PDMS sponge was then immersed in 1% (by weight) PVA solution for 20 min and then dried at 65 °C. This wetting and drying cycle was performed five times.
000, >99% hydrolyzed, Mowiol 28-99; Polysciences, Warrington, PA, USA). The modified PDMS sponge surfaces were hydrophilic, whereas the unmodified PDMS sponge surfaces were hydrophobic. Specifically, the as-prepared PDMS sponge was cleaned with air plasma produced using a YES G1000P system (Alttek Company USA, Huntington Beach, CA, USA) for 15 min to remove hydrophobic functional groups. The PDMS sponge was then immersed in 1% (by weight) PVA solution for 20 min and then dried at 65 °C. This wetting and drying cycle was performed five times.
2.2 Batch sorption tests
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) in a polypropylene centrifuge tube. The centrifuge tube was kept at 30 °C and shaken at 150 rpm for a specified contact time. Batch sorption tests were performed using contact times between 5 and 120 min, at Pb(II) concentrations of 10 and 20 mg L−1, and pH 5.0 ± 0.1. Following determination of the contact time required to reach adsorption equilibrium, the effect of pH on the sorption equilibrium was assessed by performing tests using a Pb(II) concentration of 50 mg L−1 and pH values between 2.5 and 6.0. The pH was adjusted by adding 0.1 M NaOH or 0.1 M HCl. The GO–PDMS sponges used in these tests were prepared using a 5 mg mL−1 GO solution, but the effect of GO loading on Pb(II) sorption was investigated using GO–PDMS sponges prepared using GO concentrations between 1 and 5 mg mL−1.
100) in a polypropylene centrifuge tube. The centrifuge tube was kept at 30 °C and shaken at 150 rpm for a specified contact time. Batch sorption tests were performed using contact times between 5 and 120 min, at Pb(II) concentrations of 10 and 20 mg L−1, and pH 5.0 ± 0.1. Following determination of the contact time required to reach adsorption equilibrium, the effect of pH on the sorption equilibrium was assessed by performing tests using a Pb(II) concentration of 50 mg L−1 and pH values between 2.5 and 6.0. The pH was adjusted by adding 0.1 M NaOH or 0.1 M HCl. The GO–PDMS sponges used in these tests were prepared using a 5 mg mL−1 GO solution, but the effect of GO loading on Pb(II) sorption was investigated using GO–PDMS sponges prepared using GO concentrations between 1 and 5 mg mL−1.Data to allow Pb(II) sorption isotherms for the GO–PDMS sponges prepared using 5 mg mL−1 GO solution to be drawn were acquired by performing tests at pH 5.0 ± 0.1 using Pb(II) concentrations of 5–80 mg L−1 and determining the Pb(II) concentrations at equilibrium. Each batch sorption test result is the mean of triplicate tests, and the error for each test was less than 5%.
