DOI:
10.1039/D0RA05063F
(Paper)
RSC Adv., 2020,
10, 26151-26164
Optimization extraction and characterization of Artemisia ordosica polysaccharide and its beneficial effects on antioxidant function and gut microbiota in rats
Received
8th June 2020
, Accepted 3rd July 2020
First published on 13th July 2020
Abstract
In this study, a novel polysaccharide was isolated from Artemisia ordosica by water-extraction-ethanol-precipitation method. The optimal extraction conditions of Artemisia ordosica polysaccharide (AOP) were determined by single factor investigation and response surface methodology optimization, and were shown as follows: a liquid–solid ratio of 15.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL g−1, extraction time of 4.3 h, extraction temperature of 60 °C. Under the optimal conditions, the extraction yield and the sugar content of the AOP were 5.56% and 52.65%. Gel permeation chromatography coupled to multi-angle laser light scattering, a refractive index detection system and ion-exchange chromatography were used to determine the characterization of AOP. These results indicated that AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), had narrow polydispersity and rod conformations, and was composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid with molar ratio of 6.87
1 mL g−1, extraction time of 4.3 h, extraction temperature of 60 °C. Under the optimal conditions, the extraction yield and the sugar content of the AOP were 5.56% and 52.65%. Gel permeation chromatography coupled to multi-angle laser light scattering, a refractive index detection system and ion-exchange chromatography were used to determine the characterization of AOP. These results indicated that AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), had narrow polydispersity and rod conformations, and was composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid with molar ratio of 6.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.67
10.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.13
54.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.49
2.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.37
18.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.83
4.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64
2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64. In addition, AOP exerted antioxidant ability in vitro and in vivo (rats). Moreover, AOP significantly modulated the composition of cecal microbiota population. Therefore, AOP is expected to be a functional ingredient for health improvement through improving antioxidant ability and modulating gut health.
2.64. In addition, AOP exerted antioxidant ability in vitro and in vivo (rats). Moreover, AOP significantly modulated the composition of cecal microbiota population. Therefore, AOP is expected to be a functional ingredient for health improvement through improving antioxidant ability and modulating gut health.
1. Introduction
Artemisia species are one of the most popular plants in Chinese traditional herbal medicine and frequently used in diseases treatment such as malaria, hepatitis, cancer, inflammation and infections by fungi, bacteria and viruses.1 Among genus Artemisia plants, Artemisia ordosica, a perennial herb, is one of the main shrubs growing in north and northwest areas in China. Owing to rich nutrients and bio-active components, Artemisia ordosica and their extracts can be used as natural Chinese herbal medicine feed additives. Our previous study demonstrated the growth promoting and immune regulatory effect of water extracts from Artemisia ordosica on broilers and piglets.2,3 However, the specific compounds of Artemisia ordosica water extracts that have the anti-inflammatory and antioxidant effect remain unclear.
Recently, the components of water extracts from Artemisia ordosica were identified, which were comprised four main chemical components, including alcohols, phenols, organic acids and saccharides.4 Due to their nontoxic properties and pharmacological activities, including antioxidation, immunomodulation, antitumor, anti-inflammation, inhibition of cardiovascular and cerebrovascular disease, the polysaccharides extracted from herb plants have attracted increasing attention.5–7 Furthermore, polysaccharides were recently extracted from Artemisia argyi and their immunomodulatory activity and antioxidant activity were confirmed.8,9 Moreover, growing evidences have indicated that polysaccharide has prebiotic potential through altering the composition and abundance of beneficial gut microbiota. It has shown that the gut microbiota plays a critical role in nutrient uptake, utilization, and metabolism, which may contribute to metabolic diseases.10–12
Hence, based on our previous study of Artemisia ordosica in animals2,3 and the findings of other researchers, we hypothesized that Artemisia ordosica polysaccharide (AOP) in diets could affect antioxidative capacity and gut microbiota in animals and further exploration is imperative. The objective of this study was to investigate the optimized extraction conditions of AOP. After that, the antioxidative capacity of AOP in vitro and in vivo was investigated. In addition, the effects of AOP on gut microbe structure in rats were researched as well. The results may ultimately contribute to the developing and application for AOP.
2. Materials and methods
2.1. Preparation of AOP
Artemisia ordosica was collected from Erdos (Inner Mongolia, China) in July. The AOP was prepared by using water-extraction-ethanol-precipitation method. Briefly, the whole plant (without root) was washed with distilled water and placed in the shade to dry at room temperature. The whole dried plant was grounded into powders to pass through a 60-mesh sieve. The powder of Artemisia ordosica firstly degreased by petroleum ether in the Soxhlet apparatus for 12 h. Then 200 g of Artemisia ordosica powder was steeped in 3.08 L distilled water for 4.3 h under 60 °C. The aqueous extract was filtered through a 0.45 μm filter and concentrated to 1/5 of the original volume. Anhydrous ethanol was added to the concentrated supernatants (ratio 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for precipitation of polysaccharide for 48 h at 4 °C. The sediment was collected by centrifugation (12
1, v/v) for precipitation of polysaccharide for 48 h at 4 °C. The sediment was collected by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 15 min) and washed successively with petroleum ether, acetone, and ethanol. Then the sediment was dissolved in water and deproteinated twice with Sevag reagent (n-butyl alcohol
000 × g, 15 min) and washed successively with petroleum ether, acetone, and ethanol. Then the sediment was dissolved in water and deproteinated twice with Sevag reagent (n-butyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform = 1
chloroform = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Afterward, the supernatant was collected and dialyzed using a biological semipermeable membrane (molecular weight cutoff: 500 Da, Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) against distilled water at 4 °C for 48 h, with changing the distilled water every 12 h. The resulting solution was lyophilized by a vacuum evaporate to prepare the powder, and stored at −20 °C until use. The total carbohydrate content was measured by using the phenol–sulfuric acid method using glucose as the standard.13 The protein content was determined by Coomassie brilliant blue method using bovine serum albumin as the standard.14 The polyphenols content was accomplished by Folin–Ciocalteu reagent assay using gallic acid as the standard.15 The uronic acid content was evaluated using glucuronic acid as a standard.16
4). Afterward, the supernatant was collected and dialyzed using a biological semipermeable membrane (molecular weight cutoff: 500 Da, Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) against distilled water at 4 °C for 48 h, with changing the distilled water every 12 h. The resulting solution was lyophilized by a vacuum evaporate to prepare the powder, and stored at −20 °C until use. The total carbohydrate content was measured by using the phenol–sulfuric acid method using glucose as the standard.13 The protein content was determined by Coomassie brilliant blue method using bovine serum albumin as the standard.14 The polyphenols content was accomplished by Folin–Ciocalteu reagent assay using gallic acid as the standard.15 The uronic acid content was evaluated using glucuronic acid as a standard.16
2.2. Single factor evaluation
Extraction liquid–solid ratio, temperature and time were set as factors to evaluate their influences on the AOP yield. There were five treatments with six replicates of each factor, and each factor was investigated individually.
2.3. Response surface methodology optimization
Based upon the single-factor experimental results, three-factor (extraction liquid–solid ratio, temperature, and time) with three-level response surface analysis was performed to obtain the maximum AOP yield using Box–Behnken design (BBD) combined with response surface methodology (RSM).
2.4. Characterization of polysaccharide
2.4.1 Determination of average molecular weight of AOP. The AOP water solution (5 mg mL−1) was heated at 100 °C for 5 min and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min to collect the supernatant. Gel permeation chromatography (GPC) coupled to multi-angle laser light scattering (MALLS; Heleos, Wyatt Technology Corp., Santa Barbara, CA, USA), and a refractive index (RI; model RI-150; Thermo Electron Corp., Yokohama, Japan) detection system (GPC-MALLS-RI system) was used to determine the average molecular weight of AOP. The mobile phase of the GPC-MALLS-RI was 0.1 M NaNO3 with 0.02% (w/v) NaN3 at a flow rate of 0.4 mL min−1 and column oven temperature was set at 60 °C. The value of refractive index increment (dn/dc) was 0.14 mL g−1. The injection volume was 20 μL. The Mw, polydispersity (Mw/Mn) and root mean square (RMS) radius were calculated.
