Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Adsorption performance of M-doped (M = Ti and Cr) gallium nitride nanosheets towards SO2 and NO2: a DFT-D calculation
Hossein Roohi * and
Nastaran Askari Ardehjani
* and
Nastaran Askari Ardehjani
Computational Quantum Chemistry Laboratory, Department of Chemistry, Faculty of Science, University of Guilan, Rasht, Iran. E-mail: hroohi@guilan.ac.ir; Fax: +98 131 3233262
First published on 24th July 2020
Abstract
The structure, adsorption characteristics, electronic properties, and charge transfer of SO2 and NO2 molecules on metal-doped gallium nitride nanosheets (M-GaNNSs; M = Ti and Cr) were scrutinized at the Grimme-corrected PBE/double numerical plus polarization (DNP) level of theory. Two types, MGa-GaNNSs and MN-GaNNSs, of doped nanostructures were found. The MGa sites are more stable than the MN sites. The results showed that adsorption of SO2 and NO2 molecules on TiGa,N-GaNNSs is energetically more favorable than the corresponding CrGa,N-GaNNSs. The stability order of complexes is energetically predicted to be as NO2–TiGa-GaNNS > NO2–TiN-GaNNS > SO2–TiGa-GaNNS > NO2–CrN-GaNNS > SO2–TiN-GaNNS > NO2–CrGa-GaNNS > SO2–CrN-GaNNS > SO2–CrGa-GaNNS. The electron population analysis shows that charge is transferred from MGa,N-GaNNSs to the adsorbed gases. The TiGa-GaNNS is more sensitive than the other doped nanostructures to NO2 and SO2 gases. It is estimated that the sensitivity of TiGa-GaNNS to NO2 gas is more than to SO2 gas.
1. Introduction
At present, air pollution is a significant factor limiting economic progression.1 The emission of toxicant gases into the air is a serious matter due to the dangers of these air pollutants.2 The source of air pollutants might be extensive in the Earth’s environment.3 Sulfur dioxide (SO2) and nitrogen dioxide (NO2) are noteworthy gaseous pollutants, discharged from natural and industrial procedures which have major environmental effects.4–6 Thus, specific harmful gas detecting will be a major advantage to daily life for all people.7–9For the first time in 2005, boron nitride (BN) nanosheets were forecast.10 The honeycomb samples of BN sheets have analogies similar to graphene with equal numbers of alternating boron and nitrogen atoms that exhibit remarkable properties.11 The electronic properties of BN sheets can be modified by B or N vacancies, Stone–Wales defects and doping heteroatoms.12–16 In recent years, different studies have been done via surface quantum engineering of BN nanosheets.17,18 For BN modification, the doped BN nanosheets were explored for developing a sensor for detecting harmful gases.19–21
Recently, III–V nanostructures have attracted great attention for their potential applications in novel electronic,22–24 optical,25–27 and electrochemical devices.28–30 One of the III–V nanostructures were gallium nitride nanosheets (GaNNSs) which have been theoretically predicted31–33 and then experimentally discovered.34,35 It was found that the GaNNSs have many remarkable properties such as a high surface area to volume ratio, high thermal stability and a tunable band gap indicating that GaNNSs have advantages in electronic usage such as effective gas sensor applications and so on.36 There are some experimental studies focusing on the GaN based NO2 and SO2 sensors.37–40 Bishop et al.41 suggested a double Schottky junction NO2 gas sensor based on BGaN/GaN. Triet et al. synthesized Al0.27Ga0.73N/GaN-based Schottky diode sensors for SO2 gas detection.42 For example, the adsorption capabilities of gallium nitride nanosheets towards noxious gases (such as HCN, NH3, H2S, H2, CO2 and H2O) have been described.43 Therefore, most of the research studies have focused on nanomaterials for increasing the adsorption of adsorbates on GaNNSs. For this purpose, the electronic properties of GaNNS can be modified by doping which generates more reactive adsorption sites.44,45 Transition metals such as Ti, Cr, Fe, Ni and Zn have been theoretically explored as dopants in GaNNSs to increase the adsorption properties towards CO harmful gases.46 The adsorption of H2S, NH3 and SO2 molecules on pure and doped GaNNSs has been considered using first-principles calculations. The results show that the metal doped GaNNSs are more suitable for gas molecules detection compared with the pure ones.47 The doping effect of metal atoms on the electronic properties of GaNNSs was studied for tuning the optoelectronic properties, gas adsorption, hydrogen storage and catalytic reaction.48–51 The electronic and optical properties of GaNNSs as a function of thickness and strain with predictive calculations were scrutinized.52 Based on the results reported about the magnetic properties of GaNNSs, the metallic and ferromagnetic properties of GaNNSs can be attained by semi-hydrogenation.53 The chemical oxidation of GaNNSs was explored by using first-principles calculations54 that show the oxygen adsorption mechanism can be useful for application in novel semiconducting materials. So, it would be attractive to continue investigating the promising applications of GaNNSs in gas sensors.
To the best of our knowledge, this is the first report on the adsorption of SO2 and NO2 molecules on the surface of Ti and Cr doped GaNNSs. The influence of transition metals doping on the adsorption behavior of SO2 and NO2 on the metal doped GaNNSs for exploring the possibility of using the doped GaNNSs as candidates for removing and sensing of these molecules was considered herein at the Grimme-corrected PBE/double numerical plus polarization (DNP) level of theory.
2. Computational details
In this theoretical research, the double numerical plus polarization (DNP) basis sets were selected implemented in the DMol3 package.55,56 The periodic spin-unrestricted DFT calculation is employed using generalized-gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional.57 The density functional semi-core pseudopotentials (DSPP) were generated by fitting all-electron relativistic DFT results.58To consider the van der Waals (vdW) interactions, an empirical dispersion-corrected density functional theory (DFT-D) was used in the calculations. The Brillouin zone integration was sampled using a 10 × 10 × 1 Monkhorst–Pack grid. A convergence tolerance of energy of 1.0 × 10−5 Ha, maximum force of 0.001 Ha per Å and maximum displacement of 0.005 Å were employed in all the geometry optimizations. To get reliable results, the real space global orbital cutoff radius was set as high as 5.2 Å and the smearing of electronic occupations to be 0.005 Ha.