2.3 Solid and water chemistry analysis
Elemental analyses of the GO–PDMS sponges before and after the Pb(II) sorption tests were performed using an ESCALAB 250Xi X-ray photoelectron spectroscopy (XPS) instrument (Thermo Fisher Scientific, Waltham, MA, USA). The solid and liquid in a given mixture after a batch or filtration experiment were separated by centrifuging the mixture at 4000 rpm for 20 min, then the supernatant was transferred to a 15 mL polypropylene centrifuge tube for chemical analysis. The initial and final Pb(II) concentrations were determined using a Hitachi Z-5000 atomic absorption spectrometer (Hitachi High-Technologies, Tokyo, Japan). Control tests were performed in the absence of GO–PDMS to assess the potential sorption of Pb(II) onto the centrifuge tubes or other equipment during the test process. The results confirmed that negligible Pb(II) sorption occurred onto the propylene centrifuge tubes and other equipment.3. Results and discussion
3.1 Morphology and surface characteristics of the GO–PDMS sponge
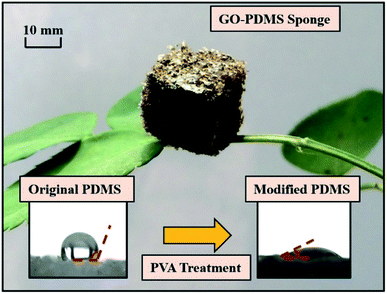
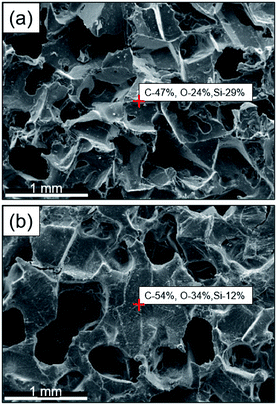
The GO–PDMS sponge was a light material with a 3D porous structure and it was easily supported by a leaf (Fig. 1). The 3D network structure was obtained using the sugar templating method. Scanning electron microscopy images of PPDMS and GO–PDMS sponges indicated that both types of sponge had porous micro-structures. The PPDMS sponge had smooth surfaces and distinct pores (Fig. 2a) but loading of GO particles onto the PPDMS sponge caused the sponge surfaces to become rough and the pores filled with GO (Fig. 2b). Additionally, the loading of GO altered the chemical composition of the sponge, resulting in a higher C content (54%) and a lower Si content (12%) in the GO–PDMS when compared with those in PDMS (47% and 29%, respectively). The PPDMS substrate could easily take on different shapes while maintaining excellent morphological integrity, meaning the GO–PDMS sponge could easily be recycled. The hydrophobicity of the original PDMS was changed by the PVA modification, which introduced hydrophilic oxygen-containing functional groups to the surfaces (Fig. 1). The original PDMS sponge (before PVA modification) had a water contact angle of 114.7° and a hydrophobic surface. PVA modification allowed water droplets to spread rapidly and penetrate the PPDMS sponge. The PPDMS sponge was hydrophilic and the water contact angle was 26.4°. The hydrophilicity and porous structure of the GO–PDMS sponge gave the material a high surface area (19.2 m2 g−1) that could sorb dissolved Pb(II).The Fourier-transform infrared spectra of PPDMS and GO–PDMS sponges indicated the presence of abundant hydroxyl groups (O–H band at 3440 cm−1), thereby indicating that PVA modification caused the PDMS sponge surface to become hydrophilic (Fig. 3). Specifically, the Fourier-transform infrared spectrum of PPDMS displayed three major stretching vibration bands at 3440, 1095, and 802 cm−1, which can be attributed to the presence of O–H, Si–O, and Si–(CH3)2 functional groups, respectively.37 Introducing GO resulted in the appearance of stretching vibration bands at 1732, 1623, and 1040 cm−1, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–O–C groups.18,38–40 These functional groups are present in GO.36 The adsorption of adsorbates onto GO–PDMS is expected to proceed via chelation reactions, involving the oxygen-containing functional groups, e.g., C
C, and C–O–C groups.18,38–40 These functional groups are present in GO.36 The adsorption of adsorbates onto GO–PDMS is expected to proceed via chelation reactions, involving the oxygen-containing functional groups, e.g., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O–C.41,42
O and C–O–C.41,42
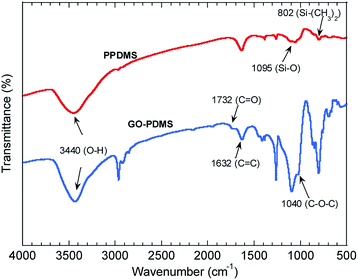 | ||
| Fig. 3 Fourier-transform infrared spectra of the polydimethylsiloxane sponge modified with polyvinyl alcohol (PPDMS) and PPDMS modified with graphene oxide (GO–PDMS). | ||
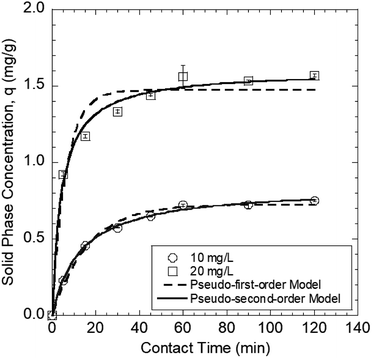
3.2 Kinetics of Pb(II) sorption onto the GO–PDMS sponge
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (1) |
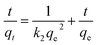 | (2) |
| Original Pb(II) concentration (mg L−1) | qe,expa (mg g−1) | Pseudo-first-order | Pseudo-second order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe,calb (mg g−1) | k1 (min−1) | tec (min) | R2 | qe,cal (mg g−1) | k2 (g mg−1 min−1) | te (min) | R2 | ||
| a qe,exp, solid-phase concentration at equilibrium determined experimentally.b qe,cal, calculated solid-phase concentration at equilibrium.c te = time to reach equilibrium. | |||||||||
| 10 | 0.75 | 0.72 | 0.062 | 24.6 | 0.99 | 0.84 | 0.092 | 81.2 | 1.0 |
| 20 | 1.57 | 1.47 | 0.16 | 63.1 | 0.96 | 1.60 | 0.15 | 71.5 | 0.95 |
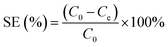 | (3) |
| qe (mg g−1) = (C0 − Ce)V/m | (4) |
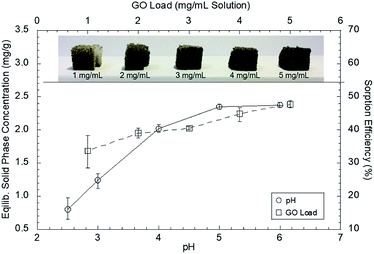
The Pb(II) concentration in the solid phase and the sorption efficiency increased as the solution pH increased from 2 to 6 (Fig. 5). The PZC of the GO–PDMS sponge was determined to be pH 3.3 from zeta potential measurements. At low pH values (<3.3), H+ is abundantly present on the GO–PDMS surface, resulting in a positively charged surface. This surface results in coulombic repulsion of heavy metal cations, thereby limiting the sorption capacity of GO–PDMS. When the pH is increased, the repulsion weakens, thus increasing the sorption capacity of the GO–PDMS. The maximum Pb(II) concentration in the solid phase (2.4 mg g−1) and the maximum sorption efficiency (47%) were observed at pH values above 5. Precipitation of Pb(II) occurred at pH 6, probably because the solution became saturated with cerussite (PbCO3) and hydrocerussite (Pb3(CO3)2(OH)2).46,47 As Pb(II) precipitation interfered with the sorption test results, experiments were not performed at pH values above 6. The subsequent batch experiments were thus performed at pH 5.0 ± 0.1 to avoid the onset of Pb(II) precipitation.
3.3 Isotherms for Pb(II) sorption onto the GO–PDMS sponge
The Pb(II) sorption isotherms for the GO–PDMS sponges were non-linear. Langmuir and Freundlich sorption isotherm models were fitted to the data, and the results are shown in Fig. 6. These non-linear models were used to evaluate the sorption process and identify the mechanism through which Pb(II) sorbed onto the GO–PDMS sponge. The Langmuir isotherm assumes that the surface of the sorbent is uniform, monolayer sorption of the sorbate occurs, there are a finite number of sorption sites, and sorption occurs with the same sorption energy at all sites. The Freundlich isotherm assumes that large numbers and various types of sorption sites act simultaneously. The Freundlich isotherm is suitable for nonideal sorption onto heterogeneous surfaces with various free energies of sorption. The Langmuir sorption isotherm model and Freundlich sorption isotherm model can be expressed as shown in eqn (5) and (6), respectively.
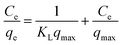 | (5) |
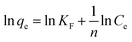 | (6) |
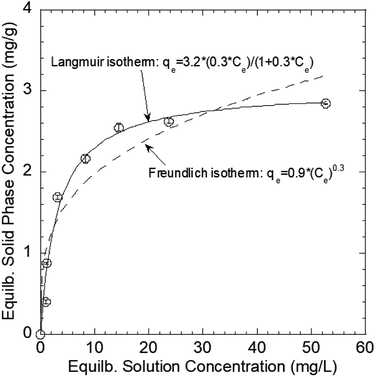 | ||
| Fig. 6 Langmuir and Freundlich sorption isotherm models for the sorption of Pb(II) onto the graphene oxide polydimethylsiloxane sponge. Error bars represent standard deviations from triplicate tests. | ||
| Langmuir fitting parameters | Freundlich fitting parameters | ||
|---|---|---|---|
| qmax (mg g−1) | 3.2 | KF [(mg g−1) (L mg−1)1/n] | 0.9 |
| KL (L mg−1) | 0.3 | 1/n | 0.3 |
| R2 | 0.98 | R2 | 0.90 |
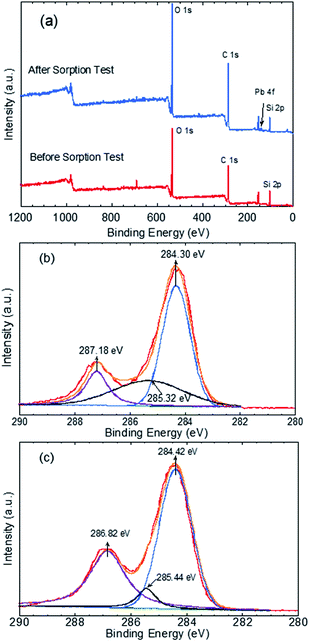
The mechanism through which Pb(II) sorbed onto the GO–PDMS sponge was evaluated by XPS to identify the elements and functional groups on the GO–PDMS sponge surface before and after sorption. Specifically, a GO–PDMS sponge that had been exposed to 80 mg L−1 Pb(II) solution and a GO–PDMS sponge that had not been exposed to Pb(II) solution were analyzed. The wide-scan spectra and the characteristic peaks before and after Pb(II) sorption are shown in Fig. 7—O 1s, C 1s, and Si 2p peaks were observed for both sponges, and these peaks reflected the elemental components of PDMS and GO. In addition, a Pb 4f peak was detected in the sponge that was exposed to Pb(II) solution, indicating the sorption of Pb(II) onto the GO–PDMS sponge. The deconvoluted C 1s peaks for both sponges are shown in Fig. 7b and c. Before sorption, the XPS pattern of GO–PDMS displayed three peaks at binding energies of 284.30, 285.32, and 287.18 eV that were assigned to C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.21,23 After the sorption tests, the binding energy of C
O bonds, respectively.21,23 After the sorption tests, the binding energy of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond shifted to 286.82 eV, suggesting that oxygen-containing groups in GO (e.g., alcohol and carboxylate groups) were actively involved in the sorption reactions. Slight shifts in the binding energies of C–C and C–O bonds (to 284.42 and 285.44 eV, respectively) were also detected, indicating that the C
O bond shifted to 286.82 eV, suggesting that oxygen-containing groups in GO (e.g., alcohol and carboxylate groups) were actively involved in the sorption reactions. Slight shifts in the binding energies of C–C and C–O bonds (to 284.42 and 285.44 eV, respectively) were also detected, indicating that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group plays a prominent role in the sorption of Pb(II) onto the GO–PDMS sponge. This observation further proves the better fitting of the sorption isotherms using the Langmuir isotherm model when compared with the Freundlich isotherm model, as the former model is based on the assumption of a surface containing a finite number of uniform sorption sites (i.e., carboxylate groups in the present study).
O functional group plays a prominent role in the sorption of Pb(II) onto the GO–PDMS sponge. This observation further proves the better fitting of the sorption isotherms using the Langmuir isotherm model when compared with the Freundlich isotherm model, as the former model is based on the assumption of a surface containing a finite number of uniform sorption sites (i.e., carboxylate groups in the present study).
3.4 Filtration experiments
The possibility of using GO–PDMS sponges to filter water to remove Pb(II) was evaluated. The simple handling and recycling of GO–PDMS sponges would be convenient when filtering water. The Pb(II) removal efficiencies achieved using GO–PDMS sponges to treat solutions containing Pb(II) at concentrations between 1 and 20 mg L−1 are shown in Fig. 8. When a GO–PDMS sponge was used to treat a 1 mg L−1 Pb(II) solution, 85% of the Pb(II) was removed after 10 filtering cycles. The removal efficiency decreased as the Pb(II) concentration in solution increased, but a rather high removal efficiency of >50% was still attained when the Pb(II) concentration in solution was 20 mg L−1. These results indicated that the GO–PDMS sponge is an ideal material for removing Pb(II) from water.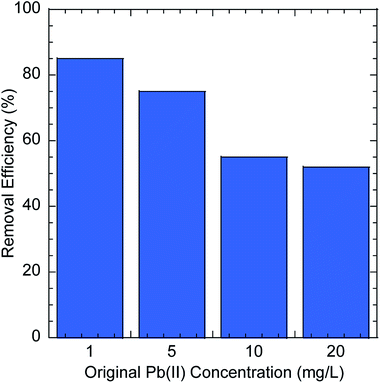 | ||
| Fig. 8 Pb(II) removal efficiencies obtained when graphene oxide polydimethylsiloxane sponges were used to filter solutions containing Pb(II) at different concentrations. | ||
4. Conclusions
An efficient sorbent to sorb Pb(II) from water was synthesized by treating a PVA-modified PDMS sponge with GO. The aim was to develop a cost-effective method for removing Pb(II) from water using GO in a material that is easy to handle and recycle. The PDMS sponge was hydrophobic, but PVA modification made the sponge surface hydrophilic. The kinetics and isotherms of Pb(II) sorption onto the GO–PDMS sponge were evaluated by performing batch sorption tests. The conclusions below were drawn from the results.■ The kinetics of Pb(II) sorption onto the GO–PDMS sponge indicated that equilibrium was reached within 60 min. The data were described well using a pseudo-second-order model. Maximum Pb(II) sorption onto the GO–PDMS sponge was found at pH > 5. Increasing the GO loading on the PDMS sponge increased the Pb(II) sorption capacity of the GO–PDMS sponge.
■ The Pb(II) sorption isotherm for the GO–PDMS sponge was non-linear and fitted well using the Langmuir isotherm model. Pb(II) sorbed onto the GO–PDMS sponge as a homogeneous monolayer.
■ The GO–PDMS sponge can be used to filter water to remove Pb(II) and is easily handled and disposed of. A Pb(II) removal efficiency of up to 85% was achieved when the GO–PDMS sponge was used to filter a dilute Pb(II) solution (1 mg L−1) and a Pb(II) removal efficiency of 50% was achieved when the GO–PDMS sponge was used to filter a more concentrated solution (20 mg L−1).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research is supported by the National Natural Science Foundation of China (Grant No. 21677117, 21777131, and 41701347).Notes and references
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- M. Yusuf, F. M. Elfghi, S. A. Zaidi, E. C. Abdullah and M. A. Khan, RSC Adv., 2015, 5, 50392–50420 RSC.
- C. J. Madadrang, H. Y. Kim, G. Gao, N. Wang, J. Zhu, H. Feng, M. Gorring, M. L. Kasner and S. Hou, ACS Appl. Mater. Interfaces, 2012, 4, 1186–1193 CrossRef CAS PubMed.
- L. F. Chen, H. W. Liang, Y. Lu, C. H. Cui and S. H. Yu, Langmuir, 2011, 27, 8998–9004 CrossRef CAS PubMed.
- M. Machida, B. Fotoohi, Y. Amamo and L. Mercier, Appl. Surf. Sci., 2012, 258, 7389–7394 CrossRef CAS.
- A. A. Edathil, I. Shittu, J. Hisham Zain, F. Banat and M. A. Haija, J. Environ. Chem. Eng., 2018, 6, 2390–2400 CrossRef CAS.
- V. K. Gupta, P. J. M. Carrott, M. M. L. Ribeiro Carrott and P. J. M. Suhas Carrott, Crit. Rev. Environ. Sci. Technol., 2009, 39, 783–842 CrossRef.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- Y. Shen, Q. Fang and B. Chen, Environ. Sci. Technol., 2014, 49, 67–84 CrossRef PubMed.
- Z. Niu, L. Liu, L. Zhang and X. Chen, Small, 2014, 10, 3434–3441 CrossRef CAS PubMed.
- M. Zbair, Z. Anfar, H. Ahsaine and H. Khallok, RSC Adv., 2019, 9(10), 5756–5769 RSC.
- R. El Haouti, Z. Anfar, A. Chennah, E. Amaterz, M. Zbair, N. El Alem, A. BenlhachemI and M. Ezahri, Groundwater for Sustainable Development, 2019, vol. 8, pp. 1–9 Search PubMed.
- X. Gong, G. Liu, Y. Li, D. Y. W. Yu and W. Y. Teoh, Chem. Mater., 2016, 28, 8082–8118 CrossRef CAS.
- G. K. Ramesha, A. Vijaya Kumara, H. B. Muralidhara and S. Sampath, J. Colloid Interface Sci., 2011, 361, 270–277 CrossRef CAS PubMed.
- W. Peng, H. Li, Y. Liu and S. Song, J. Mol. Liq., 2017, 230, 496–504 CrossRef CAS.
- I. Duru, D. Ege and A. R. Kamali, J. Mater. Sci., 2016, 51, 6097–6116 CrossRef CAS.
- R. Sitko, E. Turek, B. Zawisza, E. Malicka, E. Talik, J. Heimann, A. Gagor, B. Feist and R. Wrzalik, Dalton Trans., 2013, 42, 5682–5689 RSC.
- L. Liu, S. Liu, Q. Zhang, C. Li, C. Bao, X. Liu and P. Xiao, J. Chem. Eng. Data, 2013, 58, 209–216 CrossRef CAS.
- G. Zhao, X. Ren, X. Gao, X. Tan, J. Li, C. Chen, Y. Huang and X. Wang, Dalton Trans., 2011, 40, 10945–10952 RSC.
- S. Park, S.-O. Kang, E. Jung, S. Park and H. S. Park, RSC Adv., 2014, 4, 899–902 RSC.
- G. Zhou, C. Liu, Y. Tang, S. Luo, Z. Zeng, Y. Liu, R. Xu and L. Chu, Chem. Eng. J., 2015, 280, 275–282 CrossRef CAS.
- G. Zhou, J. Luo, C. Liu, L. Chu, J. Ma, Y. Tang, Z. Zeng and S. Luo, Water Res., 2016, 89, 151–160 CrossRef CAS PubMed.
- Y. Chen, X. Song, T. Zhao, Y. Xiao, Y. Wang and X. Chen, J. Hazard. Mater., 2018, 343, 298–303 CrossRef CAS PubMed.
- A. A. Chavan, H. Li, A. Scarpellini, S. Marras, L. Manna, A. Athanassiou and D. Fragouli, ACS Appl. Mater. Interfaces, 2015, 7, 14778–14784 CrossRef CAS PubMed.
- A. A. Chavan, J. Pinto, I. Liakos, I. S. Bayer, S. Lauciello, A. Athanassiou and D. Fragouli, ACS Sustainable Chem. Eng., 2016, 4, 5495–5502 CrossRef CAS.
- S. J. Choi, T. H. Kwon, H. Im, D. I. Moon, D. J. Baek, M. L. Seol, J. P. Duarte and Y. K. Choi, ACS Appl. Mater. Interfaces, 2011, 3, 4552–4556 CrossRef CAS PubMed.
- X. Zhao, L. Li, B. Li, J. Zhang and A. Wang, J. Mater. Chem. A, 2014, 2, 18281–18287 RSC.
- Z. Lei, Y. Deng and C. Wang, RSC Adv., 2016, 6, 106928–106934 RSC.
- D. Zhu, S. Handschuh-Wang and X. Zhou, J. Mater. Chem. A, 2017, 5, 16467–16497 RSC.
- M. Zbair, Z. Anfar, H. Ahsaine and H. Khallok, RSC Adv., 2019, 9(2), 1084–1094 RSC.
- Z. Anfar, M. Zbair, H. Ahsiane, A. Jada and N. El Alem, RSC Adv., 2020, 10(19), 11371–11380 RSC.
- H. Bi, X. Xie, K. Yin, Y. Zhou, S. Wan, L. He, F. Xu, F. Banhart, L. Sun and R. S. Ruoff, Adv. Funct. Mater., 2012, 22, 4421–4425 CrossRef CAS.
- H. Sun, Z. Zhu, W. Liang, B. Yang, X. Qin, X. Zhao, C. Pei, P. La and A. Li, RSC Adv., 2014, 4, 30587 RSC.
- T. Zhou, J. Yang, D. Zhu, J. Zheng, S. Handschuh-Wang, X. Zhou, J. Zhang, Y. Liu, Z. Liu, C. He and X. Zhou, Adv. Sci., 2017, 4, 1700028 CrossRef PubMed.
- J. William, S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- S. Liang, Y. Li, J. Yang, J. Zhang, C. He, Y. Liu and X. Zhou, Adv. Mater. Technol., 2016, 1, 1600117 CrossRef.
- J. Guo, J. Wang, S. Zhang, X. Ma, Z. Qiu, X. Peng, J. Ying, Y. Wang and G. Wu, New J. Chem., 2017, 41, 90–96 RSC.
- W.-W. Lei, H. Li, L.-Y. Shi, Y.-F. Diao, Y.-L. Zhang, R. Ran and W. Ni, Appl. Surf. Sci., 2017, 404, 230–237 CrossRef CAS.
- S. Liu, F. Yao, O. Oderinde, Z. Zhang and G. Fu, Carbohydr. Polym., 2017, 174, 392–399 CrossRef CAS PubMed.
- G. Liu, S. Gui, H. Zhou, F. Zeng, Y. Zhou and H. Ye, Dalton Trans., 2014, 43(19), 6977–6980 RSC.
- E. Aliyev, V. Filiz, M.-M. Khan, Y.-J. Lee, C. Abetz and V. Abetz, Nanomaterials, 2019, 9(8), 1180 CrossRef CAS.
- X. Ren, J. Li, X. Tan and X. Wang, Dalton Trans., 2013, 42(15), 5266–5274 RSC.
- J. Chen, S. Bradshaw, C. Benson, J. Tinjum, and T. Edil, GeoCongress 2012: State of the Art and Practice in Geotechnical Engineering, 2012, pp. 3729–3738 Search PubMed.
- M. Kapnisti, F. Noli, P. Misaelides, G. Vourlias, D. Karfaridis and A. Hatzidimitriou, Chem. Eng. J., 2018, 342, 184–195 CrossRef CAS.
- N. Fiol and I. Villaescusa, Environ. Chem. Lett., 2008, 7, 79–84 CrossRef.
- A. Ponizovskii and E. Mironenko, Eurasian Soil Sci., 2001, 34(4), 371–381 Search PubMed.
- J. Pierrand, J. Rimbault and M. Aplincourt, Water Res., 2002, 36, 879–890 CrossRef.
- N. Zhang, H. Qiu, Y. Si, W. Wang and J. Gao, Carbon, 2011, 49, 827–837 CrossRef CAS.
- Z. Anfar, M. Zbair, H. Ahsaine, Y. Abdellaoui, A. El Fakir, E. Amaterz, A. Jada and N. El Alem, ChemistrySelect, 2019, 4(17), 4981–4994 CrossRef CAS.
- Z. Anfar, A. Amedlous, A. El Fakir, M. Zbair, H. Ahsaine, A. Jada and N. El Alem, Chemosphere, 2019, 236, 124351 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2020 |