Open Access Article
Open Access ArticleFlotation separation of specularite from chlorite using propyl gallate as a collector
Xiangpeng Gao a,
Fugang Zhaob,
Mingyang Li
a,
Fugang Zhaob,
Mingyang Li *ac and
Yiming Hu*a
*ac and
Yiming Hu*a
aKey Laboratory of Metallurgical Emission Reduction & Resources Recycling, Anhui University of Technology, Ministry of Education, Ma'anshan 243002, China. E-mail: my.l@ahut.edu.cn; yiminghu@ahut.edu.cn
bSinosteel Maanshan Institute of Mining Research Co., Ltd., Ma'anshan 243071, China
cFaculty of Land and Resource Engineering, Kunming University of Science and Technology, Kunming 650093, China
First published on 13th May 2020
Abstract
Separation of specularite from iron-containing silicate iron ore is challenging due to the similar surface properties of minerals and gangues. In this work, propyl gallate (PG) was applied as a chelating collector to separate specularite from chlorite. The flotation results indicated that collector sodium oleate (NaOL) shows little selectivity for the separation of specularite and chlorite. In contrast, the separation of specularite can be achieved with no depressant required when PG was used as the collector. The optimal separation results were obtained for single mineral flotation with recoveries of 87.11% and 6.98% for specularite and chlorite, respectively, and for mixed mineral flotation with 65.13% TFe grade and 76.28% TFe recovery, when the slurry pH was 8 and PG concentration was 40 mg L−1. FT-IR and XPS analyses indicated that PG could be favorably adsorbed on specularite via phenolic hydroxyl groups, and molecular dynamic simulation results further elucidated the adsorption mechanism. This research suggested that the chelating flotation collector could be effective in the separation of minerals from iron-containing silicate iron ores.
1. Introduction
Iron ores, such as hematite, magnetite, specularite, etc., are the basic materials of the iron and steel industries.1,2 Due to the fast development of the economy and urbanization, the consumption of iron and steel requires more efficient separation methods to improve the grade of the ore.3 Froth flotation has been proven to be one of the most effective processing methods for the separation of refractory iron ores.4,5One of the refractory iron ores is iron-containing silicate type iron ore. It is difficult to separate in mineral processing due to the existence of the iron element in iron-containing silicates and iron ores, which leads to similar magnetic and floatability properties of targeted minerals and gangues.6,7 It has been proven that the chlorite depressed by starch could not be floated effectively in reverse flotation route using anionic collector sodium oleate, and the addition of sodium alginate could improve the separation efficiency.8 Besides, the sodium oleate also is invalid for the separation of hematite and aegirite; for example, the recovery of aegirite maintained around 80% while hematite around 90% with sodium oleate 1 × 10−4 mol L−1 at pH = 4–9.9 Meanwhile, as for specularite and aegirite separation, these two minerals showed similar floatability when cationic collector dodecylamine was used.7,10 Moreover, traditional depressants, including starch and carboxymethyl cellulose, were ineffective of selective depressing iron-containing silicates for the flotation separation of iron ores from amphibole or chlorite.1,6,10,11 Hence, effective flotation reagents are urgently needed to solve the vexing beneficiation problems.
Chelating collectors can form metal chelates with minerals, which is more stable with metal than ionic and covalent bonds, has shown great potential in selective flotation.11,12 As shown in Fig. 1, propyl gallate (PG), as known as propyl 3,4,5-trihydroxybenzoate, is an ester synthesized by condensation of gallic acid and propanol, which shows excellent solubility in a slurry. It appears as a fine white crystalline powder and widely used as an antioxidant to foods to prevent oxidation.13 PG represents both hydrophobic and hydrophilic properties and has been applied as a chelating collector in the selective flotation of many minerals. PG can be used to collect scheelite and fluorite from calcite through oxygen atoms on the phenolic hydroxyl groups of PG binding to Ca atoms on the surface of the two minerals.14,15 Benzene ring in a collector molecule usually contributes to the frontier orbital. It is advantageous for donating electrons to the polar group, which enhances its reactivity and selectivity in flotation.12 For example, PG was an effective collector separating diaspore from kaolinite, in which PG chelated with Al atoms on the diaspore surfaces by forming five-member rings through its ortho oxygens.16
 | ||
| Fig. 1 Molecule structure of propyl gallate (PG) (the colour representation is as follows: white-hydrogen atoms; grey-carbon atoms; red-oxygen atoms). | ||
Beside chelating with Ca and Al, PG shows excellent chelating property with Fe.17 Due to that Fe concentration in specularite is much more than that of chlorite, which should make PG show more affinity to specularite and a potential collector for specularite/chlorite flotation. The use of PG to separate specularite from chlorite has not been reported. In this work, PG was applied as the chelating collector for specularite and chloride separation via forth flotation. The results indicated that PG showed excellent collecting property and selectivity for specularite through both hydrogen bonding interaction and chemical adsorption, while the collecting property for chlorite was quite faint. PG has excellent potentialities for iron-containing silicate type iron ore flotation.
2. Experimental
2.1 Materials
The pure chlorite and specularite samples were recieved from Yuanjiacun Mine (Shanxi Province, China) and Lilou Mine (Anhui Province, China), respectively. Both minerals were crushed before dry grinding by a porcelain ball mill. Then the grounded minerals were dry sieved into two size fractions samples (−74 + 37 μm and −37 μm).For flotation and XRD analyses, the coarse size fraction sample (−74 + 37 μm) was used. The fine size fraction mineral sample (−37 μm) was thereafter grounded to −2 μm for FT-IR, zeta potential, and XPS analyses.
Analytical-grade propyl gallate (PG), sodium oleate (NaOL), starch, potassium chloride, sodium hydroxide, and hydrochloric acid were purchased from Adamas-beta® (Shanghai Titan Scientific Co.) and used as received.
2.2 Methods
3. Results and discussion
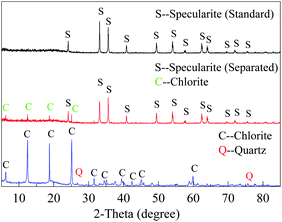
3.1 XRD and elemental analysis
Fig. 2 illustrated the XRD patterns of specularite and chlorite samples. XRD peaks of specularite mainly correspond to the (012) and (024) Bragg's reflections of hexagonal structure of iron oxide while chlorite peaks are assigned to the monoclinic structure of (Mg, Fe)6(Si, Al)4O10(OH)8.22 The X-ray fluorescence (XRF) results were shown in Table 1. The results indicated that both minerals have high purity, only with tiny amounts of quartz in the chlorite sample.| Ore sample | Content of each component/% | ||||||
|---|---|---|---|---|---|---|---|
| Fe2O3 | SiO2 | Al2O3 | MgO | TiO2 | CaO | Na2O | |
| Specularite (standard) | 95.74 | 3.36 | 0.38 | 0.23 | — | — | — |
| Specularite (separated) | 93.04 | 2.76 | 1.79 | 1.35 | 0.06 | 0.07 | 0.02 |
| Chlorite | 30.23 | 27.18 | 18.24 | 13.88 | 0.61 | 0.77 | 0.05 |
3.2 Microflotation tests
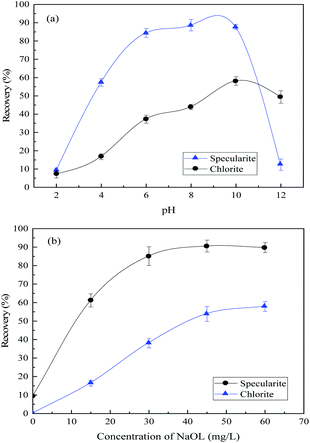 | ||
| Fig. 3 Recovery of specularite and chlorite by NaOL as the collector (a) as a function of pH values with NaOL concentration of 45 mg L−1; (b) as a function of NaOL dosage at pH 8. | ||
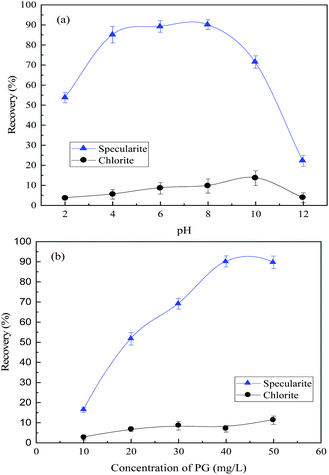
The separation of specularite and chlorite by collector NaOL is not very effective. Hence, an efficient collector for specularite and chlorite separation in the neutral condition is urgently needed. Fig. 4(a) shows the floatability of two minerals as a function of pH values with 30 mg L−1 of PG. The recovery of chlorite remains lower than 15% under all pH conditions, while the floatability of specularite maintained high position, and the optimum recovery of specularite (90.17%) can be observed in faintly acid and neutral conditions (pH 4–8). By careful consideration, the slurry pH value was better determined as 8. Moreover, the effect of PG concentration was examined at pH 8 and results in Fig. 4(b) revealed that the floatability of specularite increased sharply with increasing of PG concentration from 10 mg L−1 to 40 mg L−1, and specularite can be quantitatively recovered at 90.17% with PG dosage of 40 mg L−1. While the recovery of chlorite remained low (less than 10%) under experimental conditions, which consisted with the results in Fig. 4(a).
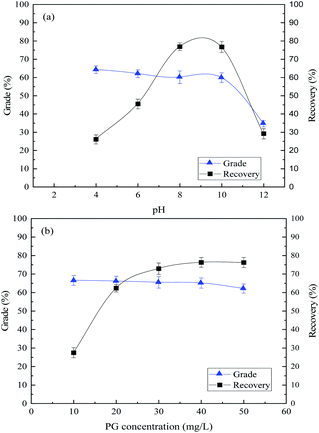 | ||
| Fig. 5 Flotation of mixed minerals. (a) Effect of slurry pH with 40 mg L−1 PG; (b) effect of PG concentration at pH 8. | ||
Fig. 5(b) demonstrated that the TFe grade of concentrate decreased from 65.52% to 62.17% slightly, and the TFe recovery increased from 27.44% to 76.20% as the PG concentration increased. In summary, the optimum flotation can be achieved with a 65.13% TFe grade and 76.28% TFe recovery in concentrate, when the corresponding slurry pH was 8 and PG concentration of 40 mg L−1. The XRF analysis results of the concentrate are shown in Table 1, in which minor of chlorite mixed in the concentrate. The flotation results demonstrated that PG could separate specularite from chlorite effectively as a collector. The optimum flotation separation results with NaOL, CY-23 and PG as the collector, respectively, are shown in Table 2. Table 2 reveals that, compared with NaOL and CY-23, PG shows the best separation properties with higher TFe grade and recovery and lower consumption, which indicates PG is an excellent collector for specularite/chlorite separation.
3.3 Zeta potential measurements
Zeta potential of specularite and chlorite were recorded with and without PG adsorption (40 mg L−1) to investigate the interactions between PG and the minerals. As indicated in Fig. 6, the results suggested that zeta potential values of these two minerals are highly depended on the pH values in which the surface charge of two minerals decreases with increasing pH value. Fig. 6(a) depicted that the iso-electric point (IEP) of specularite was 5.06, which was coincident with previous studies.7,23 After PG was adsorbed on specularite, the zeta potential value shifted towards the negative direction significantly, and its extent enlarged in the pH range of 4–8. The IEP of specularite after PG adsorption shifted left to 2.54, which revealed that PG could adsorb onto the specularite surface strongly.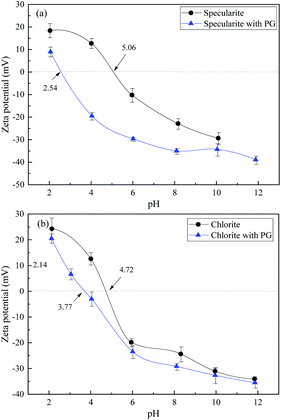 | ||
| Fig. 6 Zeta potentials of specularite (a) and chlorite (b) as a function of pH in the absence and presence of 30 mg L−1 of PG. | ||
As shown in Fig. 6(b), the IEP of chlorite was 4.72, which was consistent with previous studies.24 The zeta potential of chlorite after PG adsorption coincided with raw chlorite, illustrating that only a feeble reaction occurred between chlorite surfaces and PG molecule. In comparison, the involvement of PG brought the zeta potential of specularite more negative than chlorite, supporting that more amount of PG was adsorbed onto specularite than chlorite.
The specularite surface was negatively charged when the pH value is higher than 5.06, which was due to adsorption of hydroxyl on the mineral surface generated from comminution. When the pH value increased to around 8, ions were the main existing form of PG due to the protonation of hydroxyl groups.15,17 In this case, electrostatic forces should not exist between specularite surfaces and PG. Thus the high recovery of specularite was mainly due to the chemical or hydrogen bond adsorption between PG and specularite.
3.4 FT-IR spectra analysis
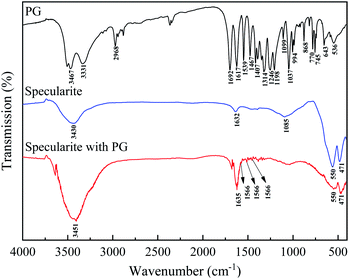
FT-IR spectra of PG, specularite, and specularite after PG adsorption were recorded in Fig. 7.Characteristic peaks for PG include: O–H stretching vibration at 3467 and 3331 cm−1;13,16,25 C–H stretching vibrations of benzene ring at 2968 cm−1;14 1692 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of ester; several consistent peaks at 1617, 1539 and 1467 cm−1 due to C
O stretching of ester; several consistent peaks at 1617, 1539 and 1467 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching in phenyl group and phenolic C–H bending vibration, respectively;26,27 1314 cm−1 attributed to phenol O–H bending; 1246, 1198, and 1099 cm−1 caused by C–O–C stretching from aromatic ester;28 994, 868, 770, and 745 cm−1 attributed to C
C stretching in phenyl group and phenolic C–H bending vibration, respectively;26,27 1314 cm−1 attributed to phenol O–H bending; 1246, 1198, and 1099 cm−1 caused by C–O–C stretching from aromatic ester;28 994, 868, 770, and 745 cm−1 attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending and angular deflections of the C–H fragment, respectively.26 For specularite, peaks at 3430 and 1632 cm−1 are attributed to absorbed water. Peaks at 550 and 471 cm−1 are due to the formation of hematite.29 After PG adsorption, the –OH stretching vibration bands (3520–3330 cm−1) of PG becomes unobvious in specularite after PG adsorption, demonstrating that the –OH group deprotonated and formed the O–Fe bonds. The C
C bending and angular deflections of the C–H fragment, respectively.26 For specularite, peaks at 3430 and 1632 cm−1 are attributed to absorbed water. Peaks at 550 and 471 cm−1 are due to the formation of hematite.29 After PG adsorption, the –OH stretching vibration bands (3520–3330 cm−1) of PG becomes unobvious in specularite after PG adsorption, demonstrating that the –OH group deprotonated and formed the O–Fe bonds. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration peak (1670 cm−1) and C–O–C stretching peaks (1250–1000 cm−1) indicated the interaction of between the ester group and Fe atoms in specularite did not occur. The C
O stretching vibration peak (1670 cm−1) and C–O–C stretching peaks (1250–1000 cm−1) indicated the interaction of between the ester group and Fe atoms in specularite did not occur. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching peaks in phenyl group shifted from 1617, 1539 and 1467 cm−1 to 1566, 1503 and 1444 cm−1, which was mainly caused by transfers of the conjugative π electrons through after the chelation between deprotonated hydroxyl of PG with Fe atoms.17
C stretching peaks in phenyl group shifted from 1617, 1539 and 1467 cm−1 to 1566, 1503 and 1444 cm−1, which was mainly caused by transfers of the conjugative π electrons through after the chelation between deprotonated hydroxyl of PG with Fe atoms.17
3.5 XPS analysis
In order to affirm the adsorption mechanism, XPS analyses were conducted for specularite before and after PG adsorption to evaluate the electron migration in the adsorption process. High-resolution spectra of C 1s, O 1s, and Fe 2p with their deconvoluted fitting curves were illustrated in Fig. 8. C–C peak at 284.8 eV was used to calibrate the binding energy scale for all measurements. Two other peaks in C 1s (Fig. 8(a)) correspond to carbon contamination, and their intensity increased after PG adsorption in Fig. 8(b), which refers to the ester and carboxyl groups on PG molecules.30 O 1s exists in two forms on specularite sample, 530.4 and 532.3 eV for metal oxide and silicate, respectively.31–33 After PG adsorption, two peaks both shifted to lower binding energies, indicating that oxygen atoms act as electron acceptors during the adsorption process. Two new peaks at 530.5 and 531.2 eV can be observed in Fig. 8(d), which are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O, respectively. Fe 2p region contain split spin–orbit components of Fe 2p1/2 and Fe 2p3/2 with a 13.3 eV difference of binding energy.34–36 Deconvoluted peaks in Fig. 8(e) correspond to FeO, Fe2O3, and Fe3+ satellite, which all shifted to lower binding energies due to the acceptance of electrons from PG. XPS results concluded that oxygen and iron atoms were the electron acceptors in the adsorption of PG, which was consisted with the FT-IR results.
O and C–O, respectively. Fe 2p region contain split spin–orbit components of Fe 2p1/2 and Fe 2p3/2 with a 13.3 eV difference of binding energy.34–36 Deconvoluted peaks in Fig. 8(e) correspond to FeO, Fe2O3, and Fe3+ satellite, which all shifted to lower binding energies due to the acceptance of electrons from PG. XPS results concluded that oxygen and iron atoms were the electron acceptors in the adsorption of PG, which was consisted with the FT-IR results.
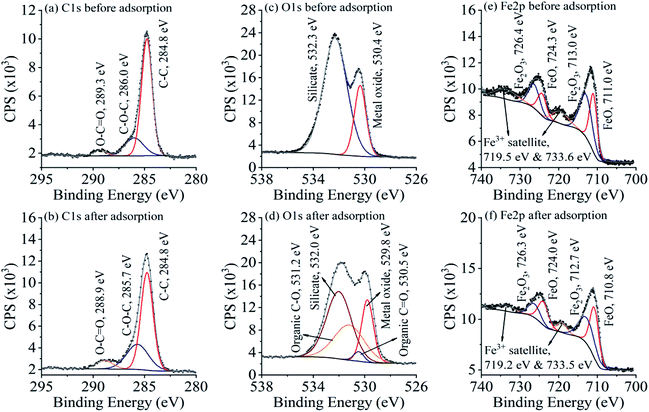 | ||
| Fig. 8 XPS spectra of C 1s (a and b), O 1s (c and d), and Fe 2p (e and f) before and after PG adsorption with their deconvoluted fitting curves. | ||
3.6 Molecular dynamics simulation
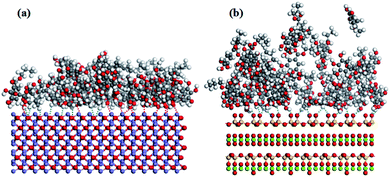
The equilibrium snapshots of PG on the (001) surface of specularite and chlorite was further studied by MDS. Fig. 9 showed the equilibrium configuration of PG on each mineral. The results from Fig. 9(a) indicated that the adsorption of PG on specularite mainly occurred by the chelate bond between O atoms in polar components of PG and Fe atoms on specularite (red line) and hydrogen bond between H atoms in PG and O atoms on specularite (blue line). As observed in Fig. 9(b), PG adsorbed on chlorite surface mainly due to the hydrogen bond between H atoms in PG and O atoms on chlorite. By contrast, PG was closer to the specularite surface than chlorite. Moreover, PG was aggregated on the specularite surface and formed a compact configuration compared to the adsorption on chlorite. The results suggested that PG was more favourably adsorbed on specularite, which was consistent with FT-IR and XPS analyses.Fig. 10 presented the relative concentration of PG along the z-direction, which was normal to the (001) surface of specularite and chlorite. The distribution of PG on specularite and chlorite surface was quantified through the relative concentration calculation of atoms O in the de-protonated hydroxyl in the para-position and H atoms in benzene ring and hydrocarbon chain of PG. The nearest atoms to the surface should be the primary action point.20 After PG acted with specularite, atoms O distributed at a distance of 1–15 Å with the strongest one being at 2.85 Å than that of H atoms 2.93 Å. The results indicated that PG adsorbed on the specularite surface via atoms O acting with surface Fe. While as for chlorite, the most reliable bond distance of O and H atoms was 3.34 Å and 3.19 Å, respectively, which means that PG ions adsorbed on chlorite surface mainly with H atoms. By comparing these two models with the same kind of atoms, the distance of most atoms O and H in PG on the specularite surface was smaller than that of chlorite. In other words, PG had stronger interaction with specularite than chlorite.
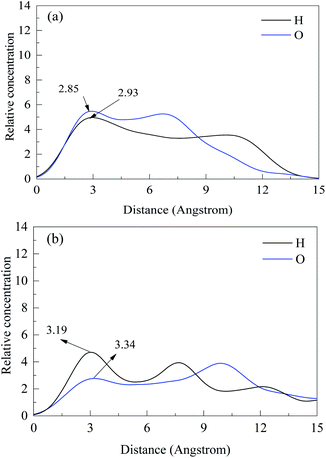 | ||
| Fig. 10 z-Direction relative concentration profiles of hydrogen and oxygen in PG on (a) specularite and (b) chlorite. | ||
Adsorption energy of PG on specularite and chlorite (001) surface was further calculated to compare PG's affinity to these two minerals. The results revealed that the adsorption energy of PG on specularite and chlorite was −1.03 eV and −0.24 eV, respectively, which indicates that the affinity of PG to specularite is much more than to chlorite. The affinity of PG to the two minerals is concordant with the experiments, detection, and MD simulation results above.
As a phyllosilicate mineral, chlorite is comprised of the silicon–oxy tetrahedron and metallic oxide with the ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The content Fe of chlorite is less than specularite, and the high solubility of metallic oxide on chlorite surface in slurry makes further efforts of the decreasing content of surface Fe. Due to that, PG adsorbs onto minerals mainly via chemical adsorption between O atoms in the de-protonated hydroxyl and Fe atoms on the mineral surface. Therefore, the difference in surface Fe content causes the selectivity difference for specularite and chlorite. Based on these ratiocinations, PG also should be a valid collector for separating specularite or hematite from iron-containing silicates, such as aegirite, grunerite, ferrodolomite, etc., especially for separating iron oxide from gangue quartz.
1. The content Fe of chlorite is less than specularite, and the high solubility of metallic oxide on chlorite surface in slurry makes further efforts of the decreasing content of surface Fe. Due to that, PG adsorbs onto minerals mainly via chemical adsorption between O atoms in the de-protonated hydroxyl and Fe atoms on the mineral surface. Therefore, the difference in surface Fe content causes the selectivity difference for specularite and chlorite. Based on these ratiocinations, PG also should be a valid collector for separating specularite or hematite from iron-containing silicates, such as aegirite, grunerite, ferrodolomite, etc., especially for separating iron oxide from gangue quartz.
4. Conclusions
Specularite and chlorite were separated by flotation using PG as a facile collector. The results indicated that the optimum separation could be achieved for single mineral flotation with recovery 87.11% for specularite and 6.98% for chlorite, and for mixed minerals, the optimum results were obtained with 65.13% TFe grade and 76.28% TFe recovery respectively at pH 8 and PG concentration 40 mg L−1, which is superior to NaOL as a conventional collector. Zeta potential, FT-IR, and XPS analyses have demonstrated that PG can be favorably adsorbed on specularite than chlorite by interactions with oxygen and iron atoms, resulting in the recoveries difference of two minerals. Molecular dynamics calculation further elucidated the binding configurations of PG on specularite and chlorite, which is highly consistent with characterization results. In conclusion, PG could be an efficient collector to float iron ore from iron-containing silicates.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully appreciate the financial support from the National Natural Science Foundation of China (grant numbers 51904001, 51674001), the Key Research and Development Projects of Anhui Province (grant numbers 201904a07020054, 201904a07020044); and Key Laboratory of Metallurgical Emission Reduction & Resources Recycling (grant number JFK19-01 and JFK20-05).Notes and references
- L. O. Filippov, V. V. Severov and I. V. Filippova, Int. J. Miner. Process., 2014, 127, 62–69 CrossRef CAS.
- W. Liu, W. Liu, Q. Zhao, Y. Shen, X. Wang, B. Wang and X. Peng, J. Mol. Liq., 2020, 301, 112428 CrossRef CAS.
- X. Zhang, X. Gu, Y. Han, N. Parra-Álvarez, V. Claremboux and S. K. Kawatra, Miner. Process. Extr. Metall. Rev., 2019, 1–29 Search PubMed.
- L. O. Filippov, I. V. Filippova and V. V. Severov, Miner. Eng., 2010, 23, 91–98 CrossRef CAS.
- A. C. Araujo, P. R. M. Viana and A. E. C. Peres, Miner. Eng., 2005, 18, 219–224 CrossRef CAS.
- L. O. Filippov, V. V. Severov and I. V. Filippova, Int. J. Miner. Process., 2013, 123, 120–128 CrossRef CAS.
- M. Li, J. Liu, X. Gao, Y. Hu, X. Tong, F. Zhao and Q. Yuan, Minerals, 2019, 9, 782 CrossRef.
- Y. Fu, W. Yin, B. Yang, C. Li, Z. Zhu and D. Li, Int. J. Miner., Metall. Mater., 2018, 25, 1113–1122 CrossRef CAS.
- G. Mei, X. Mai and Y. Yu, Met. Mine., 2002, 18–20 Search PubMed.
- M. Li, J. Liu, Y. Hu, X. Gao, Q. Yuan and F. Zhao, Carbohydr. Polym., 2020, 240, 116334 CrossRef CAS.
- X. Yang, S. Liu, G. Liu and H. Zhong, J. Ind. Eng. Chem., 2017, 46, 404–415 CrossRef CAS.
- Y. Li, Y. Liu, J. Chen and C. Zhao, Miner. Eng., 2020, 146, 106133 CrossRef.
- A. D. Wilson and D. T. Lewis, Analyst, 1963, 88, 585–589 RSC.
- J. Gao, W. Sun, Y. Hu, L. Wang, R. Liu, Z. Gao, P. Chen, H. Tang, W. Jiang and F. Lyu, Chem. Eng. Sci., 2019, 193, 255–263 CrossRef CAS.
- F. Lyu, J. Gao, N. Sun, R. Liu, X. Sun, X. Cao, L. Wang and W. Sun, Miner. Eng., 2019, 131, 66–72 CrossRef CAS.
- F. Lyu, W. Sun, S. A. Khoso, C. Zhang, R. Liu, L. Wang and J. Gao, Miner. Eng., 2019, 133, 19–26 CrossRef.
- N. Binbuga, K. Chambers, W. P. Henry and T. P. Schultz, Holzforschung, 2005, 59, 205–209 CAS.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 1, 1993, 2, 799–805 RSC.
- E. J. Silvester, W. J. Bruckard and J. T. Woodcock, Miner. Process. Extr. Metall., 2011, 120, 65–70 CrossRef CAS.
- L. Li, H. Hao, Z. Yuan and J. Liu, Appl. Surf. Sci., 2017, 419, 557–563 CrossRef CAS.
- S. S. Rath, H. Sahoo, B. Das and B. K. Mishra, Miner. Eng., 2014, 69, 57–64 CrossRef CAS.
- C. H. Veloso, L. O. Filippov, I. V. Filippova, S. Ouvrard and A. C. Araujo, Miner. Eng., 2018, 125, 133–139 CrossRef CAS.
- W. Liu, X. Peng, W. Liu, X. Wang, Q. Zhao and B. Wang, Powder Technol., 2020, 360, 1117–1125 CrossRef CAS.
- T. Yue and X. Wu, Minerals, 2018, 8, 85 CrossRef.
- W. Jiang, Y. Zhou and Y. Zhang, Chem, 2018, 2018, 1–6 CrossRef.
- D. Fornasiero and J. Ralston, Int. J. Miner. Process., 2005, 76, 75–81 CrossRef CAS.
- U. L. Kala, S. Suma, M. R. P. Kurup, S. Krishnan and R. P. John, Polyhedron, 2007, 26, 1427–1435 CrossRef CAS.
- W. Wang, Q. Chen, C. Jiang, D. Yang, X. Liu and S. Xu, Colloids Surf., A, 2007, 301, 73–79 CrossRef CAS.
- M. I. F. Barbosa, R. S. Corrêa, K. M. de Oliveira, C. Rodrigues, J. Ellena, O. R. Nascimento, V. P. C. Rocha, F. R. Nonato, T. S. Macedo, J. M. Barbosa-Filho, M. B. P. Soares and A. A. Batista, J. Inorg. Biochem., 2014, 136, 33–39 CrossRef CAS PubMed.
- M. Massoni, J. C. T. Clavijo, L. Colina-Vegas, W. Villarreal, J. S. M. Dias, G. A. F. da Silva, M. Ionta, M. Soares, J. Ellena, A. C. Dorigueto, M. I. F. Barbosa and A. A. Batista, Polyhedron, 2017, 129, 214–221 CrossRef CAS.
- Y. Wang, A. Muramatsu and T. Sugimoto, Colloids Surf., A, 1998, 134, 281–297 CrossRef CAS.
- M. C. Burrell, Y. S. Liu and H. S. Cole, J. Vac. Sci. Technol., A, 1986, 4, 2459–2462 CrossRef CAS.
- X. Gao, J. Liu, M. Li, C. Guo, H. Long, Y. Zhang and L. Xin, Chem. Eng. J., 2020, 385, 123897 CrossRef.
- J. Liu, M. Ejtemaei, A. V. Nguyen, S. Wen and Y. Zeng, Miner. Eng., 2020, 145, 106058 CrossRef CAS.
- M. Mullet, V. Khare and C. Ruby, Surf. Interface Anal., 2008, 40, 323–328 CrossRef CAS.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |