Open Access Article
Open Access ArticleRadical scavenging activity of carbon nanotubes: toward appropriate selection of a radical initiator†
Taiyo Shimizu ,
Ryoichi Kishi*,
Takeo Yamada and
Kenji Hata
,
Ryoichi Kishi*,
Takeo Yamada and
Kenji Hata
CNT-Application Research Center, National Institute of Advanced Industrial Science and Technology (AIST) Tsukuba Central, 5 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: r-kishi@aist.go.jp
First published on 10th August 2020
Abstract
Radical scavenging activities are attractive properties not only for scientific fields e.g. biomedicine, but for the materials industry. In this study, we report that carbon nanotubes (CNTs) can scavenge radicals from organic peroxides, while radicals from azo-type radical initiators exhibit only a few effects from the presence of CNTs. In addition, experimental results suggest the possibility that captured peroxide radicals generate active radical sites on the CNT surface, from which polymerization can take place. These results indicate the importance of selecting an appropriate radical initiator.
Introduction
Carbon materials generally show radical scavenging activities for various radical species. Carbon black, which is one of the most common forms of carbon material, has been characterized in terms of radical scavenging activity for several decades. For instance, Ohkita et al., studied the effect of the presence of furnace blacks on the radical polymerization of vinyl monomers in 1975.1 These studies extended to the surface modification of carbon black with radical reactions, which can successfully impart functionalities to them.2 Carbon fiber also shows radical scavenging activities in a few studies, which report increased thermal stability of polymer composites.3 The radical scavenging mechanism of these carbon materials, having a rather amorphous structure, is basically interpreted as being derived from quinone1 or phenolic hydroxyl4 groups on their surface.Among carbon materials, fullerenes and their derivatives exhibit excellent radical scavenging activities.5 High electron affinity derived from their unique chemical structures gives high reactivity of carbon–carbon double bonds against radicals. For example, the scavenging activity of fullerene C60 reaches 34 methyl radicals for each molecule.6 This remarkably high scavenging activity is the origin of the name “radical sponge”. Because of this high activity against radicals, fullerenes are candidate material for anti-oxidative applications, and some of their derivatives are adopted to commercialized products such as cosmetics.7
In some recent research, carbon nanotubes (CNTs) have turned out to improve the thermal stability of their polymer composite materials. These phenomena presumably be derived from radical scavenging activities of CNTs,8–10 and some of research exploiting density functional theory calculation supports that single-walled carbon nanotubes can spontaneously react with radicals.11 In addition to the excellent properties such as high mechanical strength, electrical and thermal conductivities, these radical scavenging activities will enhance the versatility of CNTs. However, very limited numbers of research for experimentally pursuing the details of radical scavenging have been reported so far,12,13 and thus the mechanism has not been elucidated yet. Radical scavenging activities of CNTs have a lot of potential not only for superior thermally stable nanocomposites, but for the realization of facile surface functionalization utilizing radical reactions such as polymer grafting. In addition to increasing demand of CNT in industrial usage, revealing the mechanism of these potential activities will extend the industrially applicable fields of CNTs.
In this research, we investigated the radical scavenging activities of CNTs under radical polymerization reactions. By using commonly used initiators such as azobisisobutyronitrile (AIBN) or benzoyl peroxide (BPO), the effect of the coexisting CNT on the radical polymerization has been investigated. Surprisingly, we found that CNTs strongly inhibit the polymerization of styrene initiated by BPO, while almost no effect on the yield of polymerization initiated by AIBN can be observed. Furthermore, obtained experimental results suggest the possibility that polymerization can take place from active radical sites on CNT surface presumably generated by captured BPO radicals.
Experimental
Materials
In most part of this study, we used single-walled CNTs so-called “super-growth” CNTs (SGCNTs) synthesized by a water-assisted chemical vapor deposition method according to the literature.14 The other CNTs were purchased from Nanocyl SA (multi-walled CNT NC7000, Belgium) and Cnano Technology (multi-walled CNT Flotube9000, China). We used the average diameters, average wall numbers and specific surface areas of each CNTs reported by Kobashi et al.,15 and calculated area-to-volume ratios from these values. The diameters and wall numbers were determined from the results of transmission electron microscope image analysis, and specific surface areas were obtained from a BET method on the N2 adsorption isotherms at 77 K. The average diameters, average wall numbers, specific surface areas, and area-to-volume ratios were 3.7 nm, 1.2, 1080 m2 g−1, and 0.54 nm−1 for SGCNT, 8.7 nm, 6.7, 280 m2 g−1, and 0.23 nm−1 for Nanocyl, and 9.7 nm, 8.9, 210 m2 g−1, and 0.21 nm−1 for Cnano, respectively.15 Benzoyl peroxide (BPO), azobisisobutylonitrile (AIBN) and lauroyl peroxide (LPO) were purchased from Kanto Chemical Co., Inc., Wako Pure Chemical Corporation and ARKEMA Yoshitomi. Ltd., respectively. Styrene (stabilized with 30 ppm 4-tert-butylcatechol), methanol, and chloroform were purchased from Tokyo Chemical Industry Co., Ltd. Obtained materials were used without further purification except styrene, which was purified with activated alumina before use to remove inhibitor.Bulk polymerization of styrene in the presence of CNT
General experimental scheme in this research is shown in Fig. 1. First, CNTs were dispersed into styrene of 2.73 g (3.0 mL) for 120 min by using a probe sonicator, and then radical initiator (0.05 mol%) was dissolved into the CNT-dispersed styrene. Under a N2 bubbling condition with vigorous stirring at ca. 300 rpm, CNT-dispersed styrene was first deoxygenated for more than 15 min, and after that radical polymerization was initiated by heating at 62.7 °C with oil bath and was kept for 6 h. After the polymerization, CNTs were removed by filtration from the reaction system, and the collected CNTs were washed several times with chloroform in order to remove adsorbed styrene and polystyrene (PSt). The filtrate was poured into methanol as a poor solvent for polymerized styrene, and the precipitated PSt was collected by filtration and washed with methanol for several times, and then dried under vacuum at 60 °C for 1 day.Measurement
Yields of PSt were calculated from the weight of dried PSt collected from the precipitation resulting from the addition of methanol. Thermogravimetric analysis-mass spectrometry (TG-MS) was measured by using the combination system with thermogravimetric analysis system (Netzsch, STA449 F5), gas chromatograph analyzer (which only works as a pathway for evolved gas in this study, Agilent Technologies, 7890B) and mass spectrometer (JEOL, JMS-Q1500). Operation was conducted at heating rate at 5 °C min−1 under 100 mL min−1 of He flow. X-ray photoelectron spectroscopy (XPS) was conducted by using PHI-5000 VersaProbe (ULVAC-PHI). The molecular weight distribution was measured by gel permeation chromatography (GPC) on a Tosoh HLC-8320 GPC system equipped with refractive index detector and Tosoh TSK-GEL G6000HHR + G4000HHR columns. Tetrahydrofuran (THF) was used as eluent, and standard polystyrene was used for molecular weight calibration.Results and discussion
We found that the degree of radical scavenging by CNTs depends on the type of radicals, and CNTs strongly suppress the polymerization of styrene initiated by a certain type of radical initiator. The yield of PSt (calculated from the weight of PSt collected after precipitation by methanol) is shown in Fig. 2. In the case of AIBN systems, the yield of PSt initiated by AIBN only shows slight decrease in the presence of SGCNTs. There is also no obvious change in the molecular weight distributions of obtained PSt (Fig. S1†). These results indicate that both the radicals from AIBN (2-cyano-2-propyl radical) and the active propagating PSt radicals are less reactive to the CNTs. On the other hand, the yield reaches almost zero when BPO was used. Another organic peroxide initiator, LPO, exhibited the similar trend to BPO, shown also in Fig. 2. The radicals from peroxide initiators (benzoyloxy and lauroyloxy radicals) are thus considered to be reactive to CNT surface and be readily captured, which will be experimentally confirmed in BPO systems in the latter part of this paper. Although there are some research mentioning that CNTs preferentially scavenge RO˙ radicals,13,16 these high inhibiting activity for polymerization has been observed for the first time, as far as we know. These results reveal that appropriate selection of radical initiator has critical importance for the radical polymerization in the presence of CNTs. Hereafter, we focus on the results of BPO systems in this paper.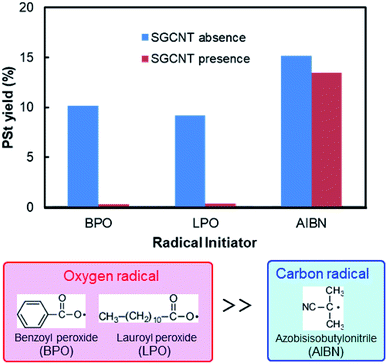 | ||
| Fig. 2 Yields of PSt with and without the presence of SGCNTs initiated by three types of radical initiators. | ||
This scavenging nature is presumably a common property for the surface of CNTs. Fig. S2† shows the yield of styrene in the presence of other kinds of CNTs. At the same weight fraction of CNT, scavenging activities depends on the kinds of CNT (the yield is 0.1% for SGCNT, 5.6% for Nanocyl and 6.6% for Cnano, shown in Fig. S2a†). On the other hand, when the same amount of CNT surface is existing in the reaction system, the yields were almost same (0.1%), shown in Fig. S2b.† Thus, when the total amount of surface area is same, radical scavenging activity of CNT is almost identical. In other words, scavenging efficiency per weight is higher when using CNTs with higher specific surface area, such as SGCNT.
As mentioned above, the efficiency of radical scavenging by CNTs is remarkably high. Fig. 3a clearly shows that the yield of styrene initiated by BPO drastically drops with increasing concentration of SGCNT. Addition of SGCNT at the concentration above 0.10 wt% results in no polymerization of styrene. This result means that in the condition studied in this research, even small amount of CNT can effectively scavenge the radicals in BPO systems. This remarkable scavenging activity indicates that for fabrication process of CNT-polymer composites such as in situ polymerization,17 appropriate selection of radical initiator is required.
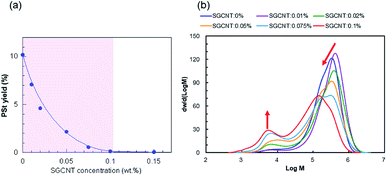 | ||
| Fig. 3 (a) Yields of PSt in the presence of different amount of SGCNTs in BPO systems. (b) Molecular weight distributions of PSt corresponding to the purple area shown in (a). | ||
In order to clarify the effect of the CNTs, which decreases the yields as the concentration increases, on PSt structure, we carried out GPC analysis also on the collected PSt in BPO systems. Molecular weight distributions of PSt in BPO systems obtained from GPC are shown in Fig. 3b. Basically, all chromatograms show bimodal distributions with high and low molecular weight components. In the case of radical polymerization of styrene, termination virtually always stems from the recombination of two active polymer chains because of e.g. small steric effects and only two available hydrogen atoms for abstraction, and the contribution from disproportionation is negligible.18 It is reasonable that we presume the high molecular weight component is derived from the recombination of two active polymers with high molecular weight. As the concentration of SGCNT increases, the high molecular weight component of PSt decreases and the low molecular weight component increases (the ratio of the peak area is shown in Fig. S3†). One reasonable interpretation of this increasing low molecular weight component in BPO systems is that the chain transfer reactions from active propagating PSt radicals to all substances (including monomers, polymers, initiators, and CNTs) took place. In general, chain transfer reactions are less conspicuous when the concentration of active radical species in the reaction system is high. In addition, the active propagating PSt radials are less reactive to CNT surface, as explained in the results of AIBN systems. However, in contrast to AIBN, the radicals from BPO can be captured onto the CNT surface, resulting in decrease in the concentration of active radical species in the reaction system. In this situation, more conspicuous chain transfer reactions of active propagating PSt radicals can take place, and the effect on the molecular weight distributions becomes consequently more highlighted, resulting in the emergence of bimodal distributions. Furthermore, the chain transfer constant of styrene of BPO is higher as compared to that of AIBN,19 which also supports the above interpretation.
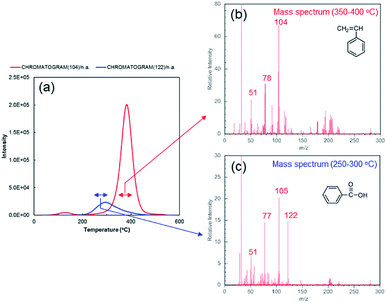
Intriguingly, we found that while no polymerized styrene can be collected at the conditions where excess amount of CNT exists in the reaction system (concentration of SGCNTs = 0.15 wt%), the polymerized styrene do exist on the CNT surface. Fig. 4a shows the results of thermogravimetric analysis-mass spectrometry (TG-MS) on the collected SGCNT at the condition where no polymerized styrene can be collected. The evolved gas from CNTs during heating under inert atmosphere was analysed by mass spectrometer. The peak of ion current intensity on m/z = 104 is located around 400 °C, and the mass spectrum around that temperature range is shown in Fig. 4b. We interpret that this peak is mainly derived from polymerized styrene. From Fig. S4,† we can clearly see that the mass spectra of standard PSt displays characteristic peaks at m/z = 51, 78, 104 and 208, which is corresponding to the peaks shown in Fig. 4b (although the peak of m/z = 208 is obscure probably due to complicated reactions between PSt and radicals such as hydrogen atom abstraction). In this case, PSt only exist on the SGCNT surface because there was no polymerized styrene collected from the reaction system. The possibility of physical adsorption of PSt on the SGCNT surface has been eliminated by chloroform extraction, and the detected PSt should have been chemically bonded to the SGCNT surface. From all of the above experimental results, we believe that there is a possibility that PSt on SGCNT surface were derived only from the polymerization initiated at active radical sites on SGCNT surface, not from the grafting by active polymer chains of PSt. Some research related to radical grafting on CNT were reported,20 however, most of them utilize some functional groups on the CNTs for the formation of chemical bonds, and limited amount of research exploits the reactivity of (pristine) CNT surface against radicals.21,22 In addition, as far as we know, there is no reports that pristine CNT surface can generate active radical site where monomer species can start polymerization. These experimental results suggest the potential way for facile surface modification of CNTs. Capture of radicals from BPO on the CNT surface was confirmed as follows. The peak of ion current intensity of m/z = 122, shown in Fig. 4a, is located close to 300 °C, and around that temperature range (250 °C to 300 °C), the mass spectra shown in Fig. 4c indicate that the main compound liberated from the SGCNT surface should be benzoic acid, the main peaks of which are m/z = 51, 77, 105 and 122.23 In order to confirm that this peak is derived from the derivative of BPO radical, we conducted the control experiment. In the case of carbon black, radicals from BPO are fixed onto the carbon black surface in the solvent containing no hydrogen atom in the molecular structure, such as tetrachloromethane.24,25 Similarly, we performed the radical reactions between BPO and SGCNTs in solvent instead of styrene. In the case of reaction between SGCNT and BPO in tetrachloromethane, the evolved gas from collected SGCNTs showed similar total mass spectra at that temperature range (Fig. S5†). Obviously, benzoyloxy radicals from BPO were captured on the SGCNT surface, resulting in the formation of benzoic acid. X-ray photoelectron spectra shown in Fig. S6† also indicate that BPO radicals are fixed onto the CNT surface owing to the increase in oxygen atoms.
From all of the above results, we assume that the reactions among BPO radicals, styrene and SGCNT with sufficient amount (above 0.10 wt%, where the conversion of PSt is zero) are as follows. At an earlier stage of the reaction, most of BPO radicals are captured onto the SGCNT surface. Although limited amount of BPO radicals might start the polymerization of styrene, the active radical species are deactivated before the polymerizing chains can sufficiently grow, resulting in no polymerized styrene can be collected. The captured BPO radicals on SGCNTs generates active radical sites, which can react with styrene. According to the results of TG-MS shown in Fig. 4, it is obvious that PSt is existing on SGCNTs after the reaction even after washing with chloroform. Thus, the PSt should have been bonded covalently, not adsorbed with physical interaction, onto the SGCNT surface. Due to the experimental fact that there were no collectable polymers in the reaction system, our interpretation on this phenomenon is that the PSt chemically-bonded to the SGCNT surface is derived from the “grafting-from” mechanism not by “grafting to”. This discovery should be useful for facile functionalization of CNT without any pre-modifications.
Conclusions
In this work, we found that carbon nanotubes (CNTs) show high radical scavenging activity toward radicals from benzoyl peroxide (BPO), while radicals from azobisisobutyronitrile (AIBN) can polymerize styrene almost regardless of the presence of CNT in the reaction system. Depending on the kinds of CNTs, the efficiency of radical scavenging per weight differs. However, when the total amount of CNT surface area is identical, there is no distinct difference in the scavenging efficiency. The yield of polystyrene drastically drops with increasing amount of CNTs, and there is no polymerization observed at the concentration of CNT of 0.15 wt%, when the super-growth CNTs (SGCNTs) were used. Even at this condition, polymerized styrene existed on the reacted CNT surface, which suggests the possibility that captured radical species from BPO generate active radical sites on the CNT surface, from which polymerization of styrene can take place. All of the result shown in this report indicates that appropriate selection of radical initiator and reaction conditions are required for the radical reaction system in the presence of CNTs and exhibit the possibility for realizing facile functionalization of CNT surface utilizing radical reactions without pre-treatment.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Aki Yoneyama is acknowledged for her experimental supports. We appreciate Dr D. N. Futaba for constructive discussion.References
- K. Ohkita, N. Tsubokawa, E. Saitoh, M. Noda and N. Takashina, Carbon, 1975, 13, 443–448 CrossRef CAS.
- N. Tsubokawa, Bull. Chem. Soc. Jpn., 2002, 75, 2115–2136 CrossRef CAS.
- X. Colin, C. Marais and J. Verdu, Compos. Sci. Technol., 2005, 65, 117–127 CrossRef CAS.
- J. Mwila, M. Miraftab and A. R. Horrocks, Polym. Degrad. Stab., 1994, 44, 351–356 CrossRef CAS.
- P. J. Krusic, E. Wasserman, P. N. Keizer, J. R. Morton and K. F. Preston, Science, 1991, 254, 1183–1185 CrossRef CAS PubMed.
- C. N. McEwen, R. G. McKay and B. S. Larsen, J. Am. Chem. Soc., 1992, 114, 4412–4414 CrossRef CAS.
- S. Z. Mousavi, S. Nafisi and H. I. Maibach, Nanomedicine, 2017, 13, 1071–1087 CrossRef CAS PubMed.
- P. C. P. Watts, P. K. Fearon, W. K. Hsu, N. C. Billingham, H. W. Kroto and D. R. M. Walton, J. Mater. Chem., 2003, 13, 491–495 RSC.
- X. Shi, B. Jiang, J. Wang and Y. Yang, Carbon, 2012, 50, 1005–1013 CrossRef CAS.
- S. Ata, S. Tomonoh, T. Yamada and K. Hata, Polymer, 2017, 119, 112–117 CrossRef CAS.
- A. Galano, Nanoscale, 2010, 2, 373–380 RSC.
- I. Fenoglio, M. Tomatis, D. Lison, J. Muller, A. Fonseca, J. B. Nagy and B. Fubini, Free Radical Biol. Med., 2006, 40, 1227–1233 CrossRef CAS PubMed.
- S. Yamane, S. Ata, L. Chen, H. Sato, T. Yamada, K. Hata and J. Mizukado, Chem. Phys. Lett., 2017, 670, 32–36 CrossRef CAS.
- K. Hata, D. Futaba, K. Mizuno, T. Namai, M. Yumura and S. Iijima, Science, 2004, 306, 1362–1365 CrossRef CAS PubMed.
- K. Kobashi, S. Ata, T. Yamada, D. N. Futaba, T. Okazaki and K. Hata, ACS Appl. Nano Mater., 2019, 2, 4043–4047 CrossRef CAS.
- P. S. Engel, W. E. Billups, D. W. Abmayr Jr, K. Tsvaygboym and R. Wang, J. Phys. Chem. C, 2008, 112, 695–700 CrossRef CAS.
- N. K. Shrivastava and B. B. Khatua, Carbon, 2011, 49, 4571–4579 CrossRef CAS.
- G. Ayrey, F. G. Levitt and R. J. Mazza, Polymer, 1965, 6, 157–171 CrossRef CAS.
- D. H. Johnson and A. V. Tobolsky, J. Am. Chem. Soc., 1952, 74, 938–943 CrossRef CAS.
- S. Qin, D. Qin, W. T. Ford, D. E. Resasco and J. E. Herrera, Macromolecules, 2004, 37, 752–757 CrossRef CAS.
- Y. Ying, R. K. Saini, F. Liang, A. K. Sadana and W. E. Billups, Org. Lett., 2003, 5, 1471–1473 CrossRef CAS PubMed.
- T. Gunji, M. Akazawa, K. Arimitsu and Y. Abe, Chem. Lett., 2006, 35, 630–631 CrossRef CAS.
- Spectral Database for Organic Compounds, SDBS, https://sdbs.db.aist.go.jp/sdbs/cgi-bin/landingpage?sdbsno=673, accessed April 2020 Search PubMed.
- K. Ohkita, H. Kasahara, N. Ishizuki and Y. Itagaki, Nippon Gomu Kyokaishi, 1963, 36, 361–367 CrossRef.
- J. B. Donnet, Carbon, 1968, 6, 161–176 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional data on yields of PSt, molecular weight distributions, thermogravimetric analysis-mass spectrometry and X-ray photoelectron spectroscopy. See DOI: 10.1039/d0ra03922e |
| This journal is © The Royal Society of Chemistry 2020 |