000 × g for 10 min to collect the supernatant. Gel permeation chromatography (GPC) coupled to multi-angle laser light scattering (MALLS; Heleos, Wyatt Technology Corp., Santa Barbara, CA, USA), and a refractive index (RI; model RI-150; Thermo Electron Corp., Yokohama, Japan) detection system (GPC-MALLS-RI system) was used to determine the average molecular weight of AOP. The mobile phase of the GPC-MALLS-RI was 0.1 M NaNO3 with 0.02% (w/v) NaN3 at a flow rate of 0.4 mL min−1 and column oven temperature was set at 60 °C. The value of refractive index increment (dn/dc) was 0.14 mL g−1. The injection volume was 20 μL. The Mw, polydispersity (Mw/Mn) and root mean square (RMS) radius were calculated.
2.4.2 Monosaccharide composition of AOP. The AOP sample (5 mg) was dissolved in 2 M trifluoroacetic acid (TFA, 4 mL) in a sealed tube, and kept at 121 °C for 2 h to complete hydrolysis. The excess TFA was removed by rotary evaporation. The residue was re-dissolved in methanol (2 mL) and dried with nitrogen using nitrogen blowing instrument for three times. Then the residue was re-dissolved in ultrapure water in chromatographic bottle and measured by ion-exchange chromatography (Dionex ICS 5000) equipped with a CarboPac PA20 analytic column (250 × 4 mm; Dionex). Gradient elution was carried out according to the following process: 0–25 min, 1% 500 mM NaOH; 25.1–40 min, 10% 500 Mm NaOH and 90% ultrapure water; and 40.1–50 min, 100% 500 mM NaOH. All procedures were conducted at a constant flow rate of 0.5 mL min−1 at a column temperature of 30 °C. The injection volume was 20 μL. Fucose, arabinose, galactose, glucose, xylose, mannose, fructose, ribose, galacturonic acid and glucuronic acid were used as the monosaccharide standards.
2.5. Antioxidative activity assay for AOP
2.5.1 Assay of DPPH free radical scavenging activity. The DPPH free radical scavenging activity of AOP was performed by spectroscopic method.17 Briefly, various concentrations (0.0, 0.2, 0.4, 0.6, 0.8 and 1.0 mg mL−1) of AOP were added into ethanol containing DPPH. The reaction mixture was shaken vigorously and the absorbance of remaining DPPH was measured at 517 nm after 30 min at room temperature in dark. Vitamin C (Vc) was used as positive control. The samples were analyzed in triplicate. DPPH free radical scavenging activity of AOP was calculated according to the following equation:
| DPPH free radical scavenging ability (%) = [1 − (A1 − A2)/A0] × 100% |
In the equation, A1 was the absorbance of AOP samples; A2 was the absorbance of the samples under conditions as A1 with distilled water instead of DPPH; A0 was the absorbance of the distilled water instead of samples.
2.5.2 Assay of hydroxyl radical scavenging activity. Hydroxyl radical scavenging activity of AOP was evaluated by the reported method.17 In brief, 500 μL of FeSO4 (9.0 mM), 500 μL of H2O2 (8.8 mM) with 500 μL of AOP at the concentrations of 0.0, 0.2, 0.4, 0.8 and 1.0 mg mL−1, respectively, were mixed to react for 10 min at 25 °C, then the mixture was mixed with 500 μL salicylic acid ethanol (9.0 mM), and the absorbance at 510 nm was measured after reaction at 25 °C for 30 min. Vc was used as positive control. The samples were analyzed in triplicate. Hydroxyl radical scavenging ability of AOP was calculated according to the following equation:
| Hydroxyl radical scavenging ability (%) = [1 − (A1 − A2)/A0] × 100% |
In the equation, A1 was the absorbance of AOP samples; A2 was the absorbance of the samples under conditions as A1 with distilled water instead of FeSO4; A0 was the absorbance of the distilled water instead of samples.
2.5.3 Assay of ABTS free radical scavenging activity. ABTS radical scavenging activity of AOP was evaluated according to the reported method.17 ABTS solution (200 μL) was mixed with 20 μL of different concentrations of AOP solution (0.0, 0.2, 0.4, 0.6, 0.8 and 1.0 mg mL−1) into a 96-well plate. The absorbance at 734 nm was measured after reaction for 6 min at 25 °C. Vc was used as positive control. The samples were analyzed in triplicate. The radical scavenging activity was determined by comparing the absorbance with which of blank (100%) containing only ABTS and solvent.
2.5.4 Reducing power assay. The reducing power (RP) was evaluated by the method according to Wan et al. (2016)18 with some modifications. Briefly, each sample (0.75 mL) was blended with PBS (200 mM, pH 6.6, 0.75 mL) and 1% potassium ferricyanide (0.75 mL). After incubation for 20 min at 50 °C, the mixed solution was added 10% trichloroacetic acid (0.75 mL), then centrifugated at 1500 × g for 10 min. The supernatant (1.5 mL) was subsequently mixed with distilled water (1.5 mL) and 0.1% ferric chloride (0.4 mL). The absorbance at 700 nm of the solution was measured. An increased absorbance of the sample indicated increased reducing power.
2.6. Animals and experimental protocol
Male Wistar rats were obtained from Experimental Animal Center of Inner Mongolia Medical University (Hohhot, China). Twelve rats were randomly divided into two groups (n = 6 per group), each group was supplied experimental diet which was the basal diet contained (1) no additive (CON) or (2) 300 mg per kg AOP for 21 days. During the entire experimental period, all rats were fed a standardized pellet feed and water ad libitum and housed under standard laboratory conditions with a constant 12 h light and 12 h dark at room temperature maintained at 23 ± 1 °C. All rats were individually weighed on day 1 and day 21, after the fasting for 12 h. Feed intake and diet residue of each rat was weighed daily. Average daily body weight gain (ADG), average daily feed intake (ADFI) and the ratio of feed to gain (F/G) were calculated.
By the end of the study, the rats were starved for 12 h and then sacrificed for blood collection and tissue sampling under anesthesia using sodium pentobarbital. Serum sample was separated by centrifugation at 3000 × g for 10 min and stored at −20 °C until analysis. Liver samples and cecal content were frozen in liquid nitrogen and stored at −80 °C until analysis. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) in the serum were determined by the automatic analyzer (HITACHI 747, Tokyo, Japan). All animal procedures were performed in accordance with the National Standard Guideline for Ethical Review of Animal Welfare (GB/T 35892-2018) and approved by the Animal Care and Use Committee of Inner Mongolia Agricultural University.
2.6.1 Assay of antioxidant indices in serum and tissue samples. Liver tissue was minced and homogenized (10% w/v) in saline, then centrifuged at 3000 × g for 10 min at 4 °C. The resulting supernatant and serum were used to determine total antioxidant capacity (T-AOC), enzymes activities including catalase (CAT), superoxide dismutase (SOD) and glutathione peroxidase (GPx), and the indicator of lipid peroxidation malondialdehyde (MDA). Antioxidant indexes and protein were measured by commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Data were expressed as U per mg of protein for tissues and U mL−1 for serum. All procedures were conducted in accordance with the manufacturer's instructions.
2.6.2 RNA preparation and fluorescence quantitative real-time PCR. Total RNA in liver tissue was isolated by using the Trizol extraction method according to the manufacturer's instructions. RNA was quantitatively and qualitatively determined by using an ultraviolet spectrophotometer at 260 nm and 280 nm. RNA integrity was analyzed by using horizontal electrophoresis through 1.5% agarose gel. Genomic DNA contamination was removed by using DNase provided with the kit. Synthesis of cDNA was conducted with the PrimeScriptRT reagent Kit with gDNA Eraser (Takara Bio Inc., Otsu, Japan) according to the manufacturer's instructions. The quantitative real-time PCR (qPCR), with the help of TB Green (TB Premix Ex Taq II, Perfect Real Time, Takara Bio Inc.) according to the manufacturer's instructions, was performed on an Illumina real-time PCR machine in two steps: a cycle of initial denaturing at 95 °C for 30 s, followed by 40 cycles involving denaturation at 95 °C for 5 s and annealing at 60 °C for 30 s. A subsequent dissociation stages produced a melting curve to verify the specificity of the amplified products. The target genes including CAT, GPx, SOD1, SOD2 and their primer sequences are tabulated in Table 1, which were designed by Shanghai Sangon Biotech (Shanghai, China). β-Actin was used as reference gene and the relative fold difference in the mRNA expression levels was calculated using the 2−ΔΔCT.
Table 1 Primer sequences and parameter
| Genes |
GenBank no. |
Primer sequences (5′–3′) |
Length (bp) |
Ta (°C) |
| β-Actin |
NM_031144.2 |
F. CCTAAGGCCAACCGTGAAAA |
103 |
60 |
| R. CAGAGGCATACAGGGACAACAC |
| CAT |
NM_012520.2 |
F. GAACATTGCCAACCACCTGAAAG |
85 |
60 |
| R. GTAGTCAGGGTGGACGTCAGTGAA |
| GPx1 |
NM_001037471 |
F.CTTCCCCAATCTGCCCTACTTA |
112 |
60 |
| R.CTCCTCCTCTGTCTCTCCACAC |
| SOD1 |
NM_017050.1 |
F. GGCAAAGGTGGAAATGAAGAAA |
136 |
60 |
| R. CAGTTTAGCAGGACAGCAGATGAG |
| SOD2 |
NM_174294 |
F. CCAGGAGCAAAACCAAGAAC |
148 |
60 |
| R. TGCAACTGGTTTCTGTTGGT |
2.6.3 DNA extraction and MiSeq sequencing. Cecal contents genomic DNA was extracted using the EZNA® Soil NDA Kit (Omega Bio-Tek, Inc., GA, USA) in accordance with kit protocols. The resulting DNA was amplified with specific bacterial primers targeting the V3–V4 hypervariable region of the bacterial 16S rRNA using universal primers: 338F (5-ACTCCTACGGGAGGCAGCAG-3), and 806R (-GGACTACHVGGGTWTCTAAT-3). PCR reactions were performed on a thermocycler PCR system in following steps: 95 °C for 180 s; followed by 40 cycles involving 95 °C for 30 s, 60 °C for 30 s, 72 °C for 45 s; then 72 °C for 10 min. The PCR products were checked on a 2% agarose gel and purified according to the manufacturer's instructions. The amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, USA) and quantified using QuantiFluor™-ST fluorometer (Promega, Madison, USA), and then sequenced on a MiSeq platform (IIIumina, San Diego, USA) according to the protocols in a Shanghai MajorBio Bio-Pharm Technology Co. Ltd (Shanghai, China).
2.7. Statistical analysis
Values were expressed as mean ± standard deviation (SD). The one-way analysis of variance (ANOVA) and Student's t test were used to detect significant differences. Values of P < 0.05 are considered to be statistically significant.
3. Results and discussion
3.1. BBD and RSM optimization
Previous study indicated that water-extraction-ethanol-precipitation method of plant polysaccharide is commonly used for its economical nature, convenient use and is strongly related to extraction liquid–solid ratio, time and temperature.19 In addition, polysaccharide yield and activities may reduce due to the excessive extraction time or temperature.20 Hence, polysaccharide yield and DPPH radical scavenging ability (%) under different extraction conditions (liquid–solid ratio, temperature and extraction time) were determined to obtain the maximum yield without decreasing antioxidant ability. As shown in Fig. 1, the maximum yield of polysaccharide was obtained at the liquid–solid ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1A), temperature of 60 °C (Fig. 1B) and extraction time of 4 h (Fig. 1C). Of note, in the present study, the extraction yield of AOP significantly increased with the increase of extraction temperature from 20 to 60 °C and significantly decreased when the extraction temperature increased from 60 to 100 °C. An increasing extraction temperature could increase the molecular movement, resulting in accelerating the transfer of intracellular substances from the cells.21 However, higher temperature can cause the degradation of some thermo-sensitive materials, such as polysaccharide, resulting in declined yield.22 In addition, DPPH radical scavenging ability (%) was higher under this condition as well (Fig. 1A–C). Therefore, a liquid–solid ratio of 10
1 (Fig. 1A), temperature of 60 °C (Fig. 1B) and extraction time of 4 h (Fig. 1C). Of note, in the present study, the extraction yield of AOP significantly increased with the increase of extraction temperature from 20 to 60 °C and significantly decreased when the extraction temperature increased from 60 to 100 °C. An increasing extraction temperature could increase the molecular movement, resulting in accelerating the transfer of intracellular substances from the cells.21 However, higher temperature can cause the degradation of some thermo-sensitive materials, such as polysaccharide, resulting in declined yield.22 In addition, DPPH radical scavenging ability (%) was higher under this condition as well (Fig. 1A–C). Therefore, a liquid–solid ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20
1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a temperature range between 40–80 °C and an extraction time of 2–6 h was selected for the BBD (Table 2). The experimental scheme was arranged by BBD and experimental results were shown in Table 3. The response surface plots and contour plots between the factors and AOP yield were shown in Fig. 2A–F, which depicted the interactions between two variables by keeping the other variables at their zero levels for AOP yield. The shapes of the contour plots, circular or elliptical, indicate whether the mutual interactions between variables are significant or not.23 In addition, the analysis of variance was conducted and detailed in Table 4. From Fig. 2 and Table 4, it could be seen that two interaction coefficients (A and C) and all quadratic term coefficients (A2, B2 and C2) were significant with low P values (P < 0.05). Moreover, the model was highly significant (Table 4), which indicated that the predicted model more highly illustrated the pattern of the interactions between variables.24
1, a temperature range between 40–80 °C and an extraction time of 2–6 h was selected for the BBD (Table 2). The experimental scheme was arranged by BBD and experimental results were shown in Table 3. The response surface plots and contour plots between the factors and AOP yield were shown in Fig. 2A–F, which depicted the interactions between two variables by keeping the other variables at their zero levels for AOP yield. The shapes of the contour plots, circular or elliptical, indicate whether the mutual interactions between variables are significant or not.23 In addition, the analysis of variance was conducted and detailed in Table 4. From Fig. 2 and Table 4, it could be seen that two interaction coefficients (A and C) and all quadratic term coefficients (A2, B2 and C2) were significant with low P values (P < 0.05). Moreover, the model was highly significant (Table 4), which indicated that the predicted model more highly illustrated the pattern of the interactions between variables.24
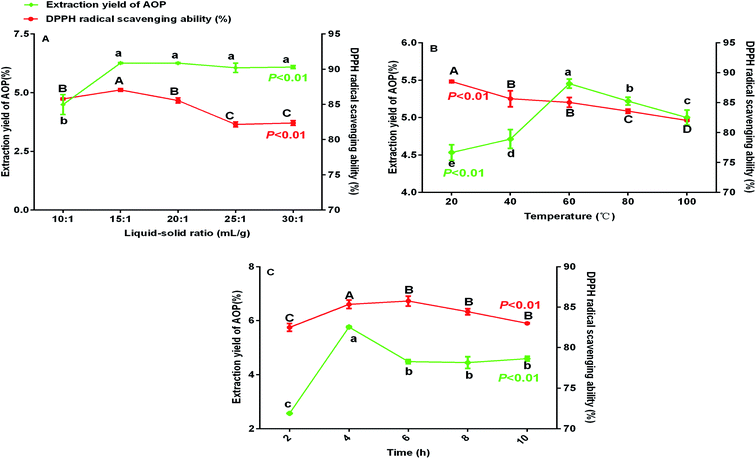 |
| | Fig. 1 Extraction yield and DPPH radical scavenging ability (%) of AOP under different extraction conditions (A: liquid–solid ratio, B: temperature and C: time). | |
Table 2 Levels and factors of response surface design
| Factors |
Levels |
| −1 |
0 |
1 |
| A: liquid–solid ratio (mL g−1) |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| B: temperature (°C) |
40 |
60 |
80 |
| C: times (h) |
2 |
4 |
6 |
Table 3 Box–Behnken design and response for extraction yield of AOP
| Run |
Independent variable |
Response |
| A (liquid–solid ratio, mL g−1) |
B (temperature, °C) |
C (time, h) |
Extraction yield (%) |
| 1 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
2 |
3.65 |
| 2 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
6 |
3.10 |
| 3 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
4 |
5.50 |
| 4 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
6 |
3.00 |
| 5 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
6 |
3.20 |
| 6 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
4 |
3.50 |
| 7 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
4 |
5.70 |
| 8 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
2 |
3.70 |
| 9 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
6 |
4.14 |
| 10 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
2 |
2.80 |
| 11 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
4 |
5.90 |
| 12 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
4 |
3.10 |
| 13 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
4 |
6.00 |
| 14 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
60 |
4 |
6.10 |
| 15 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
4 |
2.80 |
| 16 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
80 |
2 |
3.43 |
| 17 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
4 |
3.40 |
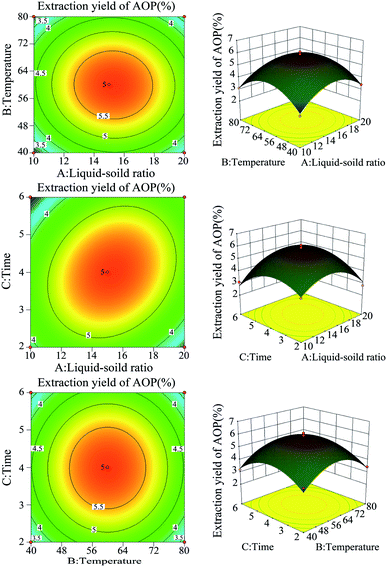 |
| | Fig. 2 The response surface plots and contour plots between the factors and AOP yield. | |
Table 4 Analysis of variance of the regression model
| Source |
Sum of squares |
df |
Mean square |
F value |
P-value |
| Model |
23.67 |
9 |
2.63 |
24.96 |
0.0002 |
| A-liquid–soild |
0.16 |
1 |
0.16 |
1.54 |
0.2547 |
| B-temperature |
8.45 × 10−5 |
1 |
8.45 × 10−5 |
8.019 × 10−4 |
0.9782 |
| C-time |
2.485 × 10−3 |
1 |
2.485 × 10−3 |
0.024 |
0.8823 |
| AB |
0.01 |
1 |
0.01 |
0.095 |
0.7670 |
| AC |
0.94 |
1 |
0.94 |
8.92 |
0.0203 |
| BC |
1.690 × 10−4 |
1 |
1.690 × 10−4 |
1.604 × 10−3 |
0.9692 |
| A2 |
6.71 |
1 |
6.71 |
63.70 |
<0.0001 |
| B2 |
7.99 |
1 |
7.99 |
75.81 |
<0.0001 |
| C2 |
5.50 |
1 |
5.50 |
52.17 |
0.0002 |
| Residual |
0.74 |
7 |
0.11 |
|
|
| Lack of fit |
0.51 |
3 |
0.17 |
2.91 |
0.1647 |
| Pure error |
0.23 |
4 |
0.058 |
|
|
| Cor total |
24.41 |
16 |
|
|
|
By applying multiple regression analysis on the experimental data, the quadratic fitting linear regression equation for AOP extraction was as follows: Y = −20.3751 + 1.37973A + 0.41990B + 1.53956C − 0.0005AB + 0.0448475AC + 0.0001625BC − 0.050505A2 − 0.00344344B2 − 0.28566C2, where Y denotes AOP extraction yield (%), A denotes the liquid–solid ratio (mL g−1), B denotes the extraction temperature (°C), and C denotes the extraction time (h). The variance of the quadratic regression model showed that the determination coefficient (R2) was 0.9309, indicating that the calculated model was able to explain 93.09% of the result in the case of AOP yield.25 The optimum extraction conditions of AOP were as follows: liquid–solid ratio of 15.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; extraction time of 4.28 h and extraction temperature of 59.96 °C. The prediction yield of AOP was estimated to be up to 5.84%. The extraction experiment was repeated three times under the optimal extraction condition (4.3 h, 60 °C, and liquid–solid ratio of 15.4
1; extraction time of 4.28 h and extraction temperature of 59.96 °C. The prediction yield of AOP was estimated to be up to 5.84%. The extraction experiment was repeated three times under the optimal extraction condition (4.3 h, 60 °C, and liquid–solid ratio of 15.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to verify the reliability of the results, and the extractive rate of AOP was 5.56% (Table 5), which was very close to the predicted value. It was suggested that the optimum conditions for AOP extraction could be effectively performed in practice. The carbohydrate content, protein content, uronic acid content and phenolic content for AOP were 52.65%, 2.39%, 4.27% and 0.11% (Table 5).
1) to verify the reliability of the results, and the extractive rate of AOP was 5.56% (Table 5), which was very close to the predicted value. It was suggested that the optimum conditions for AOP extraction could be effectively performed in practice. The carbohydrate content, protein content, uronic acid content and phenolic content for AOP were 52.65%, 2.39%, 4.27% and 0.11% (Table 5).
Table 5 Extraction yields and chemical components analysis of AOP
| |
Extraction yield (%) |
Carbohydrate (%) |
Protein (%) |
Uronic acid (%) |
Phenolic (%) |
| AOP |
5.56 ± 0.41 |
52.65 ± 5.23 |
2.39 ± 0.09 |
4.27 ± 0.49 |
0.11 ± 0.002 |
3.2. Molecular characteristics of polysaccharide
The RI-MALS superimposed chromatograms for the AOP are shown in Fig. 3. As shown in the RI chromatogram, the polysaccharide had two major peaks at the elution time of 69–71 min and 72–73 min (Fig. 3A). Hence, the average Mw value of AOP were 2.1 kDa (62.6%) and 1.5 kDa (37.4%). Previous studies reported that molecular weight was strongly related to the viscosity and solubility of polysaccharide solution. High molecular weight polysaccharide generally shares a number of characteristics with high viscosity and poor solubility, which limits its application.26,27 Thus, AOP with moderate molecular weight would be favorable for its application. The AOP also had narrow polydispersity (PD = 1.058 and 1.063), close to 1, which indicated that AOP was homogeneity.28 The conformation plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rg–log
Rg–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mw indicated the rod conformations was adopted for AOP with a slope of 0.78 (Fig. 3B). The value of the line slope of the line when plotting log
Mw indicated the rod conformations was adopted for AOP with a slope of 0.78 (Fig. 3B). The value of the line slope of the line when plotting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rg versus log
Rg versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M can be used to obtain conformational information of polymers in solution, e.g., the value of v is approximately 0.3 for hard sphere conformation, approximately 0.5 for random coil, and approximately 1 for rigid rod.29,30 The conformation plot of log
M can be used to obtain conformational information of polymers in solution, e.g., the value of v is approximately 0.3 for hard sphere conformation, approximately 0.5 for random coil, and approximately 1 for rigid rod.29,30 The conformation plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rg–log
Rg–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mw indicated the rigid rod conformations were adopted for AOP.
Mw indicated the rigid rod conformations were adopted for AOP.
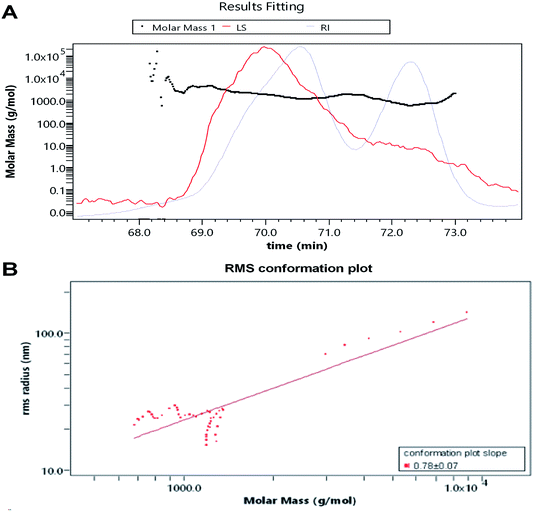 |
| | Fig. 3 Average molecular weight of AOP obtained from GPC-MALS-RI. (A) average molecular weight; (B) root mean square conformation plot. | |
3.3. Monosaccharide composition assay
The monosaccharide composition of AOP was determined by ion chromatography. On the basis of the elution time of monosaccharide standards (Fig. 4A), the AOP composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid monohydrate with molar ratio of 6.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.67
10.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.13
54.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.49
2.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.37
18.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.83
4.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64
2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64 (Fig. 4B). It has been reported that polysaccharide could modulate the composition and abundance of beneficial microbiota through altering fermentation behavior, which is greatly influenced by their monosaccharide compositions (see following sections).31
2.64 (Fig. 4B). It has been reported that polysaccharide could modulate the composition and abundance of beneficial microbiota through altering fermentation behavior, which is greatly influenced by their monosaccharide compositions (see following sections).31
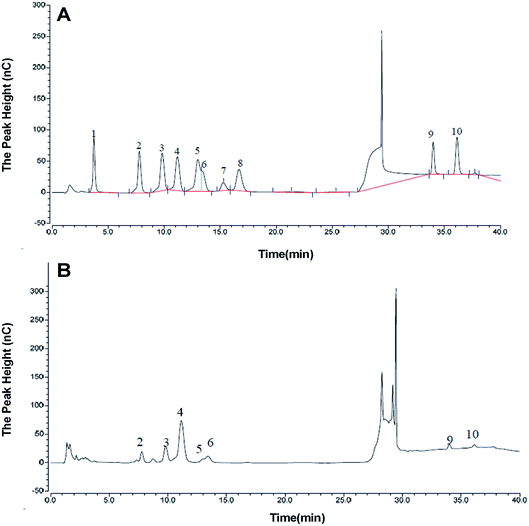 |
| | Fig. 4 Monosaccharide composition of AOP obtained from ion chromatography. a,b (A) Monosaccharide standards; (B) AOP. | |
3.4. Antioxidant activity of AOP in vitro
Plant polysaccharide is with powerful antioxidant capable of mopping up free radicals.32 DPPH radical is one of common radicals and usually used to evaluate the capacity of natural antioxidant.33 The DPPH radical scavenging activities of AOP at different concentrations were shown in Fig. 5A. It could be seen that AOP showed does-dependent radical scavenging capacity along with increasing concentration. At a concentration of 1.0 mg mL−1, the DPPH radical scavenging activity for AOP was 58.60 ± 1.59% which was far lower than positive control (Vc).
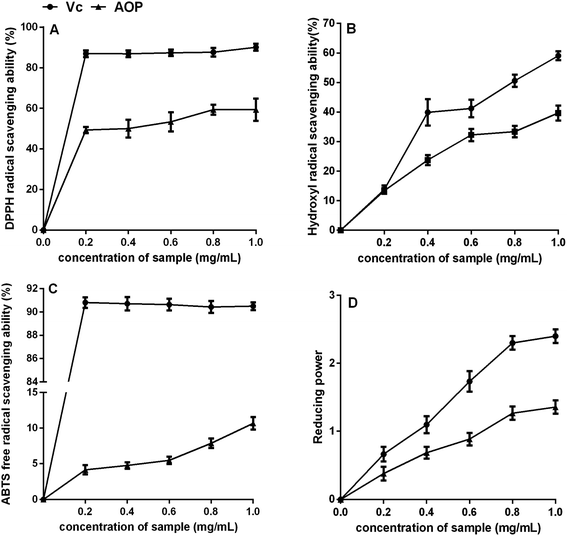 |
| | Fig. 5 Antioxidant activity of AOP in vitro. (A) DPPH radical scavenging activity; (B) hydroxyl radical scavenging activity; (C) ABTS radical scavenging activity; (D) reducing power. | |
Uncontrolled production of hydroxyl radical may cause damage to numerous biological substances, which led to diseases.34 Thus, seeking a natural antioxidant to scavenge hydroxyl radical is obtained increasing attention. The scavenging effect of AOP on hydroxyl radical was determined (Fig. 5B). Concentration-dependent effect of radical-scavenging activity was observed. At a concentration of 1.0 mg mL−1, the hydroxyl radical scavenging activity of AOP was 38.20 ± 2.06%, which suggested a satisfying radical scavenging activity.
ABTS radical scavenging activity is an important index to evaluate the total antioxidant power of polysaccharide.17 The ABTS radical-scavenging effect of AOP was presented in Fig. 5C. Concentration-dependent effect of radical scavenging activity was observed as well. Compared to Vc, AOP exhibited lower ABTS radical scavenging activity at all tested concentrations.
The reducing power of AOP was investigated and the results are shown in Fig. 5D. Both AOP and Vc demonstrated evident reducing effects in a concentration-dependent manner at concentration range of 0–1.0 mg mL−1. The reducing power of the AOP was a little lower than that of Vc, but it was similar with Artemisia annua L. enzymatic treated,18 indicating that Artemisia species possessed antioxidant activity in vitro.
3.5. Growth performance of rats
As shown in Table 6, dietary supplementation with AOP significantly increased ADG and G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F. However, the effect of AOP on the ADFI was not detected (P > 0.05). Our previous study indicated that Artemisia argyi aqueous extract and Artemisia annua L. aqueous extract could improve growth performance of broilers.35,36 The present study was further confirmed the growth prompting effect of polysaccharide as the main component in the plant aqueous extract.
F. However, the effect of AOP on the ADFI was not detected (P > 0.05). Our previous study indicated that Artemisia argyi aqueous extract and Artemisia annua L. aqueous extract could improve growth performance of broilers.35,36 The present study was further confirmed the growth prompting effect of polysaccharide as the main component in the plant aqueous extract.
Table 6 Effects of AOP on growth performance and serum biochemical indexes of ratsa,b
| Items |
CON |
AOP |
P-value |
| Different letter within the same line denote significant differences between diets. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase. |
| Growth performance |
| ADG, g d−1 |
3.42 ± 0.89B |
4.20 ± 0.35A |
0.02 |
| ADFI, g d−1 |
20.40 ± 0.36 |
20.61 ± 0.55 |
0.56 |
G![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F, g g−1 F, g g−1 |
0.16 ± 0.01B |
0.23 ± 0.02A |
0.002 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Serum biochemical indexes |
| ALT (U L−1) |
92.42 ± 2.00 |
87.81 ± 1.94 |
0.14 |
| AST (U L−1) |
160.8 ± 20.38 |
133.3 ± 10.08 |
0.31 |
| ALP (U L−1) |
239.8 ± 9.62A |
144.5 ± 4.02B |
0.004 |
3.6. Hepatotoxicity of AOP
The effect of AOP on liver injury was evaluated by determining AST, ALT and ALP activities in serum. As shown in Table 6, the levels of serum ALT and AST activities did not affect by AOP feeding. AOP supplementation could significantly decrease ALP levels. ALP is intracellular and only a small amount is present in serum. The increasing ALP activity in serum, which is caused by the breakdown of cell in certain disease conditions, is the symptoms of hepatobiliary sickness.37 Therefore, we can reasonably arrive at the suggestion that AOP is beneficial in protecting liver.
3.7. Antioxidative activities and gene expressions in serum and liver
Overproduction of free radicals in the body will cause damage to T cell, decrease immune function and bring cardiovascular and cerebrovascular disease risks.38 Studies have found that some polysaccharides can not only inhibit above disease, but also inhibit lipid peroxidation, enhance the body's ability to scavenge free radicals and anti-aging.39,40 AOP is a highly promising source of antioxidants, which is confirmed in the present study by measuring scavenging ability to free radicals. Meanwhile, the antioxidant activities of AOP in vivo, including lipid peroxidation indicator (MDA), antioxidant enzyme activities (CAT, SOD and GSPx) and T-AOC, were determined at an animal level. In serum, compared with the control group, AOP supplementation significantly increased the activities of SOD and GPx (P < 0.05). There was no significant difference between AOP and control group on the levels of T-AOC, CAT and MDA (Table 7).
Table 7 Effects of AOP on serum and liver antioxidative activities and the corresponding gene expressionsa
| Items |
CON |
AOP |
P-value |
| Different letter within the same line denote significant differences between diets. |
| Serum |
| T-AOC, U mL−1 |
8.51 ± 0.67 |
9.49 ± 0.46 |
0.30 |
| SOD, U mL−1 |
277.0 ± 2.76B |
302.3 ± 2.03A |
0.01 |
| CAT, U mL−1 |
4.60 ± 0.09 |
5.39 ± 0.24 |
0.16 |
| GPx, U mL−1 |
1810 ± 88.29B |
2216 ± 94.89A |
0.01 |
| MDA, nmol mL−1 |
4.11 ± 0.21 |
4.49 ± 0.46 |
0.49 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Liver |
| T-AOC, U per mg prot. |
1.69 ± 0.14B |
2.45 ± 0.33A |
0.09 |
| SOD, U per mg prot. |
259.1 ± 8.54B |
328.9 ± 23.37A |
0.04 |
| CAT, U per mg prot. |
45.81 ± 3.81B |
70.31 ± 1.06A |
0.001 |
| GPx, U per mg prot. |
244.1 ± 8.76B |
276.2 ± 7.00A |
0.03 |
| MDA, nmol per mg prot. |
6.367 ± 0.16 |
4.76 ± 0.70 |
0.14 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
| Genes in liver |
| SOD1 |
1.00 ± 0.00B |
1.48 ± 0.13A |
0.03 |
| SOD2 |
1.00 ± 0.00B |
1.44 ± 0.06A |
0.002 |
| CAT |
1.00 ± 0.00B |
1.38 ± 0.10A |
0.04 |
| GPx1 |
1.00 ± 0.00 |
1.17 ± 0.16 |
0.23 |
The activities of antioxidant enzymes in liver and the corresponding gene expressions were shown in Table 7. Compare to those of control group, CAT and SOD activity of AOP group were increased, and the corresponding gene expressions (CAT, SOD1 and SOD2) were up-regulated with AOP addition as well (P < 0.05). In addition, AOP supplementation enhanced GPx activity (P < 0.05), however, the GPx gene expression didn't show significant difference between the two groups. The results indicated that AOP with remarkable antioxidant activity has important significance in natural medicine research and production.
3.8. Overall structural change of gut microbiota
3.8.1 Sequencing coverage and bacterial diversity. Twelve samples of caecum microbiome DNA (n = 6 for per group) were used to sequence the V3–V4 regions of the 16S rRNA gene by Illumina Miseq sequencer after PCR and quality check with Agilent 2200 TapeStation and Qubit 2.0. Sequences shorter than 200 bp were removed, and 708![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056 sequences were gained with an average length of 432 bp. A total of 555 OTUs were generated via clustering analysis for high-quality sequences at a 97% similarity cutoff. The rarefaction and Shannon index curves that were generated from the OTUs revealed that the data had covered most of the diversity and new phylotypes (Fig. 6), suggesting that high sampling coverage was captured with the sequencing depth, such that further increasing the sequencing depth was unlikely to achieve more gut microbiota diversity. Furthermore, the diets played a key role in shaping phylogenetic diversity based on the α-diversity. Thereinto, Shannon index was significantly increased (p < 0.05) after AOP treatment (Table 8). The principal component analysis (PCA) was used to analyze the composition changes in the gut microbiota. The results revealed that AOP diet caused significant separation of fecal microbiota for CON and AOP diet groups (Fig. 7).
056 sequences were gained with an average length of 432 bp. A total of 555 OTUs were generated via clustering analysis for high-quality sequences at a 97% similarity cutoff. The rarefaction and Shannon index curves that were generated from the OTUs revealed that the data had covered most of the diversity and new phylotypes (Fig. 6), suggesting that high sampling coverage was captured with the sequencing depth, such that further increasing the sequencing depth was unlikely to achieve more gut microbiota diversity. Furthermore, the diets played a key role in shaping phylogenetic diversity based on the α-diversity. Thereinto, Shannon index was significantly increased (p < 0.05) after AOP treatment (Table 8). The principal component analysis (PCA) was used to analyze the composition changes in the gut microbiota. The results revealed that AOP diet caused significant separation of fecal microbiota for CON and AOP diet groups (Fig. 7).
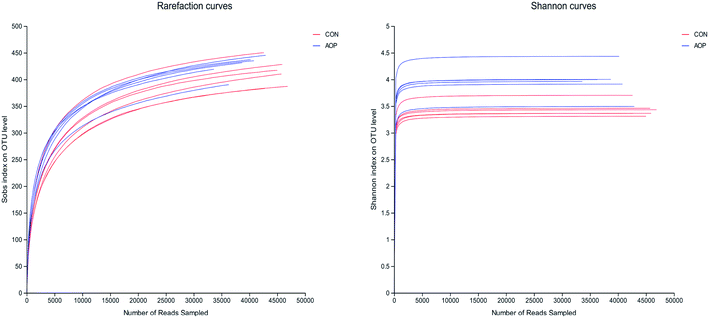 |
| | Fig. 6 The rarefaction and Shannon index curves. | |
Table 8 Alpha diversity indexes of cecal bacteria in rats among different groupsa
| Estimators |
CON |
AOP |
P-value |
| Different letter within the same line denote significant differences between diets. |
| Sobs |
412.50 ± 25.27 |
426.17 ± 19.66 |
0.32 |
| Shannon |
3.44 ± 0.14B |
3.96 ± 0.29A |
0.002 |
| Simpson |
0.11 ± 0.02 |
0.08 ± 0.03 |
0.12 |
| Ace |
438.59 ± 27.79 |
455.93 ± 14.14 |
0.20 |
| Chao |
443.67 ± 30.07 |
459.02 ± 10.76 |
0.27 |
| Coverage |
0.99 ± 0.0001 |
0.99 ± 0.0002 |
0.86 |
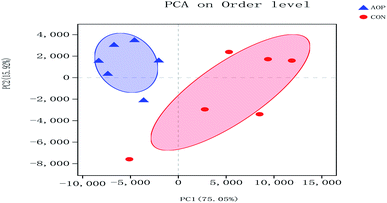 |
| | Fig. 7 Principal coordinate analysis (PCA) of caecum microbiome in rats in control group (CON) vs. polysaccharides group (AOP). | |
3.8.2 Changes in microbial composition after supplementation administration. Considerable studies showed that herbal medicine plants exerted beneficial ability against diseases by modulating gut microbiota.41,42 Hence, in order to investigate the structural response of gut microbiota to AOP feeding, the bacterial communities in rats were analyzed at the phylum and genus levels. At the phylum level, among the host microbiota, Firmicutes and Bacteroidetes were the predominant phyla in all treatment groups, followed by far less abundant Verrucomicrobia and Actinobacteria (Fig. 8). In general, gastrointestinal digestive enzymes can't digest polysaccharide. However, most of them can be fermented by gut microbiota. Short-chain fatty acids (SCFA) are main end products of the fermentation.43,44 High level of SCFA is unfavorable to several potentially pathogenic bacteria,45 but they can promote the proliferation of Bacteroides, Ruminococcus, Lactobacillus, Prevotella, and Butyricicoccus,46,47 thereby benefit the host. Unfortunately, short-chained fatty acids in hindgut were not detecting in this study. Based on previous study, polysaccharide can up-regulated short-chained fatty acids, stimulating the growth of beneficial microbes, such as Ruminococcaceae, Bacteroidetes, Lactobacillus48 and Prevotella.49 In the current study, AOP administration tended to increase the relative abundance of Bacteroidetes (P = 0.09). Bacteroidetes have been reported to be able to generate several SCFAs (e.g. acetic and propionic acid) by a range of glycoside hydrolases and carbohydrate metabolic pathways.50 These results indicate that AOP presenting the growth of SCFA-producing bacteria in the gut, such as Bacteroidetes.
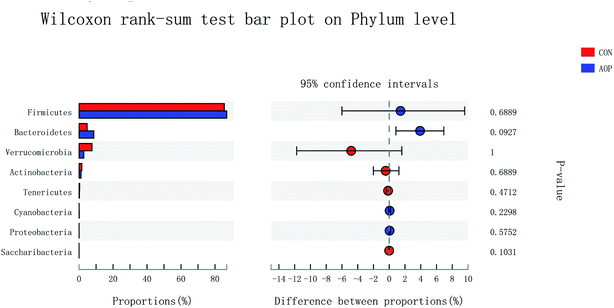 |
| | Fig. 8 Comparison of the bacteria (phylum level) present within the caecum of rats in CON and AOP groups. | |
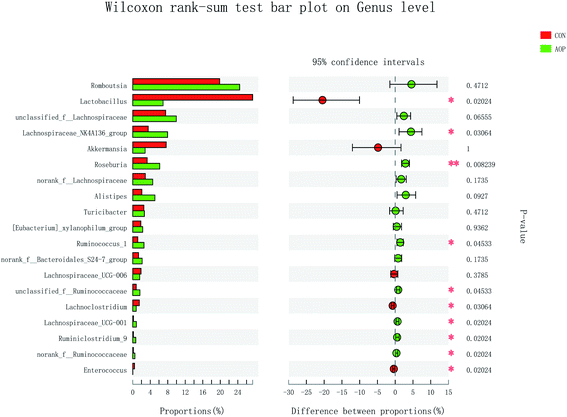
On genus level, the gut microbial community structure could be obviously changed by AOP feeding. In the present study, AOP increased the relative abundance of unclassified_f_Lachnospiraceae (P = 0.06), Lachnospiraceae_NK4A136_group (P = 0.03), Roseburia (P = 0.008), Alistipes (P = 0.09), Ruminococcus_1 (P = 0.04), unclassified_f_Ruminococcaceae (P = 0.04), Lachnospiraceae_UCG-001 (P = 0.02), Ruminiclostridium_9 (P = 0.02) and norank_f__Ruminococcaceae (P = 0.02) and decreased the relative abundance of Lactobacillus (P = 0.09), Lachnoclostridium (P = 0.09) and Enterococcus (P = 0.09) (Fig. 9). The relative abundance of bacteria in the AOP group was significantly different, indicating that AOP fermentation played a significant impact on the microbial communities. This result is similar to the findings of Fu et al. (2018),28 who reported that Sargassum thunbergii polysaccharide significantly increased the beneficial Bifidobacterium, Roseburia, Parasutterella and Fusicatenibacter. Ruminococcus and Roseburia have been well demonstrated to be the main butyric acid producers, responsible for degradation of polysaccharide and fibres.51,52 Therefore, AOP could potentially be a functional food modulating the composition and abundance of beneficial gut microbiota.
 |
| | Fig. 9 Comparison of the bacteria (genus level) present within the caecum of rats in CON and AOP groups. | |
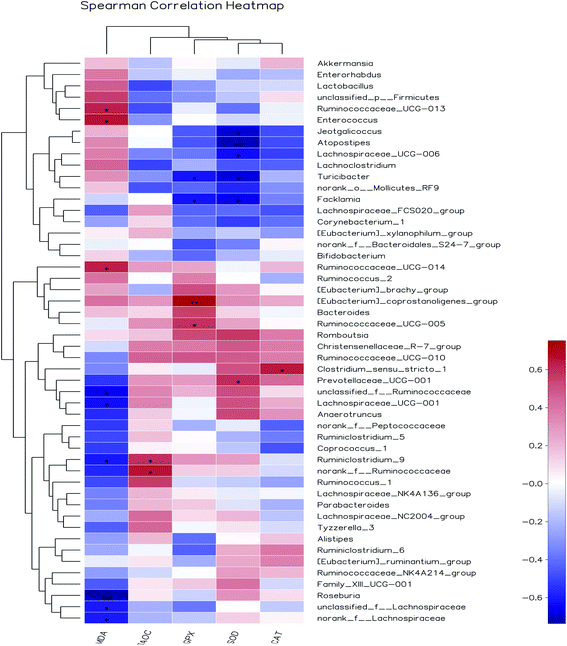
3.8.3 Correlation between gut bacteria and antioxidant indexes in vivo. Increasing evidences demonstrated that herbal medicines could modulate gut microbiota, thereby ameliorate inflammatory status and metabolic syndrome.53 However, so far, limited studies have been done to illustrate the correlation between gut bacteria and antioxidant function. Pearson's correlation coefficient (r) was used to determine if any significant correlations could be observed between antioxidant indexes in serum of the rats fed the different diets and any of the most relatively abundant bacterial genera (Fig. 10). The MDA content was significantly and positively associated with the abundance of Ruminococcaceae_UCG-013, Enterococcus and Ruminococcaceae_UCG-014, while negatively correlated with unclassified_f__Ruminococcaceae, Lachnospiraceae_UCG-001, Ruminiclostridium_9, Roseburia, unclassified_f__Lachnospiraceae and norank_f__Lachnospiraceae. The T-AOC was positively correlated with members of the Ruminiclostridium_9 and norank_f__Ruminococcaceae. The GPx activity was significantly and positively associated with the abundance of [Eubacterium]_coprostanoligenes_group and Ruminococcaceae_UCG-005, while negatively correlated with Turicibacter and Facklamia. SOD activity was positively associated with the abundance of Prevotellaceae_UCG-001, while negatively correlated with Jeotgalicoccus, Atopostipes, Lachnospiraceae_UCG-006, Turicibacter and Facklamia. CAT activity was positively associated with the abundance of Clostridium_sensu_stricto_1. Among them, the relative abundance of unclassified_f_Ruminococcaceae and unclassified_f_Lachnospiraceae were increased by AOP diets and negatively correlated with MDA content. Based on the present findings, we believed that the antioxidant effect of AOP was associated with the alteration of gut microbiota composition. Further in-depth research will be needed to understand the mechanism of crosstalk of gut microbiota and antioxidant function.
 |
| | Fig. 10 Heat maps showing correlations between the relative abundance of sequences assigned to each bacterial genus and antioxidant indexes. Pearson's correlation coefficients (r) are given, with r < 0 indicating a negative correlation (blue), r = 0 indicating no correlation (white) and r > 0 indicating a positive correlation (red). | |
4. Conclusion
The optimum extraction conditions were as follows: a liquid–solid ratio of 15.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL g−1, extraction time of 4.3 h, and extraction temperature of 60 °C. AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), has narrow polydispersity and rod conformations, and is composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid monohydrate with molar ratio of 6.87
1 mL g−1, extraction time of 4.3 h, and extraction temperature of 60 °C. AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), has narrow polydispersity and rod conformations, and is composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid monohydrate with molar ratio of 6.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.67
10.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.13
54.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.49
2.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.37
18.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.83
4.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64
2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64. In addition, AOP could exert the ability to scavenge radicals in vitro. Furthermore, it was found that the AOP enhanced the activities of SOD, CAT, and GPx in the liver and serum of rats, and up-regulated the corresponding gene expressions. Moreover, AOP significantly modulated the composition of cecal bacterial populations. Therefore, AOP is expected to be a functional ingredient for health improvement through modulating the gut health.
2.64. In addition, AOP could exert the ability to scavenge radicals in vitro. Furthermore, it was found that the AOP enhanced the activities of SOD, CAT, and GPx in the liver and serum of rats, and up-regulated the corresponding gene expressions. Moreover, AOP significantly modulated the composition of cecal bacterial populations. Therefore, AOP is expected to be a functional ingredient for health improvement through modulating the gut health.
Abbreviations
| AOP | Artemisia ordosica polysaccharide |
| BBD | Box–Behnken design |
| RSM | Response surface methodology |
| GPC-MALLS-RI | Gel permeation chromatography coupled to multi-angle laser light scattering, a refractive index detection system |
| Mn | Number-average molecular weight |
| Mw | Weight-average molecular weight |
| TFA | Trifluoroacetic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azinobis[3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt |
| RP | Reducing power |
| ADG | Average daily body weight gain |
| ADFI | Average daily feed intake |
| F/G | Ratio of feed to gain |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP | Alkaline phosphatase |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase, |
| T-AOC | Total antioxidant capacity |
| MDA | Malondialdehyde |
| RMS | Root mean square |
| PD | Polydispersity |
| PCA | Principal component analysis |
| SCFA | Short-chain fatty acids |
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
This work was supported by National Natural Science Foundation (Project No. 31960667).
References
- C. W. Wright, Artemisia, New York, 1st edn, 2002 Search PubMed.
- K. Li, P. Zhang, B. Shi, J. Su, Y. Yue, M. Tong and S. Yan, Dietary Artemisia ordosica extract alleviating immune stress in broilers exposed to lipopolysaccharide, Ital. J. Anim. Sci., 2017, 16, 301–330 CrossRef CAS.
- Y. Xing, Y. Wu, C. Mao, D. Sun, S. Guo, Y. Xu, X. Jin, S. Yan and B. Shi, Water extract of Artemisia ordosica enhances antioxidant capability and immune response without affecting growth performance in weanling piglets, J. Anim. Physiol. Anim. Nutr., 2019, 103(6), 1848–1856 CrossRef CAS PubMed.
- X. Zhou, Y. Zhang, X. An, R. De Philippis, X. Ma, C. Ye and L. Chen, Identification of aqueous extracts from Artemisia ordosica and their allelopathic effects on desert soil algae, Chemoecology, 2019, 29, 61–71 CrossRef.
- C. W. Cho, C. J. Han, Y. K. Rhee, Y. C. Lee, K. S. Shin, J. S. Shin, K. T. Lee and H. D. Hong, Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression, Int. J. Biol. Macromol., 2015, 72, 519–525 CrossRef CAS PubMed.
- N. Li, L. Li, J. C. Fang, J. H. Wong, T. B. Ng, Y. Jiang, C. R. Wang, N. Y. Zhang, T. Y. Wen and L. Y. Qu, Isolation and identification of a novel polysaccharide–peptide complex with antioxidant, antiproliferative and hypoglycaemic activities from the abalone mushroom, Biosci. Rep., 2012, 32, 221–228 CrossRef CAS PubMed.
- L. J. Li, M. Y. Li, Y. T. Li, J. J. Feng, F. Q. Hao and L. Zhang, Adjuvant activity of Sargassum pallidum polysaccharides against combined new castle disease, infectious bronchitis and avian influenza inactivated vaccines, Mar. Drugs, 2012, 10, 2648–2660 CrossRef CAS PubMed.
- P. Zhang, B. Shi, T. Li, Y. Xu, X. Jin, X. Guo and S. Yan, Immunomodulatory effect of Artemisia argyi polysaccharide on peripheral blood leucocyte of broiler chickens, J. Anim. Physiol. Anim. Nutr., 2018,(Suppl. 1), 1–8 Search PubMed.
- C. Ye and Y. Lai, Optimization of extraction process and antioxidant activity of polysaccharides from leaves of Artemisia argyi Levl. et vant, J. Food Process. Preserv., 2015, 39, 1309–1317 CrossRef CAS.
- H. J. Flint, E. A. Bayer, M. T. Rincon, R. Lamed and B. A. White, Polysaccharide utilization by gut bacteria potential for new insights from genomic analysis, Nat. Rev. Microbiol., 2008, 6, 121–131 CrossRef CAS PubMed.
- P. Louis, K. P. Scott, S. H. Duncan and H. J. Flint, Understanding the effects of diet on bacterial metabolism in the large intestine, J. Appl. Microbiol., 2007, 102, 1197–1208 CrossRef CAS PubMed.
- T. W. Hand, I. Vujkovic-Cvijin, V. K. Ridaura and Y. Belkaid, Linking the microbiota, chronic disease, and the immune system, Trends Endocrinol. Metab., 2016, 27(12), 831–843 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles and J. K. Hamilton, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS PubMed.
- P. Siddhuraju and K. Becker, J. Agric. Food Chem., 2003, 51, 2144–2155 CrossRef CAS PubMed.
- T. Bitter and H. M. Muir, Anal. Biochem., 1962, 4, 330–334 CrossRef CAS PubMed.
- T. Di, G. Chen, Y. Sun, S. Ou, X. Zeng and H. Ye, Antioxidant and immune-stimulating activities in vitro of sulfated polysaccharides isolated from Gracilaria rubra, J. Funct. Foods, 2017, 28, 64–75 CrossRef CAS.
- X. L. Wan, Y. Niu, X. C. Zheng, Q. Huang, W. P. Su, J. F. Zhang, L. L. Zhang and T. Wang, Antioxidant capacities of Artemisia annua L. leaves and enzymatically treated Artemisia annua L. in vitro and in broilers, Anim. Feed Sci. Technol., 2016, 221, 27–34 CrossRef CAS.
- Y. Wang, Z. Chen, J. Mao, M. Fan and X. Wu, Optimization of ultrasonic-assisted extraction process of Poria cocos polysaccharides by response surface methodology, Carbohydr. Polym., 2009, 77, 713–717 CrossRef CAS.
- C. Wang, L. Xu, L. Huang, X. R. Li, W. Han, D. Liu, X. Cui and Y. Yang, Optimization of maca polysaccharide extraction process and its chemo-protective effects on cyclophosphamide-induced mice, J. Food Process Eng., 2018, 41, e12856 CrossRef.
- S. Tsubaki, K. Oono, M. Hiraoka, A. Onda and T. Mitani, Microwave-assisted hydrothermal extraction of sulfated polysaccharides from Ulva spp. and Monostroma latissimum, Food Chem., 2016, 210, 311–316 CrossRef CAS PubMed.
- C. Chen, L. You, A. M. Abbasi, X. Fu and R. H. Liu, Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyperglycemic activity in vitro, Carbohydr. Polym., 2015, 130, 122–132 CrossRef CAS PubMed.
- Y. L. Yan, C. H. Yu, J. Chen, X. X. Li, W. Wang and S. Q. Li, Ultrasonic-assisted extraction optimized by response surface methodology, chemical composition and antioxidant activity of polysaccharides from Tremella mesenterica, Carbohydr. Polym., 2011, 83, 217–224 CrossRef CAS.
- X. Guo, X. Zou and M. Sun, Optimization of extraction process by response surface methodology and preliminary characterization of polysaccharides from Phellinus igniarius, Carbohydr. Polym., 2010, 80, 344–349 CrossRef CAS.
- J. Liu, Y. Sun, L. Liu and C. Yu, The extraction process optimization and physicochemical properties of polysaccharides from the roots of Euphorbia fischeriana, Int. J. Biol. Macromol., 2011, 49, 0–421 Search PubMed.
- L. Wang, H. M. Liu and G. Y. Qin, Structure characterization and antioxidant activity of polysaccharides from Chinese quince seed meal, Food Chem., 2017, 234, 314–322 CrossRef CAS PubMed.
- X. Yu, C. Zhou, H. Yang, X. Huang, H. Ma, X. Qin and J. Hu, Effect of ultrasonic treatment on the degradation and inhibition cancer cell lines of polysaccharides from Porphyra yezoensis, Carbohydr. Polym., 2015, 117, 650–656 CrossRef CAS PubMed.
- X. Fu, C. Cao, B. Ren, B. Zhang, Q. Huang and C. Li, Structural characterization and, in vitro, fermentation of a novel polysaccharide from, Sargassum thunbergii, and its impact on gut microbiota, Carbohydr. Polym., 2018, 183, 230–239 CrossRef CAS PubMed.
- W. Burchard, in Branched Polymers II. Advances in Polymer Science, ed. J. Roovers, Springer, Berlin, Heidelberg, 1999, vol. 143, pp. 113–194 Search PubMed.
- L. Yang and L. M. Zhang, Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources, Carbohydr. Polym., 2009, 76, 349–361 CrossRef CAS.
- V. Salvador, C. Cherbut, J. L. Barry, D. Bertrand, C. Bonnet and J. Delort-Laval, Sugar composition of dietary fibre and short-chain fatty acid production during in vitro fermentation by human bacteria, Br. J. Nutr., 1993, 70, 189–197 CrossRef CAS PubMed.
- H. B. Wang, S. J. Wu and D. Liu, Preparation of polysaccharides from cyanobacteria Nostoc commune and their antioxidant activities, Carbohydr. Polym., 2014, 99, 553–555 CrossRef CAS PubMed.
- S. Q. Li and N. P. Shah, Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275, Food Chem., 2014, 165, 262–270 CrossRef CAS PubMed.
- P. Shao, X. X. Chen and P. L. Sun, Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri, Carbohydr. Polym., 2014, 105, 260–269 CrossRef CAS PubMed.
- P. F. Zhang, B. L. Shi, J. L. Su, Y. X. Yue, Z. X. Cao, W. B. Chu, K. Li and S. M. Yan, Relieving effect of Artemisia argyi aqueous extract on immune stress in broilers, J. Anim. Physiol. Anim. Nutr., 2016, 101, 251–258 CrossRef PubMed.
- S. Guo, J. Ma, Y. Xing, Y. Xu, X. Jin, S. Yan and B. Shi, Artemisia annua L. aqueous extract as an alternative to antibiotics improving growth performance and antioxidant function in broilers, Ital. J. Anim. Sci., 2020, 19, 399–409 CrossRef.
- L. P. James, P. R. Mayeux and J. A. Hinson, Acetaminophen-induced hepatotoxicity, