Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and molecular modeling studies of cholinesterase inhibitor dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidines]†
M. Adel Youssefa,
Siva S. Panda b,
Riham A. El-Shiekhc,
ElSayed M. Shalabyd,
Dalia R. Aboshouk
b,
Riham A. El-Shiekhc,
ElSayed M. Shalabyd,
Dalia R. Aboshouk e,
Walid Fayadf,
Nehmedo G. Fawzye and
Adel S. Girgis
e,
Walid Fayadf,
Nehmedo G. Fawzye and
Adel S. Girgis *e
*e
aDepartment of Chemistry, Faculty of Science, Helwan University, Helwan, Egypt
bDepartment of Chemistry and Physics, Augusta University, Augusta, GA 30912, USA
cDepartment of Pharmacognosy, Faculty of Pharmacy, Cairo University, Cairo 11562, Egypt
dX-Ray Crystallography Lab., Physics Division, National Research Centre, Dokki, Giza 12622, Egypt
eDepartment of Pesticide Chemistry, National Research Centre, Dokki, Giza 12622, Egypt. E-mail: girgisas10@yahoo.com
fDrug Bioassay-Cell Culture Laboratory, Pharmacognosy Department, National Research Centre, Dokki, Giza, 12622, Egypt
First published on 8th June 2020
Abstract
A set of dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidines] 8a–l was regioselectively synthesized utilizing multi-component azomethine cycloaddition reaction of 3-(arylmethylidene)pyrrolidine-2,5-diones 5a–e, isatins 6a–c and sarcosine 7. Single crystal X-ray studies of 8c add conclusive support for the structure. Compounds 8e and 8g reveal cholinesterase inhibitory properties with promising efficacy against both AChE and BChE and were found to be more selective towards AChE than BChE as indicted by the selectivity index like Donepezil (a clinically used cholinesterase inhibitory drug). Molecular modeling studies assist in understanding the bio-observations and identifying the responsible parameters behind biological properties.
Introduction
Dementia is one of the most serious health problems for older people. About 50 million people are suffering from dementia globally with 10 million new cases yearly according to the WHO (World Health Organization).1 Alzheimer's disease (AD) represents the most common cause of dementia (60–70%).1 AD is a fatal chronic neurodegenerative disease associated with memory impairment and language deficits besides high degeneration of cholinergic neurons of the central nervous system.2,3 Although no cure has been discovered for AD, a few pharmacological targets have been rationalized. Reduction of formation and aggregation of pathological hallmarks of AD (insoluble amyloid-β oligomers and tau neurofibrillary tangles) is a pharmacological target for AD. Other targets include modulation of neurotransmitter signals (cholinesterase inhibitors and N-methyl-D-aspartate receptor blockers).4 Although the full mechanism of AD is not well elucidated yet, extensive studies explained that the brain of AD patients suffers from cholinergic neuron damage. This is why acetylcholine (AC) level is considered an important therapeutic target of AD. AC is an important brain neurotransmitter with major roles in memory and maintaining consciousness.5 Acetylcholinesterase (AChE) is a catabolic enzyme capable for hydrolysis of AC. Butyrylcholinesterase (BChC) also regulates the AC levels. This is why inhibition of both cholinesterases is useful for AD patients.2,6 Tacrine (Cognex) 1 was the first cholinesterase inhibitor approved drug7 (Fig. 1). However, due to many clinically adverse effects including elevated liver transaminase levels it has been discontinued in many countries.8,9 Meanwhile, many researchers are still interested in this compound for developing analogs of lesser side effects.9–11Currently, many cholinesterase inhibitors are clinically used as AD drugs of which Galantamine (Razadyne) 2 (approved by FDA “Food and Drug Administration” on 28 Feb. 2001) for mild and moderate AD.4,12,13 Rivastigmine (Exelon) 3 (approved by FDA in 21 April 2000) is also useful for mild and moderate dementia patients caused by AD or Parkinson's disease.4,14,15 Donepezil (Aricept) 4 is also a cholinesterase inhibitor approved by FDA on 25 Nov. 1996 for AD.4,16,17 Currently, available drugs are used to manage and prevent progress of the disease over time but unfortunately not able to cure. Additionally, the drugs lack long term efficacy and also associated with severe side effects. This is why urgent need of effective anti-AD agents are still compelling.18
The present study is focused on the construction of novel dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidines] and exploring their cholinesterase properties. Rational for the targeted chemical scaffold is based on the bio-isosteric form of the indanyl nucleus of Donepezil 4 and the indolyl heterocycle of the targeted agents.19 Additionally many natural and synthetic indole containing-compounds show promising cholinesterase inhibitory properties20–23 including mono- and bis-spiro-indoles.24–27 The biologically active spiro-indoles developed by our group also prompted the current study.28–30
Results and discussion
Chemistry
Synthetic route towards the targeted dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-triones 8a–l is depicted in Scheme 1. Azomethine ylides generated from the reaction of refluxing isatins 6a–c and sarcosine 7 in ethanol reacted regioselectively with 3-(arylmethylidene)pyrrolidine-2,5-diones 5a–e31–33 affording solely the corresponding dispiro analogs 8a–l (TLC monitor). The non-stabilized azomethine ylide is formed in situ due to the applied reaction conditions (refluxing ethanol) through CO2 elimination from spiro-oxazalidinone. The latter is formed via condensation of amino acid (sarcosine) with isatin(s).34 The IR spectrum of compound 8a (example of the synthesized compounds) shows strong bands at ν = 1786, 1713 cm−1 assignable for the stretching vibration of carbonyl groups. 1H-NMR spectrum of 8a reveals the diastereotopic methylene protons of pyrrolidinedionyl H2C-4′′ and pyrrolidinyl H2C-5′ at δH = 2.37, 2.71 and 3.49, 3.84, respectively. The methine pyrrolidinyl HC-4′ is shown as a triplet signal at δH = 4.39. 13C-NMR spectrum of 8a reveals the pyrrolidinedionyl CH2 (C-4′′) and pyrrolidinyl CH2 (C-5′) at δC = 36.7, 58.4, respectively. The spiro carbons are shown at δC = 61.0, 77.6 assignable for C-3′ (C-3′′) and C-3 (C-2′), respectively. The methyl and methine (C-4′) carbons are revealed at δC = 34.6, 48.4, respectively. Additionally the carbonyl carbons are exhibited at δC = 173.0, 177.1 and 177.5. HSQC of compounds 8b and 8g support these interpretations (ESI Fig. S1–S38† show the spectral charts of the synthesized compounds). Single crystal X-ray study of compound 8c supports the structure (Fig. 2).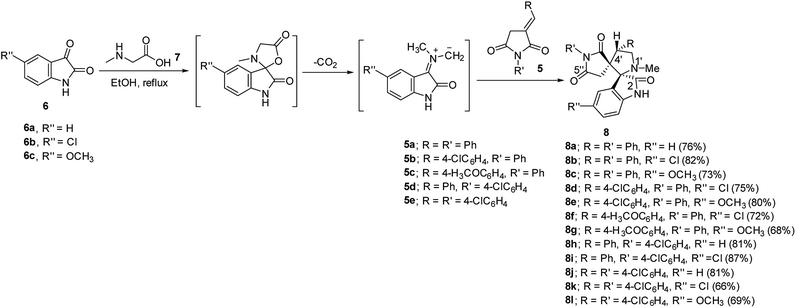 | ||
| Scheme 1 Synthetic route towards the targeted dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-triones 8a–l. | ||
 | ||
| Fig. 2 An ORTEP view of 8c showing the atom-numbering scheme. H atoms are shown as small spheres of arbitrary radii. | ||
X-ray studies
The ORTEP view of compound 8c is shown in Fig. 2. The compound is in the monoclinic system and space group P21/c with four molecules in the unit cell and one molecule in the asymmetric unit of the crystallized form. Two spiro linkages exist in 8c attaching the central pyrrolidine ring to the pyrrolidinedione at C12 and to the indolyl heterocycle at C23. In general, the geometric parameters including both bond lengths and angles (ESI Tables S1–S3†) are in good agreement with the pre-determined structures having similar rings and moieties.35,36The two phenyl rings (C4 → C9 and C15 → C20) as well as the indolyl heterocycle are planar conformations. The two pyrrolidinyl rings (C2–N3–C10–C12–C13) and (C12–C14–C21–N22–C23) are envelope conformations with the flap atoms being C12, lies 0.223 (3) Å out of the mean plane of the remaining four atoms, and N22, lies 0.662 (4) Å out of the mean plane of the remaining atoms, respectively. The sum of angles around the N22 atom is approximately 330° confirming its sp3 hybridization. The C14 and C23 atoms occupy axial and equatorial positions with respect to the pyrrolidinedione. In the crystal, molecules are linked together by set of intermolecular C–H⋯O hydrogen-bonding interactions forming supramolecular assemblies (Fig. 3 and Table 1).
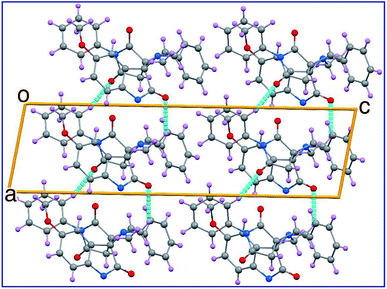 | ||
| Fig. 3 Crystal packing in the unit cell of 8c showing some hydrogen-bond interactions as dashed lines. | ||
Biological studies
| Entry | Compd. | IC50 of AChE (μM) ± SD | IC50 of BChE (μM) ± SD | SI(AChE/BChE) | SI(BChE/AChE) |
|---|---|---|---|---|---|
| 1 | 8a | 116.58 ± 12.28 | 81.75 ± 9.55 | 1.43 | 0.70 |
| 2 | 8b | 13.60 ± 0.62 | 42.45 ± 6.26 | 0.32 | 3.12 |
| 3 | 8c | 102.89 ± 10.13 | 71.75 ± 2.58 | 1.43 | 0.70 |
| 4 | 8d | 24.30 ± 4.08 | 21.33 ± 2.81 | 1.14 | 0.88 |
| 5 | 8e | 3.35 ± 0.03 | 5.63 ± 0.60 | 0.60 | 1.68 |
| 6 | 8f | 12.25 ± 1.72 | 20.10 ± 0.16 | 0.61 | 1.64 |
| 7 | 8g | 3.15 ± 0.63 | 4.74 ± 0.43 | 0.66 | 1.50 |
| 8 | 8h | 6.27 ± 0.08 | 5.34 ± 0.76 | 1.17 | 0.85 |
| 9 | 8i | 39.41 ± 2.23 | 35.12 ± 0.11 | 1.12 | 0.89 |
| 10 | 8j | 20.98 ± 1.54 | 13.58 ± 0.37 | 1.54 | 0.65 |
| 11 | 8k | 13.93 ± 0.18 | 10.22 ± 0.76 | 1.36 | 0.73 |
| 12 | 8l | 21.97 ± 2.82 | 35.54 ± 0.33 | 0.62 | 1.62 |
| 13 | Donepezil | 0.59 ± 0.08 | 0.77 ± 0.01 | 0.77 | 1.31 |
Structure–activity relationship (SAR) based on the exhibited biological observations explain that, attachment of phenyl ring at pyrrolidinyl C-4′ seems more favorable than the p-chlorophenyl ring for AChE inhibitory properties (8k is an exception) as shown in pairs 8b/8d and 8h/8j (IC50 = 13.60, 24.30, 6.27, 20.98 μM, for 8b, 8d, 8h and 8j, respectively). Meanwhile, when p-methoxyphenyl ring is considered at pyrrolidinyl C-4′, better biologically active agents are optimized as shown in pairs 8b/8f and 8c/8g (IC50 = 13.60, 12.25, 102.89, 3.15 μM, for 8b, 8f, 8c and 8g, respectively). However, different SAR was noticed for BChE inhibitory properties. Where, the p-chlorophenyl ring containing compounds at pyrrolidinyl C-4′ are of higher BChE inhibitory properties than those of phenyl ring (compound 8h is an exception) as shown in pairs 8b/8d, 8c/8e and 8i/8k (IC50 = 42.45, 21.33, 71.75, 5.63, 35.12, 10.22 μM, for 8b, 8d, 8c, 8e, 8i and 8k, respectively). The same observation was also noticed for p-methoxyphenyl ring relative to phenyl ring due to BChE inhibitory properties similar to that of AChE as shown in pairs 8b/8f and 8c/8g (IC50 = 42.45, 20.10, 71.75 and 4.74 μM, for 8b, 8f, 8c and 8g, respectively). Molecular modeling studies can make the biological properties more understandable and identify the rules optimizing biological properties.
Molecular modeling studies
Molecular modeling is an efficient technique useful for validating biological properties and identifying the parameters necessary for bio-properties beside its unique importance for developing/estimating novel hits/leads.Goodness of the QSAR models was supported by the comparative values of the correlation coefficient (R2) with their cross validation of leave one-out (R2cvOO) and leave many-out (R2cvMO) [R2 = 0.923, 0.979; R2cvOO = 0.882, 0.936; R2cvMO = 0.904, 0.956, for AChE and BChE models, respectively]. Standard deviation (s2) and Fisher criteria (F) values of the models also support their goodness (s2 = 0.026, 0.005; F = 31.948, 122.564, for AChE and BChE models, respectively). Additionally, the correlations of the observed and predicted values “specially the high potent synthesized compounds” add good support for the attained models.
The 3D-pharmacophore modelling of the synthesized agents as BChE inhibitors exhibits three chemical features (hydrophobic “H”, hydrogen bonding acceptor “HBA” and hydrogen bonding donor “HBD”). The aryl ring attached to the pyrrolidinyl N-1′′ is mapped with the hydrophobic “H”. Meanwhile, the pyrrolidinyl carbonyl at C-2′′ and indolyl N-1 are mapped with the HBA and HBD, receptively. Again, mapping of the aryl ring at N-1′′ supported its importance for the revealed BChE inhibitory properties.
Conclusion
In conclusion it can be stated that, the synthesized dispiro-compounds are good cholinesterase inhibitors especially compounds 8e and 8g which show good potency and selectivity index towards AChE over BChE similar to Donepezil (clinically used cholinesterase inhibitory drug). The attained QSAR and 3D-pharmacophore models are good enough to be considered for developing novel effective hits/leads with enhanced potency/efficacy considering the elements controlling bio-observations (mainly the aryl rings attached to the pyrrolidinedione). Additionally, the multi-component azomethine cycloaddition procedure is an accessible technique for developing the targeted dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidines] in good yield (66–82%) and high regioselectivity.Experimental
Melting points were determined on a capillary point apparatus (Stuart SMP3) equipped with a digital thermometer. IR spectra (KBr) were recorded on a Shimadzu FT-IR 8400S spectrophotometer. Reactions were monitored using thin layer chromatography (TLC) on 0.2 mm silica gel F254 plates (Merck) utilizing various solvents for elution. The chemical structures of the synthesized compounds were characterized by nuclear magnetic resonance spectra (1H-NMR, 13C-NMR) and determined on a Bruker NMR spectrometer (500 MHz, 125 MHz for 1H and 13C, respectively). 13C-NMR spectra are fully decoupled. Chemical shifts were reported in parts per million (ppm) using the deuterated solvent peak or tetramethylsilane as an internal standard. Colorimetric enzyme inhibitory assays were performed in 96-well plates and the absorbance was recorded utilizing a microplate reader (Infinite F50, Tecan, Switzerland).Synthesis of dispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidines] 8a–l (general procedure)
A mixture of equimolar amount of the appropriate 5a–e (5 mmol), isatin 6a–c and sarcosine 7 in absolute ethanol (25 mL) was boiled under reflux for the specific time. The separated solid while boiling, was collected and crystallized from a suitable solvent affording the corresponding 8a–g,i–k. In case of 8h,l the clear reaction mixture was stored at room temperature (20–25 °C) overnight. So, the separated solid was collected and purified by crystallization from a suitable solvent.1′-Methyl-1′′,4′-diphenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8a)
It was obtained from the reaction of 5a with 6a and sarcosine for 10 h as colorless microcrystals from n-butanol with mp 236–238 °C and yield 76% (1.65 g). IR: νmax/cm−1 3480, 3067, 2870, 1786, 1713, 1616, 1597. 1H-NMR (DMSO-d6) δ (ppm): 2.10 (s, 3H, NCH3), 2.37 (d, J = 18.3 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.71 (d, J = 18.3 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.49 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.84 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.39 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.75 (dd, J = 1.8, 7.7 Hz, 2H, arom. H), 6.89 (d, J = 7.7 Hz, 1H, arom. H), 6.99 (t, J = 7.6 Hz, 1H, arom. H), 7.21 (d, J = 7.4 Hz, 1H, arom. H), 7.30–7.43 (m, 7H, arom. H), 7.49 (d, J = 7.4 Hz, 2H, arom. H), 10.78 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.7 [pyrrolidinedionyl CH2 (C-4′′)], 48.4 [pyrrolidinyl CH (C-4′)], 58.4 [pyrrolidinyl CH2 (C-5′)], 61.0 [C-3′ (C-3′′)], 77.6 [C-3 (C-2′)], 110.0, 122.0, 125.0, 126.5, 126.8, 127.4, 128.4, 128.6, 128.7, 129.9, 131.6, 137.9, 142.7 (arom. C), 173.0, 177.1, 177.5 (CO). Anal. calcd for C27H23N3O3 (437.50): C, 74.13; H, 5.30; N, 9.60. Found: C, 74.24; H, 5.49; N, 9.68.5-Chloro-1′-methyl-1′′,4′-diphenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8b)
It was obtained from the reaction of 5a with 6b and sarcosine for 12 h as colorless microcrystals from N,N-dimethylformamide–water (2–1 v/v) with mp 253–255 °C and yield 82% (1.94 g). IR: νmax/cm−1 3472, 3063, 2874, 1782, 1713, 1616, 1597. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.39 (d, J = 18.2 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.70 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.51 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.80 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.37 (t, J = 9.0 Hz, 1H, pyrrolidinyl HC-4′), 6.82 (dd, J = 1.6, 8.0 Hz, 2H, arom. H), 6.91 (d, J = 8.3 Hz, 1H, arom. H), 7.19 (d, J = 2.2 Hz, 1H, arom. H), 7.33 (t, J = 7.3 Hz, 1H, arom. H), 7.37–7.43 (m, 6H, arom. H), 7.48 (d, J = 7.4 Hz, 2H, arom. H), 10.94 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.5 [pyrrolidinedionyl CH2 (C-4′′)], 48.5 [pyrrolidinyl CH (C-4′)], 58.3 [pyrrolidinyl CH2 (C-5′)], 61.0 [C-3′ (C-3′′)], 77.5 [C-3 (C-2′)], 111.5, 126.2, 126.3, 126.6, 127.0, 127.4, 128.5, 128.6, 128.7, 129.9, 130.0, 131.5, 137.6, 141.7 (arom. C), 172.8, 176.7, 177.4 (CO). Anal. calcd for C27H22ClN3O3 (471.94): C, 68.72; H, 4.70; N, 8.90. Found: C, 68.96; H, 4.84; N, 9.06.5-Methoxy-1′-methyl-1′′,4′-diphenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8c)
It was obtained from the reaction of 5a with 6c and sarcosine for 12 h as colorless microcrystals from n-butanol with mp 253–255 °C and yield 73% (1.70 g). IR: νmax/cm−1 3472, 3063, 2870, 1778, 1713, 1697, 1605, 1497. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.36 (d, J = 18.2 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.64 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.49 (t, J = 8.5 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.55 (s, 3H, OCH3), 3.83 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.37 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.73–6.75 (m, 3H, arom. H), 6.82 (d, J = 8.4 Hz, 1H, arom. H), 6.90 (dd, J = 2.6, 8.5 Hz, 1H, arom. H), 7.31–7.42 (m, 6H, arom. H), 7.51 (d, J = 7.4 Hz, 2H, arom. H), 10.63 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.8 [pyrrolidinedionyl CH2 (C-4′′)], 48.1 [pyrrolidinyl CH (C-4′)], 55.1 (OCH3), 58.7 [pyrrolidinyl CH2 (C-5′)], 61.4 [C-3′ (C-3′′)], 77.9 [C-3 (C-2′)], 110.4, 113.2, 114.6, 126.2, 126.7, 127.3, 128.4, 128.57, 128.61, 130.1, 131.6, 135.8, 138.1, 154.9 (arom. C), 172.9, 177.0, 177.6 (CO). Anal. calcd for C28H25N3O4 (467.53): C, 71.93; H, 5.39; N, 8.99. Found: C, 71.99; H, 5.60; N, 9.22.5-Chloro-4′-(4-chlorophenyl)-1′-methyl-1′′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8d)
It was obtained from the reaction of 5b with 6b and sarcosine for 12 h as colorless microcrystals from n-butanol with mp 232–234 °C and yield 75% (1.89 g). IR: νmax/cm−1 3487, 3059, 2874, 1790, 1713, 1620, 1597. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.42 (d, J = 18.1 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.67 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.52 (t, J = 8.7 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.74 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.37 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.83 (dd, J = 1.5, 8.0 Hz, 2H, arom. H), 6.92 (d, J = 8.3 Hz, 1H, arom. H), 7.16 (d, J = 2.2 Hz, 1H, arom. H), 7.38–7.41 (m, 4H, arom. H), 7.47 (d, J = 8.5 Hz, 2H, arom. H), 7.53 (d, J = 8.5 Hz, 2H, arom. H), 10.97 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.6 [pyrrolidinedionyl CH2 (C-4′′)], 47.7 [pyrrolidinyl CH (C-4′)], 58.7 [pyrrolidinyl CH2 (C-5′)], 61.0 [C-3′ (C-3′′)], 77.6 [C-3 (C-2′)], 111.6, 126.2, 126.3, 126.6, 126.9, 128.46, 128.54, 128.7, 130.0, 131.5, 132.0, 132.1, 136.8, 141.7 (arom. C), 172.8, 176.8, 177.3 (CO). Anal. calcd for C27H21Cl2N3O3 (506.38): C, 64.04; H, 4.18; N, 8.30. Found: C, 63.74; H, 4.35; N, 8.44.4′-(4-Chlorophenyl)-5-methoxy-1′-methyl-1′′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8e)
It was obtained from the reaction of 5b with 6c and sarcosine for 14 h as colorless microcrystals from n-butanol with mp 228–230 °C and yield 80% (2.00 g). IR: νmax/cm−1 3487, 3059, 2874, 1786, 1713, 1601, 1489. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.39 (d, J = 18.1 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.62 (d, J = 18.1 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.51 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.54 (s, 3H, OCH3), 3.77 (t, J = 9.2 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.37 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.70 (d, J = 2.5 Hz, 1H, arom. H), 6.75 (dd, J = 2.0, 7.4 Hz, 2H, arom. H), 6.83 (d, J = 8.4 Hz, 1H, arom. H), 6.90 (dd, J = 2.6, 8.5 Hz, 1H, arom. H), 7.36–7.38 (m, 3H, arom. H), 7.47 (d, J = 8.5 Hz, 2H, arom. H), 7.55 (d, J = 8.4 Hz, 2H, arom. H), 10.66 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.5 (NCH3), 36.9 [pyrrolidinedionyl CH2 (C-4′′)], 47.3 [pyrrolidinyl CH (C-4′)], 55.1 (OCH3), 59.0 [pyrrolidinyl CH2 (C-5′)], 61.3 [C-3′ (C-3′′)], 77.9 [C-3 (C-2′)], 110.5, 113.0, 114.6, 126.1, 126.7, 128.4, 128.5, 128.53, 131.6, 132.0, 132.1, 135.8, 137.3, 155.0 (arom. C), 172.9, 177.1, 177.4 (CO). Anal. calcd for C28H24ClN3O4 (501.97): C, 67.00; H, 4.82; N, 8.37. Found: C, 67.19; H, 5.04; N, 8.42.5-Chloro-4′-(4-methoxyphenyl)-1′-methyl-1′′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8f)
It was obtained from the reaction of 5c with 6b and sarcosine for 12 h as colorless microcrystals from n-butanol with mp 223–225 °C and yield 72% (1.80 g). IR: νmax/cm−1 3483, 3078, 2832, 1786, 1713, 1616. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.42 (d, J = 18.2 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.68 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.48 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.76 (s, 3H, OCH3), 3.76 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.32 (t, J = 9.0 Hz, 1H, pyrrolidinyl HC-4′), 6.84 (dd, J = 1.4, 8.0 Hz, 2H, arom. H), 6.91 (d, J = 8.3 Hz, 1H, arom. H), 6.97 (d, J = 8.7 Hz, 2H, arom. H), 7.21 (d, J = 2.1 Hz, 1H, arom. H), 7.37–7.42 (m, 6H, arom. H), 10.92 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.4 [pyrrolidinedionyl CH2 (C-4′′)], 48.0 [pyrrolidinyl CH (C-4′)], 55.0 (OCH3), 58.4 [pyrrolidinyl CH2 (C-5′)], 61.0 [C-3′ (C-3′′)], 77.5 [C-3 (C-2′)], 111.5, 114.0, 126.2, 126.4, 126.6, 127.1, 128.5, 128.8, 129.3, 129.8, 131.0, 131.5, 141.7, 158.5 (arom. C), 172.9, 176.7, 177.6 (CO). Anal. calcd for C28H24ClN3O4 (501.97): C, 67.00; H, 4.82; N, 8.37. Found: C, 67.20; H, 4.66; N, 8.18.5-Methoxy-4′-(4-methoxyphenyl)-1′-methyl-1′′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8g)
It was obtained from the reaction of 5c with 6c and sarcosine for 15 h as colorless microcrystals from n-butanol with mp 217–219 °C and yield 68% (1.68 g). IR: νmax/cm−1 3480, 3075, 2959, 1782, 1713, 1601. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.38 (d, J = 18.3 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.62 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.46 (t, J = 8.5 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.55 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.79 (t, J = 9.2 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.32 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.74–6.76 (m, 3H, arom. H), 6.81 (d, J = 8.4 Hz, 1H, arom. H), 6.89 (dd, J = 2.6, 8.5 Hz, 1H, arom. H), 6.97 (d, J = 8.8 Hz, 2H, arom. H), 7.37–7.43 (m, 5H, arom. H), 10.61 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.7 [pyrrolidinedionyl CH2 (C-4′′)], 47.6 [pyrrolidinyl CH (C-4′)], 55.0 (OCH3), 55.1 (OCH3), 58.8 [pyrrolidinyl CH2 (C-5′)], 61.4 [C-3′ (C-3′′)], 77.9 [C-3 (C-2′)], 110.4, 113.2, 114.0, 114.5, 126.3, 126.7, 128.4, 128.6, 129.8, 131.1, 131.6, 135.8, 154.9, 158.4 (arom. C), 173.0, 177.1, 177.7 (CO). Anal. calcd for C29H27N3O5 (497.55): C, 70.01; H, 5.47; N, 8.45. Found: C, 69.87; H, 5.40; N, 8.25.1′′-(4-Chlorophenyl)-1′-methyl-4′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8h)
It was obtained from the reaction of 5d with 6a and sarcosine for 10 h as pale yellow microcrystals from n-butanol with mp 208–210 °C (lit. 189–190 °C (ref. 33)) and yield 81% (1.90 g). IR: νmax/cm−1 3480, 3067, 2951, 1778, 1713, 1616, 1597. 1H-NMR (DMSO-d6) δ (ppm): 2.09 (s, 3H, NCH3), 2.39 (d, J = 18.2 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.67 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.49 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.82 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.37 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.79 (dd, J = 2.0, 6.8 Hz, 2H, arom. H), 6.88 (d, J = 7.7 Hz, 1H, arom. H), 6.97 (t, J = 7.6 Hz, 1H, arom. H), 7.16 (d, J = 7.4 Hz, 1H, arom. H), 7.28–7.33 (m, 2H, arom. H), 7.39–7.50 (m, 6H, arom. H), 10.78 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.5 (NCH3), 36.9 [pyrrolidinedionyl CH2 (C-4′′)], 48.2 [pyrrolidinyl CH (C-4′)], 58.6 [pyrrolidinyl CH2 (C-5′)], 61.2 [C-3′ (C-3′′)], 77.6 [C-3 (C-2′)], 110.0, 122.0, 125.0, 126.2, 127.3, 128.5, 128.6, 128.7, 129.99, 130.0, 130.3, 132.9, 138.0, 142.7 (arom. C), 172.7, 177.1, 177.3 (CO). Anal. calcd for C27H22ClN3O3 (471.94): C, 68.72; H, 4.70; N, 8.90. Found: C, 68.95; H, 4.86; N, 8.81.5-Chloro-1′′-(4-chlorophenyl)-1′-methyl-4′-phenyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8i)
It was obtained from the reaction of 5d with 6b and sarcosine for 10 h as colorless microcrystals from n-butanol with mp 240–242 °C and yield 87% (2.20 g). IR: νmax/cm−1 3476, 3102, 2839, 1778, 1713, 1620. 1H-NMR (DMSO-d6) δ (ppm): 2.12 (s, 3H, NCH3), 2.42 (d, J = 18.2 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.70 (d, J = 18.2 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.51 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.81 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.37 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.88 (d, J = 8.7 Hz, 2H, arom. H), 6.92 (d, J = 8.3 Hz, 1H, arom. H), 7.17 (d, J = 2.2 Hz, 1H, arom. H), 7.31–7.42 (m, 4H, arom. H), 7.48–7.50 (m, 4H, arom. H), 10.95 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.6 [pyrrolidinedionyl CH2 (C-4′′)], 48.5 [pyrrolidinyl CH (C-4′)], 58.5 [pyrrolidinyl CH2 (C-5′)], 61.1 [C-3′ (C-3′′)], 77.5 [C-3 (C-2′)], 111.6, 126.22, 126.24, 127.0, 127.4, 128.3, 128.6, 128.8, 129.9, 130.1, 130.3, 133.0, 137.6, 141.7 (arom. C), 172.6, 176.7, 177.2 (CO). Anal. calcd for C27H21Cl2N3O3 (506.38): C, 64.04; H, 4.18; N, 8.30. Found: C, 64.21; H, 4.26; N, 8.41.1′′,4′-Bis(4-chlorophenyl)-1′-methyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8j)
It was obtained from the reaction of 5e with 6a and sarcosine for 10 h as colorless microcrystals from n-butanol with mp 225–227 °C (lit. 224–226 °C (ref. 33)) and yield 81% (2.05 g). IR: νmax/cm−1 3487, 3063, 2878, 1790, 1717, 1620, 1597, 1493. 1H-NMR (DMSO-d6) δ (ppm): 2.08 (s, 3H, NCH3), 2.41 (d, J = 18.1 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.64 (d, J = 18.1 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.51 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.75 (t, J = 9.2 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.36 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.79 (dd, J = 1.9, 6.8 Hz, 2H, arom. H), 6.89 (d, J = 7.7 Hz, 1H, arom. H), 6.97 (t, J = 7.7 Hz, 1H, arom. H), 7.13 (d, J = 7.4 Hz, 1H, arom. H), 7.30 (dt, J = 1.0, 7.7 Hz, 1H, arom. H), 7.44–7.47 (m, 4H, arom. H), 7.53 (d, J = 8.5 Hz, 2H, arom. H), 10.80 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.5 (NCH3), 36.9 [pyrrolidinedionyl CH2 (C-4′′)], 47.4 [pyrrolidinyl CH (C-4′)], 58.9 [pyrrolidinyl CH2 (C-5′)], 61.1 [C-3′ (C-3′′)], 77.6 [C-3 (C-2′)], 110.1, 122.1, 124.8, 126.1, 128.5, 128.7, 130.1, 130.3, 132.0, 132.1, 132.9, 137.2, 142.7 (arom. C), 172.7, 177.2 (CO). Anal. calcd for C27H21Cl2N3O3 (506.38): C, 64.04; H, 4.18; N, 8.30. Found: C, 63.88; H, 3.97; N, 8.06.5-Chloro-1′′,4′-bis(4-chlorophenyl)-1′-methyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8k)
It was obtained from the reaction of 5e with 6b and sarcosine for 9 h as colorless microcrystals from n-butanol with mp 236–238 °C and yield 66% (1.77 g). IR: νmax/cm−1 3487, 3105, 2874, 1786, 1713, 1620, 1493. 1H-NMR (DMSO-d6) δ (ppm): 2.10 (s, 3H, NCH3), 2.42 (d, J = 18.1 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.65 (d, J = 18.0 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.52 (t, J = 8.7 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.72 (t, J = 9.3 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.35 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.87 (dd, J = 2.0, 6.8 Hz, 2H, arom. H), 6.91 (d, J = 8.3 Hz, 1H, arom. H), 7.11 (d, J = 2.2 Hz, 1H, arom. H), 7.37 (dd, J = 2.2, 8.3 Hz, 1H, arom. H), 7.47–7.54 (m, 6H, arom. H), 10.97 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.6 (NCH3), 36.7 [pyrrolidinedionyl CH2 (C-4′′)], 47.5 [pyrrolidinyl CH (C-4′)], 58.8 [pyrrolidinyl CH2 (C-5′)], 61.1 [C-3′ (C-3′′)], 77.5 [C-3 (C-2′)], 111.6, 126.0, 126.3, 126.9, 128.2, 128.5, 128.8, 130.0, 130.3, 132.06, 132.1, 132.9, 136.8, 141.7 (arom. C), 172.5, 176.7, 177.1 (CO). Anal. calcd for C27H20Cl3N3O3 (540.83): C, 59.96; H, 3.73; N, 7.77. Found: C, 60.10; H, 3.84; N, 7.64.1′′,4′-Bis(4-chlorophenyl)-5-methoxy-1′-methyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-pyrrolidine]-2,2′′,5′′-trione (8l)
It was obtained from the reaction of 5e with 6c and sarcosine for 12 h as colorless microcrystals from n-butanol with mp 247–249 °C and yield 69% (1.84 g). IR: νmax/cm−1 3476, 3210, 2870, 1778, 1697, 1632, 1605. 1H-NMR (DMSO-d6) δ (ppm): 2.11 (s, 3H, NCH3), 2.41 (d, J = 18.0 Hz, 1H, upfield H of pyrrolidinedionyl H2C-4′′), 2.62 (d, J = 18.0 Hz, 1H, downfield H of pyrrolidinedionyl H2C-4′′), 3.51 (t, J = 8.6 Hz, 1H, upfield H of pyrrolidinyl H2C-5′), 3.55 (s, 3H, OCH3), 3.77 (t, J = 9.2 Hz, 1H, downfield H of pyrrolidinyl H2C-5′), 4.38 (t, J = 8.9 Hz, 1H, pyrrolidinyl HC-4′), 6.68 (d, J = 2.5 Hz, 1H, arom. H), 6.78–6.91 (m, 4H, arom. H), 7.45–7.48 (m, 3H, arom. H), 7.56 (d, J = 8.5 Hz, 2H, arom. H), 10.67 (s, 1H, NH). 13C-NMR (DMSO-d6) δ (ppm): 34.5 (NCH3), 37.0 [pyrrolidinedionyl CH2 (C-4′′)], 47.2 [pyrrolidinyl CH (C-4′)], 55.1 (OCH3), 59.2 [pyrrolidinyl CH2 (C-5′)], 61.5 [C-3' (C-3′′)], 77.9 [C-3 (C-2′)], 110.5, 112.9, 114.6, 126.1, 128.4, 128.5, 128.6, 130.4, 132.0, 132.2, 132.9, 135.7, 137.3, 155.0 (arom. C), 172.7, 177.1 (CO). Anal. calcd for C28H23Cl2N3O4 (536.41): C, 62.70; H, 4.32; N, 7.83. Found: C, 62.91; H, 4.14; N, 8.00.X-ray studies
The experimental procedure was mentioned in the ESI.†Biological studies
All the biological procedures utilized obey the standards and approved by the Research Ethics Committee, National Research Centre, Egypt (associated with project ID: 12060101). All the experiments were performed following the relevant guidelines and regulations.| % Inhibition = [1 − (corrected A/corrected B)] × 100 |
Selectivity index (SI) for acetyl and butyryl cholinesterases was calculated as follow:
| SI(AChE/BChE) = IC50 (AChE)/IC50 (BChE) |
| SI(BChE/AChE) = IC50 (AChE)/IC50 (BChE) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1 potassium penicillin, 10
000 μg mL−1 potassium penicillin, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1 streptomycin sulfate and 25 μg mL−1 amphotericin B), 10% fetal bovine serum and 1% L-glutamine at 37 °C, under 5% CO2 and 95% humidity. Cells were seeded at concentration of 30
000 μg mL−1 streptomycin sulfate and 25 μg mL−1 amphotericin B), 10% fetal bovine serum and 1% L-glutamine at 37 °C, under 5% CO2 and 95% humidity. Cells were seeded at concentration of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in fresh complete growth medium in 96-well tissue culture microtiter plates for 24 h. Media was aspirated, fresh complete medium was added and cells were incubated with different concentrations of the tested compound to give a final concentration of (100, 50, 25 and 12.5 μM). 0.5% DMSO was used as negative control. Triplicate wells were prepared for each individual dose. After 72 h of incubation, medium was aspirated, 40 μl MTT salt (2.5 mg mL−1) were added to each well and incubated for further 4 h at 37 °C. To stop the reaction and dissolve the formed crystals, 150 μL of 10% sodium dodecyl sulfate (SDS) in deionized water were added to each well and incubated overnight at 37 °C. The absorbance was then measured at 570 nm and a reference wavelength of 595 nm.
000 cells per well in fresh complete growth medium in 96-well tissue culture microtiter plates for 24 h. Media was aspirated, fresh complete medium was added and cells were incubated with different concentrations of the tested compound to give a final concentration of (100, 50, 25 and 12.5 μM). 0.5% DMSO was used as negative control. Triplicate wells were prepared for each individual dose. After 72 h of incubation, medium was aspirated, 40 μl MTT salt (2.5 mg mL−1) were added to each well and incubated for further 4 h at 37 °C. To stop the reaction and dissolve the formed crystals, 150 μL of 10% sodium dodecyl sulfate (SDS) in deionized water were added to each well and incubated overnight at 37 °C. The absorbance was then measured at 570 nm and a reference wavelength of 595 nm.Data were collected as mean values for experiments performed in triplicates for each individual dose which had been measured by MTT assay. Control experiments did not exhibit significant change compared to the DMSO vehicle. The percentage of cell survival was calculated according to the following equation.
The IC50 (concentration required to produce 50% inhibition of cell growth compared to the control experiment) can was determined using Graph-Pad PRISM version-5 software. Statistical calculations for determination of the mean and standard error values were determined by SPSS 16 software. The observed anti-proliferative properties are presented ESI Fig. S39.†
Molecular modeling studies
The experimental procedures in details were mentioned in the ESI.†Conflicts of interest
There is no conflict to declare.Acknowledgements
This work was supported financially by National Research Centre, Egypt, project ID: 12060101.Notes and references
- https://www.who.int/news-room/fact-sheets/detail/dementia.
- R. Malek, R. L. Arribas, A. Palomino-Antolin, P. Totoson, C. Demougeot, T. Kobrlova, O. Soukup, I. Iriepa, I. Moraleda, D. Diez-Iriepa, J. Godyń, D. Panek, B. Malawska, M. Głuch-Lutwin, B. Mordyl, A. Siwek, F. Chabchoub, J. Marco-Contelles, K. Kiec-Kononowicz, J. Egea, C. de los Rios and L. Ismaili, New dual small molecules for Alzheimer's disease therapy combining histamine H3 receptor (H3R) antagonism and calcium channels blockade with additional cholinesterase inhibition, J. Med. Chem., 2019, 62, 11416–11422 CrossRef CAS PubMed.
- S. Rizzo, C. Rivière, L. Piazzi, A. Bisi, S. Gobbi, M. Bartolini, V. Andrisano, F. Morroni, A. Tarozzi, J.-P. Monti and A. Rampa, Benzofuran-based hybrid compounds for the inhibition of cholinesterase activity, β amyloid aggregation, and Aβ neurotoxicity, J. Med. Chem., 2008, 51, 2883–2886 CrossRef CAS PubMed.
- A. Morsy and P. C. Trippier, Current and emerging pharmacological targets for the treatment of Alzheimer's disease, J. Alzheimer's Dis., 2019, 72, S145–S176 CAS.
- X. Li, H. Wang, Z. Lu, X. Zheng, W. Ni, J. Zhu, Y. Fu, F. Lian, N. Zhang, J. Li, H. Zhang and F. Mao, Development of multifunctional primidinylthiourea derivatives as potential anti-Alzheimer agents, J. Med. Chem., 2016, 59, 8326–8344 CrossRef CAS PubMed.
- P. W. Elsinghorst, C. M. G. Tanarro and M. Gütschow, Novel heterobivalent Tacrine derivatives as cholinesterase inhibitors with notable selectivity toward butyrylcholinesterase, J. Med. Chem., 2006, 49, 7540–7544 CrossRef CAS PubMed.
- M. L. Crismon, Tacrine: first drug approved for Alzheimer's disease, Ann. Pharmacother., 1994, 28, 744–751 CrossRef CAS PubMed.
- https://www.drugbank.ca/drugs/DB00382.
- S. Hamulakova, L. Janovec, M. Hrabinova, K. Spilovska, J. Korabecny, P. Kristian, K. Kuca and J. Imrich, Synthesis and biological evaluation of novel Tacrine derivatives and Tacrine-coumarin hybrids as cholinesterase inhibitors, J. Med. Chem., 2014, 57, 7073–7084 CrossRef CAS PubMed.
- L. Fang, D. Appenroth, M. Decker, M. Kiehntopf, C. Roegler, T. Deufel, C. Fleck, S. Peng, Y. Zhang and J. Lehmann, Synthesis and biological evaluation of NO-donor-Tacrine hybrids as hepatoprotective anti-Alzheimer drug candidates, J. Med. Chem., 2008, 51, 713–716 CrossRef CAS PubMed.
- M.-K. Hu, L.-J. Wu, G. Hsia and M.-H. Yen, Homodimeric Tacrine congeners as acetylcholinesterase inhibitors, J. Med. Chem., 2002, 45, 2277–2282 CrossRef PubMed.
- https://www.drugs.com/ppa/galantamine.html.
- P. N. Tariot, P. R. Solomon, J. C. Morris, P. Kershaw, S. Lilienfeld and C. Ding, A 5-month, randomized, placebocontrolled trial of galantamine in AD, Neurology, 2000, 54, 2269–2276 CrossRef CAS PubMed.
- https://www.drugs.com/exelon.html.
- M. Rösler, R. Anand, A. Cicin-Sain, S. Gauthier, Y. Agid, P. Dal-Bianco, H. B. Stähelin and R. Hartman, Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial, BMJ, 1999, 318, 633–640 CrossRef PubMed.
- https://www.drugs.com/mtm/donepezil.html.
- S. L. Rogers, M. R. Farlow, R. S. Doody, R. Mohs and L. T. Friedhoff, A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease, Neurology, 1998, 50, 136–145 CrossRef CAS PubMed.
- M. G. Bursavich, B. A. Harrison and J.-F. Blain, Gamma secretase modulators: new Alzheimer's drugs on the horizon?, J. Med. Chem., 2016, 59, 7389–7409 CrossRef CAS PubMed.
- G. A. Patani and E. J. LaVoie, Bioisosterism: A Rational Approach in Drug Design, Chem. Rev., 1996, 96, 3147–3176 CrossRef CAS PubMed.
- G. Zhan, J. Liu, J. Zhou, B. Sun, H. A. Aisa and G. Yao, Amaryllidaceae alkaloids with new framework types from Zephyranthes candida as potent acetylcholinesterase inhibitors, Eur. J. Med. Chem., 2017, 127, 771–780 CrossRef CAS PubMed.
- M. T. Andrade, J. A. Lima, A. C. Pinto, C. M. Rezende, M. P. Carvalho and R. A. Epifanio, Indole alkaloids from Tabernaemontana australis (Müell. Arg) Miers that inhibit acetylcholinesterase enzyme, Bioorg. Med. Chem., 2005, 13, 4092–4095 CrossRef CAS PubMed.
- S. Chigurupati, M. Selvaraj, V. Mani, K. K. Selvarajan, J. I. Mohammad, B. Kaveti, H. Bera, V. R. Palanimuthu, L. K. The and M. Z. Salleh, Identification of novel acetylcholinesterase inhibitors: indolopyrazoline derivatives and molecular docking studies, Bioorg. Chem., 2016, 67, 9–17 CrossRef CAS PubMed.
- M. Atanasova, G. Stavrakov, I. Philipova, D. Zheleva, N. Yordanov and I. Doytchinova, Galantamine derivatives with indole moiety: docking, design, synthesis and acetylcholinesterase inhibitory activity, Bioorg. Med. Chem., 2015, 23, 5382–5389 CrossRef CAS PubMed.
- Y. Kia, H. Osman, R. S. Kumar, V. Murugaiyah, A. Basiri, S. Perumal, H. A. Wahab and C. S. Bing, Synthesis and discovery of novel piperidone-grafted mono- and bis-spirooxindole-hexahydropyrrolizines as potent cholinesterase inhibitors, Bioorg. Med. Chem., 2013, 21, 1696–1707 CrossRef CAS PubMed.
- Y. Kia, H. Osman, R. S. Kumar, V. Murugaiyah, A. Basiri, S. Perumal and I. A. Razak, A facile chemo-, regio- and stereoselective synthesis and cholinesterase inhibitory activity of spirooxindole–pyrrolizine–piperidine hybrids, Bioorg. Med. Chem. Lett., 2013, 23, 2979–2983 CrossRef CAS PubMed.
- Y. kia, H. Osman, R. S. Kumar, A. Basiri and V. Murugaiyah, Ionic liquid mediated synthesis of mono- and bis-spirooxindole-hexahydropyrrolidines as cholinesterase inhibitors and their molecular docking studies, Bioorg. Med. Chem., 2014, 22, 1318–1328 CrossRef CAS PubMed.
- Y. Kia, H. Osman, R. S. Kumar, A. Basiri and V. Murugaiyah, Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors, Bioorg. Med. Chem. Lett., 2014, 24, 1815–1819 CrossRef CAS PubMed.
- R. F. George, S. S. Panda, E. M. Shalaby, A. M. Srour, I. S. Ahmed Farag and A. S. Girgis, Synthesis and molecular modeling studies of indole-based antitumor agents, RSC Adv., 2016, 6, 45434–45451 RSC.
- A. S. Girgis, A. F. Mabied, J. Stawinski, L. Hegazy, R. F. George, H. Farag, E. M. Shalaby and I. S. Ahmed Farag, Synthesis and DFT studies of an antitumor active spiro-oxindole, New J. Chem., 2015, 39, 8017–8027 RSC.
- A. S. Girgis, S. S. Panda, A. M. Srour, H. Farag, N. S. M. Ismail, M. Elgendy, A. K. Abdel-Aziz and A. R. Katritzky, Rational design, synthesis and molecular modeling studies of novel anti-oncological alkaloids against melanoma, Org. Biomol. Chem., 2015, 13, 6619–6633 RSC.
- L. Yan, W. Yang, L. Li, Y. Shen and Z. Jiang, A one-pot green synthesis of alkylidenesuccinimides, Chin. J. Chem., 2011, 29, 1906–1910 CrossRef CAS.
- E. M. Shalaby, A. S. Girgis, H. Farag, A. F. Mabied and A. N. Fitch, Synthesis, X-ray powder diffraction and DFT calculations of vasorelaxant active 3-(arylmethylidene)pyrrolidine-2,5-diones, RSC Adv., 2016, 6, 112950–112959 RSC.
- A. Kaur, M. Kaur and B. Singh, One-pot regioselective synthesis of novel 1-N-methyl-spiro[2,3′]oxindole-spiro[3,3′′]-1′′-N-arylpyrrolidine-2′′,5′′-dione-4-arylpyrrolidines through multicomponent 1,3-dipolar cycloaddition reaction of azomethine ylide, J. Heterocycl. Chem., 2015, 52, 827–833 CrossRef CAS.
- A. S. Girgis, A. F. Mabied, J. Stawinski, L. Hegazy, R. F. George, H. Farag, E. M. Shalaby and I. S. Ahmed Farag, Synthesis and DFT studies of an antitumor active spiro-oxindole, New J. Chem., 2015, 39, 8017–8027 RSC.
- E. M. Shalaby, A. S. Girgis, A. M. Moustafa, A. M. ElShaabiny, B. E. M. El-Gendy, A. F. Mabied and I. S. Ahmed Farag, Regioselective synthesis, stereochemical structure, spectroscopic characterization and geometry optimization of dispiro[3H-indole-3,2′-pyrrolidine-3′,3′′-piperidines], J. Mol. Struct., 2014, 1075, 327–334 CrossRef CAS.
- I. S. Ahmed Farag, A. S. Girgis, A. A. Ramadan, A. M. Moustafa and A. F. Mabied, 5-Chloro-5′′-(4-chlorobenzylidene)-4′-(4-chlorophenyl)-1′,1′′-dimethyldispiro[indoline-3,2′-pyrrolidine-3′,3′′-piperidine]-2,4′′-dione, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2014, 70, o379–o380 CrossRef PubMed.
- N. G. Fawzy, S. S. Panda, W. Fayad, M. A. El-Manawaty, A. M. Srour and A. S. Girgis, Novel curcumin inspired antineoplastic 1-sulfonyl-4-piperidones: design, synthesis and molecular modeling studies, Anti Canc. Agents Med. Chem., 2019, 19, 1069–1078 CrossRef PubMed.
- S. S. Panda, A. S. Girgis, S. J. Thomas, J. E. Capito, R. F. George, A. Salman, M. A. El-Manawaty and A. Samir, Synthesis, pharmacological profile and 2D-QSAR studies of curcumin-amino acid conjugates as potential drug candidates, Eur. J. Med. Chem., 2020, 196, 112293 CrossRef CAS PubMed.
- I. A. Seliem, S. S. Panda, A. S. Girgis, Y. I. Nagy, R. F. George, W. Fayad, N. G. Fawzy, T. S. Ibrahim, A. M. M. Al-Mahmoudy, R. Sakhuja and Z. K. M. Abdel-samii, Design, synthesis, antimicrobial and DNA gyrase inhibitory properties of fluoroquinolone-dichloroacetic acid hybrids, Chem. Biol. Drug Des., 2020, 95, 248–259 CrossRef CAS PubMed.
- N. G. Fawzy, S. S. Panda, W. Fayad, E. M. Shalaby, A. M. Srour and A. S. Girgis, Synthesis, human topoisomerase IIα inhibitory properties and molecular modeling studies of anti-proliferative curcumin mimics, RSC Adv., 2019, 9, 33761–33774 RSC.
- A. R. Katritzky, A. S. Girgis, S. Slavov, S. R. Tala and I. Stoyanova-Slavova, QSAR modeling, synthesis and bioassay of diverse leukemia RPMI-8226 cell line active agents, Eur. J. Med. Chem., 2010, 45, 5183–5199 CrossRef CAS PubMed.
- A. M. Srour, D. H. Dawood, M. N. A. Khalil and Z. M. Nofal, Synthesis and 2D-QSAR study of dispiropyrrolodinyl-oxindole based alkaloids as cholinesterase inhibitors, Bioorg. Chem., 2019, 83, 226–234 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1988241. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra03064c |
| This journal is © The Royal Society of Chemistry 2020 |