To calculate the adsorption energies (AE) of the SO2 and NO2 molecules on the pure and metal doped GaNNSs, the following equation is given:
| AE = ET − [ES + Em] | (1) |
3. Results and discussion
3.1. Adsorption of SO2 and NO2 gas molecules over pure GaNNSs
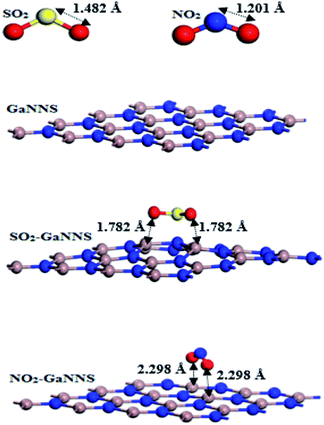
The optimized geometries of adsorbed molecules and gallium nitride nanosheets are displayed in Fig. 1. As demonstrated in Fig. 1, the calculated bond lengths of X![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (X = N and S) in free SO2 and NO2 molecules are 1.482 Å and 1.201 Å, respectively, and that of the Ga–N bond length in the optimized geometry of GaNNS is 1.861 Å.
O (X = N and S) in free SO2 and NO2 molecules are 1.482 Å and 1.201 Å, respectively, and that of the Ga–N bond length in the optimized geometry of GaNNS is 1.861 Å.
To find the most stable complexes obtained from adsorption of SO2 and NO2 gas molecules on the GaNNS, several configurations of SO2 and NO2 molecules on top of the GaNNSs are explored. The most stable adsorption complexes are illustrated in Fig. 1. After optimization, the C2 axis of the SO2 molecule is parallel to the GaNNS and that of the NO2 molecule is perpendicular to the nanosheet. As presented in Table 1, the nearest distances between the SO2 and NO2 molecules with SO2–GaNNS and NO2–GaNNS are 1.782 Å and 2.298 Å, respectively.
| Configurations | AE (kcal mol−1) | D (Å) | Q (e) | CT (e) | Band gap (eV) |
|---|---|---|---|---|---|
| Pure GaNNS | — | — | 0.39Ga (−0.39)N | — | 2.52 |
| SO2–GaNNS | −27.22 | 1.782 | 0.40 (−0.38) | 0.17 | 2.51 |
| NO2–GaNNS | −11.86 | 2.298 | 0.39 (−0.39) | 0.09 | 0.29 |
The values of the adsorption energies (AEs) are −27.22 and −11.86 kcal mol−1 for SO2–GaNNS and NO2–GaNNS complexes, in good agreement with the smaller SO2–GaNNS distance obtained. Hence, the SO2 and NO2 molecules are chemically adsorbed on the GaNNSs. The adsorption energy for the SO2 molecule on the GaNNS is comparable with those found for graphene (−6.45 kcal mol−1)59 and boron nitride nanosheet (−7.14 kcal mol−1).21 For adsorption of NO2 on graphene, the calculated adsorption energy is −11.06 kcal mol−1.41
The Hirshfeld charges on the Ga and N atoms in pristine GaNNS are 0.39e and −0.39e, respectively, which change to 0.40e and −0.38e in SO2–GaNNS, and 0.39e and −0.39e in NO2–GaNNS. The charges on S and O change from 0.38e and −0.19e in free SO2 to 0.33 e and −0.25e in SO2–GaNNS, respectively. Besides, the charges on N and O are 0.17e and −0.09e which change to 0.11e and −0.10e in NO2-GaNNS. This reveals that oxygen atoms in SO2–GaNNS and NO2–GaNNS complexes have the main contribution to charge transfer between the gas and nanosheet. The charge analyses show that the 0.17e and 0.09e charges are shifted from the GaNNSs to the SO2 and NO2 molecules, respectively, in good agreement with greater AE found for the SO2–GaNNS complex.
The negative AEs demonstrate the orbital interactions between the gases and GaNNS. The Mulliken electron populations of the total and each of the s, p and d orbitals before and after interactions are given in Table 2. Inspection of the s, p and d orbital contributions in the free gases and in the SO2–GaNNS and NO2–GaNNS complexes indicate that the p orbital of the S atom in SO2–GaNNS and O atom in NO2–GaNNS have the most contribution in the interaction of molecules with the d orbital of Ga and p orbital of N atoms in the GaNNS. Comparison of the total electron population of orbitals shows that the population increases by 0.352e for SO2 and 0.169e for NO2 after interaction of the gas with the surface. This indicates that the adsorbates will get electrons from the GaNNSs. The change in the electronic population of the orbitals in SO2–GaNNS is greater than for NO2–GaNNS, in good agreement with the greater AE and Hirshfeld charge transfer values found for SO2–GaNNS compared with NO2–GaNNS.
| Total pop. | Orbital | Total pop. | Orbital | ||||||
|---|---|---|---|---|---|---|---|---|---|
| s | p | d | s | p | d | ||||
| Free SO2 | NO2–CrGa-GaNNS | ||||||||
| S | 15.558 | 5.838 | 8.872 | 0.848 | N | 6.72 | 3.637 | 2.942 | 0.14 |
| O | 8.22 | 3.836 | 4.346 | 0.04 | O | 8.346 | 3.839 | 4.461 | 0.047 |
| Free NO2 | Cr | 13.317 | 2.592 | 6.454 | 4.272 | ||||
| N | 6.646 | 3.449 | 3.028 | 0.17 | NO2–CrN-GaNNS | ||||
| O | 8.176 | 3.851 | 4.272 | 0.055 | N | 6.759 | 3.684 | 2.951 | 0.124 |
| SO2–GaNNS | O | 8.343 | 3.852 | 4.443 | 0.05 | ||||
| S | 15.514 | 5.674 | 8.922 | 0.918 | Cr | 13.917 | 2.71 | 6.441 | 4.767 |
| O | 8.424 | 3.846 | 4.546 | 0.032 | SO2–TiGa-GaNNS | ||||
| NO2–GaNNS | S | 15.613 | 5.802 | 9.203 | 0.608 | ||||
| N | 6.669 | 3.541 | 2.972 | 0.156 | O | 8.364 | 3.834 | 4.499 | 0.031 |
| O | 8.25 | 3.841 | 4.36 | 0.049 | Ti | 11.018 | 2.433 | 6.304 | 2.281 |
| TiGa-GaNNS | SO2–TiN-GaNNS | ||||||||
| Ti | 11.244 | 2.658 | 6.202 | 2.384 | S | 15.651 | 5.82 | 9.29 | 0.542 |
| TiN-GaNNS | O | 8.384 | 3.832 | 4.522 | 0.03 | ||||
| Ti | 11.465 | 2.612 | 6.347 | 2.505 | Ti | 11.322 | 2.51 | 6.383 | 2.43 |
| CrGa-GaNNS | SO2–CrGa-GaNNS | ||||||||
| Cr | 13.357 | 2.655 | 6.345 | 4.357 | S | 15.706 | 5.81 | 9.364 | 0.533 |
| CrN-GaNNS | O | 8.399 | 3.837 | 4.531 | 0.03 | ||||
| Cr | 14.014 | 2.888 | 6.232 | 4.894 | Cr | 13.286 | 2.589 | 6.463 | 4.234 |
| NO2–TiGa-GaNNS | SO2–CrN-GaNNS | ||||||||
| N | 6.72 | 3.644 | 2.932 | 0.142 | S | 15.6 | 5.802 | 9.198 | 0.6 |
| O | 8.4 | 3.838 | 4.518 | 0.042 | O | 8.423 | 3.856 | 4.532 | 0.034 |
| Ti | 11.048 | 2.45 | 6.3 | 2.3 | Cr | 13.842 | 2.714 | 6.462 | 4.667 |
| NO2–TiN-GaNNS | |||||||||
| N | 6.734 | 3.666 | 2.934 | 0.134 | |||||
| O | 8.382 | 3.86 | 4.478 | 0.046 | |||||
| Ti | 11.386 | 2.516 | 6.44 | 2.43 | |||||
3.2. Ti and Cr doped GaNNSs
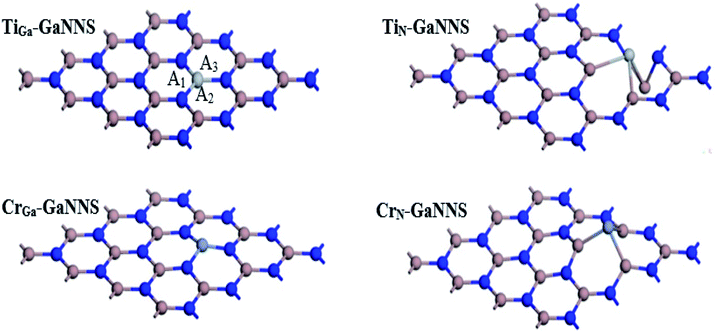
In order to investigate the effect of metal doping on the geometrical and electronic properties of the GaNNSs, one of the central atoms in the nanosheet was substituted by Ti and Cr metal atoms. Hereafter, MGa-GaNNS and MN-GaNNS denote that Ga and N atoms in GaNNS have been substituted by M metal atoms, respectively.The optimized structures of MN(Ga)-GaNNSs are illustrated in Fig. 2. The average bond distances between the metal atoms and the neighboring atoms are given in Table 3. The results show that the M–Ga bonds in MN-GaNNS nanostructures are longer than the M–N bonds in MGa-GaNNSs. For example, the Ti–Ga and Cr–Ga bonds are longer than the Ti–N and Cr–N bonds by about 0.92 and 0.62 Å, respectively. Accordingly, it is predicted that binding of the metal to the nanosheet is stronger for MGa-GaNNS than MN-GaNNS. Fig. 2 presents the three bond angles A1, A2 and A3 around the M atoms of the NS and their average values are listed in Table 3. The averages of the three bond angles are 120.0°, 119.9°, 83.1° and 87.1° in TiGa-GaNNS, CrGa-GaNNS, TiN-GaNNS and CrN-GaNNS, respectively.
| Configurations | Bond distances (Å) | Bond angle (°) | Binding energies (kcal mol−1) | Q (e) |
|---|---|---|---|---|
| GaNNS | 1.862 | 118.2 | — | — |
| TiGa-GaNNS | 1.913 | 120.0 | −330.5 | 0.30 |
| TiN-GaNNS | 2.838 | 83.1 | −236.1 | 0.26 |
| CrGa-GaNNS | 1.883 | 119.9 | −246.9 | 0.43 |
| CrN-GaNNS | 2.505 | 87.1 | −61.6 | 0.28 |
The binding energies (BEs) of TiGa-GaNNS, TiN-GaNNS, CrGa-GaNNS and CrN-GaNNS are −330.5, −236.1, −246.9 and −61.6 kcal mol−1, respectively (Table 3). It is found that the BEs increase in the order MGa-GaNNSs > MN-GaNNSs so that the value for the TiGa-GaNNS structure is greater than other ones. Therefore, the Ga sites for M doping are energetically more appropriate than N sites. The sequence of BEs for the MGa-GaNNS series is TiGa > CrGa and that of the MN-GaNNS series is TiN > CrN. The more-negative BE indicates that the adatom is easier to be incorporated into the GaNNS and M doped GaNNSs are stable.
The Hirshfeld44 charge values of M atoms for M-GaNNSs are shown in Table 3. The results show that the charge of the M atom in all M-doped GaNNSs is positive, indicating that the GaNNS in many cases acts as an electron-withdrawing support. The considerable electron transfer from the metal atom to the GaNNS leads to the strong bonding between the M atom and its neighbor atoms and stabilization of single-metal doped GaNNS. Besides, the positive charge of the M atom in MGa-GaNNSs is more than MN-GaNNSs, in good agreement with their binding energy (BE) and the average value of the M–NS bond length. Thus, because of greater transfer of charge between the nanosheet and M atoms in MGa-GaNNSs with respect to the corresponding MN-GaNNSs, the MGa-GaNNSs are predicted to be more stable than MN-GaNNSs.
3.3. Adsorption of SO2 and NO2 gas molecules over Ti-doped GaNNSs
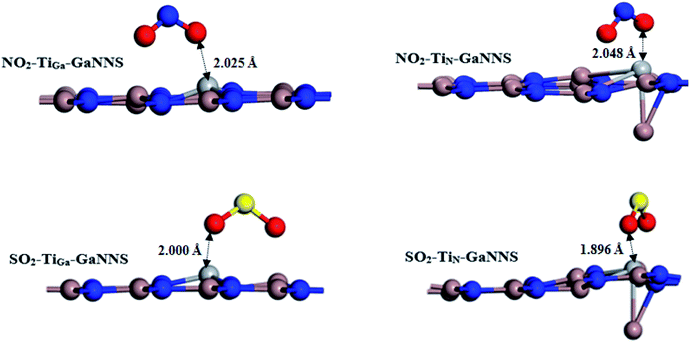
Now, we investigate the adsorption of SO2 and NO2 gas molecules on the Ti-doped GaNNSs as displayed in Fig. 3. Our results show that for the SO2–TiGa-GaNNS and NO2–TiGa-GaNNS complexes, the averages of the three binding distances (Ti–N) are 1.901 Å and 1.899 Å, respectively. For the SO2–TiN-GaNNS and NO2–TiN-GaNNS complexes, the averages of the three binding distances (Ti–Ga) are 2.983 Å and 2.949 Å, respectively. The calculated averages of the bond lengths of the S–O and N–O bonds in SO2–TiGa-GaNNS, SO2–TiN-GaNNS, NO2–TiGa-GaNNS and NO2–TiN-GaNNS have increased from 1.482 and 1.201 Å to 1.576, 1.606, 1.274 and 1.288 Å, respectively. These results show that the change in the SO2 and NO2 bond lengths upon adsorption on the TiN-GaNNS is greater than those of TiGa-GaNNS.| Configurations | AE (kcal mol−1) | D (Å) | CTa (e) |
|---|---|---|---|
| a Absolute value of the sum of atomic charges in complexed gases. | |||
| NO2 | 0.00 | ||
| NO2–TiGa-GaNNS | −76.83 | 2.025 | 0.21 |
| NO2–TiN-GaNNS | −70.68 | 2.048 | 0.25 |
| NO2–CrGa-GaNNS | −54.79 | 1.912 | 0.22 |
| NO2–CrN-GaNNS | −60.81 | 1.952 | 0.31 |
| SO2 | |||
| SO2–TiGa-GaNNS | −61.06 | 2.000 | 0.16 |
| SO2–TiN-GaNNS | −58.36 | 1.896 | 0.21 |
| SO2–CrGa-GaNNS | −40.46 | 1.834 | 0.27 |
| SO2–CrN-GaNNS | −53.28 | 2.124 | 0.26 |
The modified surface of Ti-doped GaNNSs facilitates the doped region to interact with approaching SO2 and NO2 molecules because of the higher chemical reactivity of the doped M atom. The results show that the SO2⋯Ti distance of SO2–TiGa-GaNNS complex is larger than SO2–TiN-GaNNS ones. Also, the NO2⋯Ti distance for the NO2–TiN-GaNNS complex is larger than for NO2–TiGa-GaNNS, indicating that the interaction in these complexes is stronger than in other ones.
The range of adsorption energies for SO2 and NO2 adsorbed on Ti-doped GaNNSs was between −58.36 to −61.06 and −70.68 to −76.83 kcal mol−1, respectively. The negative value of the AE indicates that adsorption of SO2 and NO2 on Ti doped GaNNSs is an exothermic process. It is found that SO2 and NO2 molecules are adsorbed on TiN-GaNNSs in the sequence NO2–TiN-GaNNS > SO2–TiN-GaNNS and on the TiGa-GaNNSs in the order NO2–TiGa-GaNNS > SO2–TiGa-GaNNS. Besides, it is found that the SO2 and NO2 adsorption energy values on TiGa-GaNNSs are greater than on TiN-GaNNSs. The obtained results indicate that the adsorption capability of TiGa-GaNNSs is greater than that of TiN-GaNNSs. Our results show that SO2 and NO2 molecules are chemically adsorbed on all TiGa,N-GaNNSs.
The Hirshfeld population analysis presents that the charges are transferred from the TiGa,N-GaNNSs complexes to the SO2 and NO2 molecules. In other words, SO2 and NO2 act as electron acceptors. There is a correlation between charge transfer values and adsorption energy in the process of adsorption of SO2 and NO2 on the GaNNSs. Comparison of charge transfer values between M-doped GaNNSs and SO2 and NO2 molecules demonstrate that the value for adsorption of NO2 is greater than that of the corresponding SO2 one, with the exception of that obtained for CrGa-GaNNS. This indicates that other parameters than charge transfer are responsible for the stability of the complexes.
The electron populations of orbitals given in Table 2 show that population of d-orbitals as well as total population of the M metals decrease upon the interaction of gases with TiGa,N-GaNNSs. Besides, total electron population of orbitals in gases increases after adsorption of gases on the surface. This finding reveals that gases will take the electrons from TiGa,N-GaNNSs. After adsorption of gases, total electron populations of orbitals of NO2 (0.522e for NO2–TiGa-GaNNS and 0.500e for NO2–TiN-GaNNS) are greater than those of SO2 (0.343e for SO2–TiGa-GaNNS and 0.421e for SO2–TiN-GaNNS), in good agreement with the greater AEs found for NO2–TiGa,N-GaNNS complexes. The decrease in total electron population of Ti before and after interaction is −0.196e and −0.079e in NO2–TiGa-GaNNS and NO2–TiN-GaNNS, respectively, in good agreement with the greater AE found for NO2–TiGa-GaNNS. Besides, the total electron population of Ti after interaction with SO2 decreases by −0.226e and −0.143e in SO2–TiGa-GaNNS and SO2–TiN-GaNNS, respectively, in good agreement with the greater AE found for SO2–TiGa-GaNNS.
3.4. Adsorption of SO2 and NO2 gas molecules over Cr-doped GaNNSs
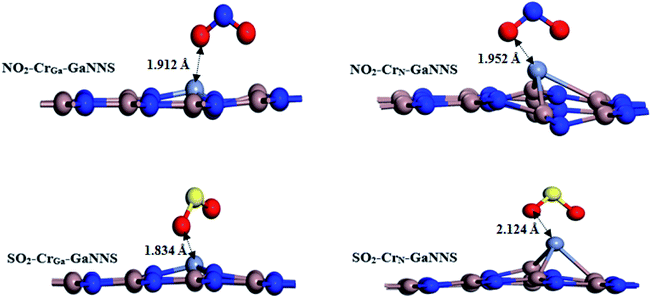
For the SO2–CrGa-GaNNS and NO2–CrGa-GaNNS complexes, the averages of three binding distances (Ti–N) are 1.834 Å and 1.912 Å, respectively. The calculated averages of bond lengths of S–O and N–O bonds in SO2–CrGa-GaNNS, SO2–CrN-GaNNS, NO2–CrGa-GaNNS and NO2–CrN-GaNNS have increased from 1.482 Å to 1.527, 1.574, 1.277 and 1.307 Å, respectively. For the SO2–CrN-GaNNS and NO2–CrN-GaNNS complexes, the averages of the three binding distances (Ti–Ga) are 2.124 Å and 1.952 Å, respectively. These results show that the change in the SO2 and NO2 bond lengths upon adsorption on the CrN-GaNNS is greater than that for CrGa-GaNNS. The optimized structures of the NO2 and SO2 adsorbed on Cr-doped GaNNSs are illustrated in Fig. 4. The modified surface of the Cr-doped GaNNSs facilitates the doped region to interact with approaching SO2 and NO2 molecules because of the higher chemical reactivity of the doped M atom. The results show that the SO2⋯Cr distance for the SO2–CrN-GaNNS complex is larger than that for the SO2–CrGa-GaNNS one. Also, the NO2⋯Cr distance for the NO2–CrN-GaNNS complex is larger than that for NO2–CrGa-GaNNS, indicating that interaction in these complexes is stronger than for other ones.The adsorption energies for SO2 adsorbed on CrGa-GaNNS and CrN-GaNNS are −40.46 and −53.28 kcal mol−1 and those for NO2 are −54.79 and −60.81 kcal mol−1, respectively. The negative value of the AE indicates that adsorption of SO2 and NO2 on Cr doped GaNNSs is an exothermic process. It is found that the ability of CrN-GaNNS towards adsorption of SO2 and NO2 molecules is in the sequence NO2–CrN-GaNNS > SO2–CrN-GaNNS and that of CrGa-GaNNS is in the order NO2–CrGa-GaNNS > SO2–CrGa-GaNNS. In addition, it is found that SO2 and NO2 adsorption energies on CrN-GaNNS are greater than on CrGa-GaNNS. The obtained results indicate that the adsorption capability of CrN-GaNNS is greater than CrGa-GaNNS. Our results show that SO2 and NO2 molecules are chemically adsorbed on all M-doped GaNNSs.
The Hirshfeld population analysis shows that the charges are transferred from the CrGa,N-GaNNS complexes to the SO2 and NO2 molecules. As can be seen in Table 4, the CT values are 0.22e, 0.31e, 0.27e and 0.26e in NO2–CrGa-GaNNS, NO2–CrN-GaNNS, SO2–CrGa-GaNNS and SO2–CrN-GaNNS, respectively. This finding reveals that the charge transferred from the nanosheet to the gas in NO2–CrN-GaNNS is greater than in other complexes, in good agreement with the greater AE obtained for these complexes. It should be noted that other parameters than charge transfer can affect the adsorption of gases.
Analysis of the electron population of orbitals involved in the interaction between gases and nanosheets given in Table 2 reveals that the total electron population of Cr decreases by −0.040e, −0.097e, −0.071e and −0.172e in NO2–CrGa-GaNNS, NO2–CrN-GaNNS, SO2–CrGa-GaNNS, SO2–CrN-GaNNS, respectively, in good agreement with the greater AE found for NO2(SO2)–CrN-GaNNSs compared with NO2(SO2)–CrGa-GaNNSs. In addition, the results show that the population of d-orbitals of Cr decreases and those of the NO2 and SO2 gases increase upon interaction with CrGa,N-GaNNSs. This finding demonstrates that gases will get electrons from CrGa,N-GaNNSs, in good agreement with the computed Hirshfeld charge transfer from the surface to adsorbed gases.
3.5. HOMO and LUMO based electronic properties
There is an obvious difference between the electronic properties of doped and un-doped GaNNSs. As can be seen in Table 5, compared to pristine GaNNS, the energy of the highest occupied molecule orbital (HOMO) increases and that of the lowest un-occupied molecular orbital (LUMO) decreases in doped GaNNS so that the amount of increase in HOMO is greater than decrease in LUMO. After adsorption of NO2, the energies of the HOMO and LUMO decrease, but the LUMO energy shows a further decrease. In the case of SO2, adsorption of gas on the Ti-doped GaNNS decreases both the HOMO and LUMO, but its adsorption on the Cr-doped GaNNS increases them. These changes in HOMO and LUMO energy levels lead to a change in the HOMO–LUMO gap and, in turn, in the electronic properties of GaNNS.| Configurations | EHOMO (eV) | ELUMO (eV) | Eg (eV) | ΔEg (eV) |
|---|---|---|---|---|
| GaNNS | −5.75 | −3.23 | 2.52 | — |
| TiGa-GaNNS | −3.76 | −3.28 | 0.47 | 2.05 |
| TiN-GaNNS | −4.50 | −3.60 | 0.90 | 1.62 |
| CrGa-GaNNS | −4.28 | −3.64 | 0.64 | 1.88 |
| CrN-GaNNS | −4.26 | −3.78 | 0.47 | 2.05 |
| NO2–GaNNS | −5.83 | −5.53 | 0.29 | 2.23 |
| NO2–TiGa-GaNNS | −5.90 | −3.62 | 2.28 | 1.81 |
| NO2–TiN-GaNNS | −4.96 | −4.01 | 0.95 | 0.05 |
| NO2–CrGa-GaNNS | −4.52 | −3.87 | 0.65 | 0.01 |
| NO2–CrN-GaNNS | −5.08 | −4.18 | 0.91 | 0.44 |
| SO2–GaNNS | −5.90 | −3.37 | 2.11 | 0.41 |
| SO2–TiGa-GaNNS | −4.69 | −3.29 | 1.39 | 0.92 |
| SO2–TiN-GaNNS | −4.59 | −3.47 | 1.11 | 0.21 |
| SO2–CrGa-GaNNS | −3.45 | −2.91 | 0.53 | 0.11 |
| SO2–CrN-GaNNS | −3.82 | −3.53 | 0.28 | 0.19 |
The results indicate that the band gap energies in both MGa,N doped GaNNSs are smaller than the pure GaNNS, making it more conductive. The results given in Table 5 demonstrate that the band gap value of pristine GaNNS is 2.52 eV that changes to 2.11 eV in SO2–GaNNS and 0.29 eV in NO2–GaNNS. Because of the greater decrease in the LUMO level in NO2–GaNNS with respect to that of SO2–GaNNS, the change in the band gap for SO2–GaNNS is lesser than for NO2–GaNNS. Therefore, it is predicted that GaNNS is more sensitive to NO2 gas than SO2 gas. So, the large changes in band gap value for GaNNS (2.52 to 0.29 eV) with NO2 gas molecule adsorption can lead to a significant change in electrical conductivity.
The electronic properties of doped GaNNSs are affected by the adsorption of SO2 and NO2 molecules. Upon adsorption of SO2 and NO2 molecules on the TiGa,N-GaNNS complexes the energy gap decreases in comparison with the pristine GaNNS. The energy gap values are in the sequences SO2–TiN-GaNNS (1.11 eV) > NO2–TiN-GaNNS (0.95 eV) and NO2–TiGa-GaNNS (2.28 eV) > SO2–TiGa-GaNNS (1.39 eV). Reduction of the band gap for SO2–TiN-GaNNS is more than for NO2–TiN-GaNNS and that for NO2–TiGa-GaNNS is more than for SO2–TiGa-GaNNS. Therefore, it can be concluded that the sensitivity of TiN-GaNNS to SO2 gas is greater than to NO2 gas. Besides, for TiGa-GaNNS, it is predicted that the sensitivity to NO2 gas is more than to SO2 gas. It is a well-known issue that reduction of the energy gap enhances the electrical conductivity. Thus, the electrical conductivities are predicted to be in the order SO2–TiN-GaNNS > NO2–TiN-GaNNS and NO2–TiGa-GaNNS > SO2–TiGa-GaNNS.
Also, after adsorption of SO2 and NO2 molecules on the CrGa,N-GaNNS complexes the band gap decreases in comparison with the pristine GaNNS. The band gap values are in sequence NO2–CrN-GaNNS (0.91 eV) > SO2–CrN-GaNNS (0.28 eV) and NO2–CrGa-GaNNS (0.65 eV) > SO2–CrGa-GaNNS (0.53 eV). The reduction in band gap for SO2–CrGa-GaNNS is more than for NO2–CrGa-GaNNS and that for NO2–CrN-GaNNS is more than for SO2–CrN-GaNNS. In consequence, the conductivities of SO2–CrGa-GaNNS and NO2–CrN-GaNNS are greater than NO2–CrGa-GaNNS and SO2–CrN-GaNNS, respectively. Also, from the changes in band gap energy values, it is forecast that the sensitivity of CrN-GaNNS to NO2 gas is more than to SO2 gas.
The top and side view plots of the electron density are illustrated in Fig. 5. An orbital overlap can be observed between the NO2 (SO2) gas molecules and the GaN sheets, revealing the occurrence of a strong chemisorption. It is clearly displayed that the electrons dominantly accumulate in the region between gases and M-GaNNSs. These results are in accordance with the obtained adsorption energies and binding distances. The orbital mixing and the charge transfer are expected to bring significant changes to the electronic structure of the GaN nanosheets which is beneficial for sensing applications. The isovalue for adsorption of gas molecules on GaNNS is 0.2e Å−3.
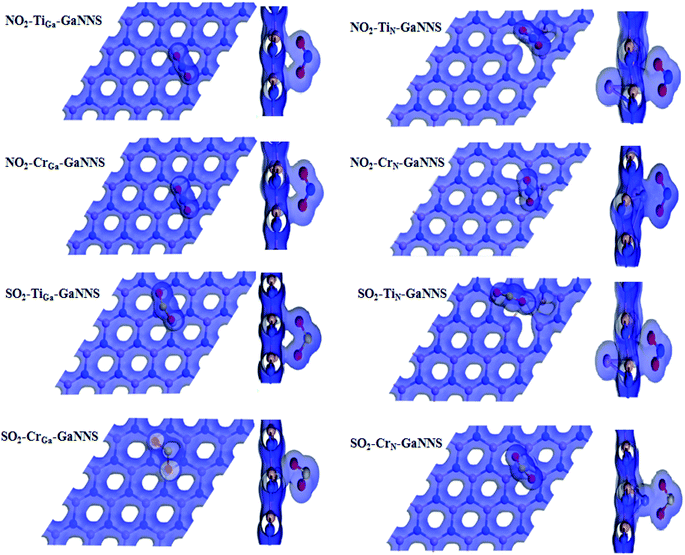 | ||
| Fig. 5 The electron density schema (top and side views) of SO2 or NO2 adsorbed on doped GaNNSs with isovalue = 0.2e Å−3. | ||
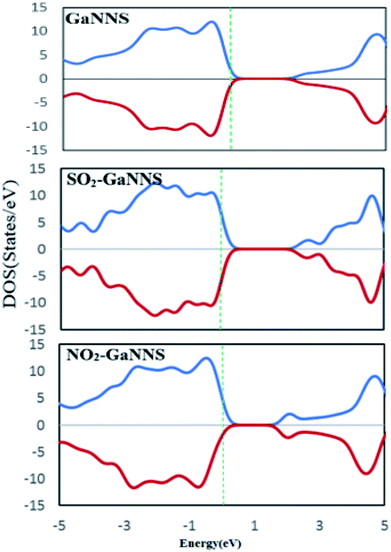
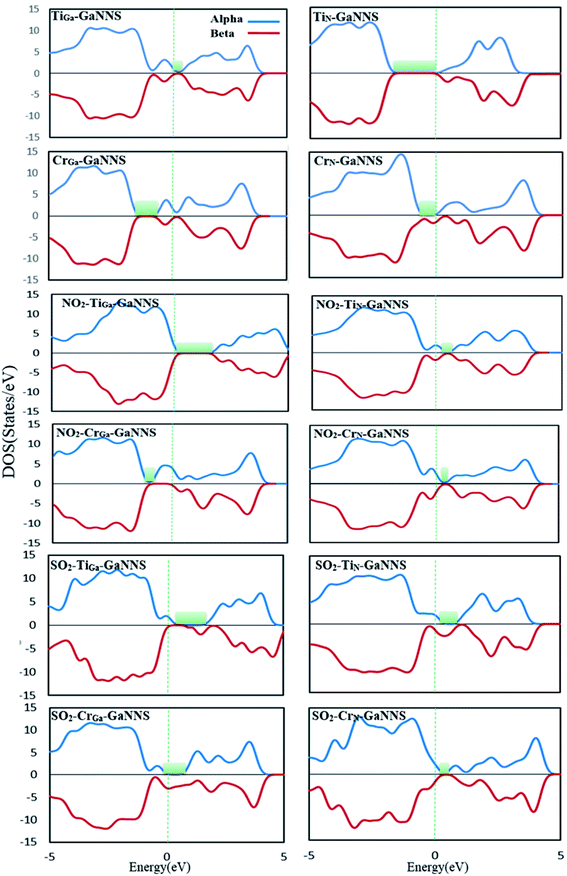
To obtain a better understanding about the electronic properties of the complexes, densities of states (DOSs) of the nanostructures are calculated and visualized in Fig. 6 and 7. As can be observed, the GaNNS, NO2–GaNNS, SO2–GaNNS, NO2–TiGa-GaNNS, NO2–TiN-GaNNS and NO2–CrN-GaNNS nanostructures are nonmagnetic systems because the spin-up and spin-down in the DOS plots are the same as each other. From this figure, it can be found that new states appear in the band gap regions of NO2–CrGa-GaNNS, SO2–TiGa-GaNNS, SO2–TiN-GaNNS, SO2–CrGa-GaNNS and SO2–CrN-GaNNS. Since the spin-up and spin-down DOSs are different, NO2–CrGa-GaNNS, SO2–TiGa-GaNNS, SO2–TiN-GaNNS, SO2–CrGa-GaNNS and SO2–CrN-GaNNS have magnetic properties.
4. Conclusions
DFT calculations are performed to consider the adsorption of sulfur dioxide and nitrogen dioxide molecules on metal-doped gallium nitride nanosheets. The results present that adsorption of NO2 on MN-GaNNS and MGa-GaNNS is energetically more favorable than that of SO2 on corresponding NSs. A brief comparison of AEs of SO2 and NO2 molecules on Ti and Cr doped GaNNSs indicates that the AEs of NO2 and SO2 on TiGa,N-GaNNSs are greater than on CrGa,N-GaNNSs. The electron populations of orbitals were calculated before and after interaction. There is a correlation between the change in electron population of orbitals of adsorbate and adsorbent and the AE obtained for complexes. The electron population analysis shows that charge is transferred from MGa,N-GaNNSs to the adsorbed gases. After the adsorption of SO2 and NO2 molecules, the electronic properties of the pure and doped GaNNSs indicate the considerable changes in the conductivity of the nanosheets. Furthermore, the sensitivity of TiGa-GaNNS is predicted to be more than for other NSs toward SO2 gas. It is estimated that the sensitivity of TiGa-GaNNS to NO2 gas is more than to SO2 gas. Therefore, these results show that GaNNS-based materials can be used as noxious gas sensors.Conflicts of interest
The all authors declare that they have no conflict of interest.Acknowledgements
This work was supported by the University of Guilan and Iran National Science Foundation through research facilities and financial grants.References
- Y. Zhang, S. Yuan, X. Feng, H. Li, J. Zhou and B. Wang, J. Am. Chem. Soc., 2016, 138, 5785–5788 CrossRef CAS PubMed.
- A. Tiwary and I. Williams, Air pollution: measurement, modelling and mitigation, CRC Press, 2018 Search PubMed.
- V. Ramanathan and Y. Feng, Atmos. Environ., 2009, 43, 37–50 CrossRef CAS.
- R. S. Scorer, Pollution in the air: problems, policies and priorities, Routledge, 2019 Search PubMed.
- J. A. Bernstein, N. Alexis, C. Barnes, I. L. Bernstein, A. Nel, D. Peden, D. Diaz-Sanchez, S. M. Tarlo and P. B. Williams, J. Allergy Clin. Immunol., 2004, 114, 1116–1123 CrossRef PubMed.
- R. Bascom, P. A. Bromberg, D. L. Costa, R. Devlin, D. W. Dockery, M. W. Frampton, W. Lambert, J. M. Samet, F. E. Speizer and M. Utell, Am. J. Respir. Crit. Care Med., 1996, 153, 477–498 CrossRef PubMed.
- P. M. Mannucci, S. Harari, I. Martinelli and M. Franchini, Intern. Emerg. Med., 2015, 10, 657–662 CrossRef PubMed.
- L. B. Lave and E. P. Seskin, Air pollution and human health, Routledge, 2013, vol. 6 Search PubMed.
- M. Kampa and E. Castanas, Environ. Pollut., 2008, 151, 362–367 CrossRef CAS PubMed.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V Khotkevich, S. V Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed.
- L. H. Li and Y. Chen, Adv. Funct. Mater., 2016, 26, 2594–2608 CrossRef CAS.
- Q. Zeng, H. Wang, W. Fu, Y. Gong, W. Zhou, P. M. Ajayan, J. Lou and Z. Liu, Small, 2015, 11, 1868–1884 CrossRef CAS PubMed.
- R. Ansari, S. Malakpour and S. Ajori, Superlattices Microstruct., 2014, 72, 230–237 CrossRef CAS.
- A. Bhattacharya, S. Bhattacharya and G. P. Das, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 35415 CrossRef.
- S. Lin, X. Ye, R. S. Johnson and H. Guo, J. Phys. Chem. C, 2013, 117, 17319–17326 CrossRef CAS.
- X. Gao, S. Wang and S. Lin, ACS Appl. Mater. Interfaces, 2016, 8, 24238–24247 CrossRef PubMed.
- Q. Tang, Z. Zhou and Z. Chen, J. Phys. Chem. C, 2011, 115, 18531–18537 CrossRef CAS.
- P. Thangasamy, M. Santhanam and M. Sathish, ACS Appl. Mater. Interfaces, 2016, 8, 18647–18651 CrossRef CAS PubMed.
- M. Samadizadeh, A. A. Peyghan and S. F. Rastegar, Chin. Chem. Lett., 2015, 26, 1042–1045 CrossRef CAS.
- M. D. Esrafili and F. A. Rad, Vacuum, 2019, 166, 127–134 CrossRef CAS.
- F. Behmagham, E. Vessally, B. Massoumi, A. Hosseinian and L. Edjlali, Superlattices Microstruct., 2016, 100, 350–357 CrossRef CAS.
- A. A. Tonkikh, E. N. Voloshina, P. Werner, H. Blumtritt, B. Senkovskiy, G. Güntherodt, S. S. P. Parkin and Y. S. Dedkov, Sci. Rep., 2016, 6, 23547 CrossRef CAS PubMed.
- N. Izyumskaya, D. O. Demchenko, S. Das, Ü. Özgür, V. Avrutin and H. Morkoç, Adv. Electron. Mater., 2017, 3, 1600485 CrossRef.
- G. B. Pinhal, N. L. Marana, G. S. L. Fabris and J. R. Sambrano, Theor. Chem. Acc., 2019, 138, 31 Search PubMed.
- H. Vovusha and B. Sanyal, RSC Adv., 2015, 5, 4599–4608 RSC.
- B. A. Ravan and H. Jafari, Comput. Condens. Matter, 2019, e00416 CrossRef.
- K. Wang, Q. Xiao, Q. Xie, L. Wang, T. He, H. Chen and J. Shi, J. Electron. Mater., 2019, 48, 5135–5142 CrossRef CAS.
- S. Y. F. Zhao, G. A. Elbaz, D. K. Bediako, C. Yu, D. K. Efetov, Y. Guo, J. Ravichandran, K.-A. Min, S. Hong, T. Taniguchi, K. Watanabe, L. E. Brus, X. Roy and P. Kim, Nano Lett., 2018, 18, 460–466 CrossRef CAS PubMed.
- T. Ouyang, Z. Qian, R. Ahuja and X. Liu, Appl. Surf. Sci., 2018, 439, 196–201 CrossRef CAS.
- A. Sengupta, Appl. Surf. Sci., 2018, 451, 141–147 CrossRef CAS.
- A. K. Singh and R. G. Hennig, Appl. Phys. Lett., 2014, 105, 51604 CrossRef.
- A. K. Singh, H. L. Zhuang and R. G. Hennig, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 245431 CrossRef.
- H. Zhang, F.-S. Meng and Y.-B. Wu, Solid State Commun., 2017, 250, 18–22 CrossRef CAS.
- Z. Y. Al Balushi, K. Wang, R. K. Ghosh, R. A. Vilá, S. M. Eichfeld, J. D. Caldwell, X. Qin, Y.-C. Lin, P. A. DeSario and G. Stone, Nat. Mater., 2016, 15, 1166 CrossRef CAS PubMed.
- N. A. Koratkar, Nat. Mater., 2016, 15, 1153 CrossRef CAS PubMed.
- Y. Yong, X. Su, H. Cui, Q. Zhou, Y. Kuang and X. Li, ACS Omega, 2017, 2(12), 8888–8895 CrossRef CAS PubMed.
- M. Lim, S. Mills, B. Lee and V. Misra, ECS J. Solid State Sci. Technol., 2015, 4, S3034–S3037 CrossRef CAS.
- Y. Halfaya, C. Bishop, A. Soltani, S. Sundaram, V. Aubry, P. L. Voss, J.-P. Salvestrini and A. Ougazzaden, Sensors, 2016, 16, 273 CrossRef PubMed.
- M. A. H. Khan, B. Thomson, R. Debnath, A. Rani, A. Motayed and M. V Rao, Nanotechnology, 2020, 31, 155504 CrossRef PubMed.
- C. Bishop, Y. Halfaya, A. Soltani, S. Sundaram, X. Li, J. Streque, Y. El Gmili, P. L. Voss, J. P. Salvestrini and A. Ougazzaden, IEEE Sens. J., 2016, 16, 6828–6838 CAS.
- C. Bishop, J.-P. Salvestrini, Y. Halfaya, S. Sundaram, Y. El Gmili, L. Pradere, J. Y. Marteau, M. B. Assouar, P. L. Voss and A. Ougazzaden, Appl. Phys. Lett., 2015, 106, 243504 CrossRef.
- N. Minh Triet, L. Thai Duy, B.-U. Hwang, A. Hanif, S. Siddiqui, K.-H. Park, C.-Y. Cho and N.-E. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 30722–30732 CrossRef CAS PubMed.
- Y. Yong, H. Cui, Q. Zhou and X. Su, RSC Adv., 2017, 7, 51027–51035 RSC.
- M. Xiao, T. Yao, Z. Ao, P. Wei, D. Wang and H. Song, Phys. Chem. Chem. Phys., 2015, 17, 8692–8698 RSC.
- G. Chen, D. Wang, J. Wen, A. Yang and J. Zhang, Int. J. Quantum Chem., 2016, 116, 1000–1005 CrossRef CAS.
- H. Roohi and N. Askari Ardehjani, New J. Chem., 2019, 43, 15280–15292 RSC.
- G.-X. Chen, H.-F. Li, D.-D. Wang, S.-Q. Li, X.-B. Fan and J.-M. Zhang, Vacuum, 2019, 165, 35–45 CrossRef CAS.
- V. Sharma and S. Srivastava, Mater. Res. Express, 2018, 5, 45001 CrossRef.
- B. Sarkar, P. Reddy, A. Klump, F. Kaess, R. Rounds, R. Kirste, S. Mita, E. Kohn, R. Collazo and Z. Sitar, J. Electron. Mater., 2018, 47, 305–311 CrossRef CAS.
- M. Zhang, T. F. Zhou, Y. M. Zhang, W. Y. Wang, W. Li, Y. Bai, K. Lian, J. F. Wang and K. Xu, J. Phys. D: Appl. Phys., 2018, 51, 65105 CrossRef.
- M. A. Reshchikov, P. Ghimire and D. O. Demchenko, Phys. Rev. B, 2018, 97, 205204 CrossRef CAS.
- N. Sanders, D. Bayerl, G. Shi, K. A. Mengle and E. Kioupakis, Nano Lett., 2017, 17(12), 7345–7349 CrossRef CAS PubMed.
- W. Xiao, L. Wang, L. Xu, Q. Wan, A. Pan and H. Deng, Phys. Status Solidi, 2011, 248, 1442–1445 CrossRef CAS.
- J. Chen, J. Zhu, J. Ning, X. Duan, D. Wang, J. Zhang and Y. Hao, Phys. Chem. Chem. Phys., 2019, 21, 6224–6228 RSC.
- B. Delley, J. Chem. Phys., 1990, 92, 508–517 CrossRef CAS.
- D. R. Hamann, M. Schlüter and C. Chiang, Phys. Rev. Lett., 1979, 43, 1494 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- B. Delley, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 155125 CrossRef.
- D. Cortes-Arriagada, N. Villegas-Escobar and D. E. Ortega, Appl. Surf. Sci., 2018, 427, 227–236 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |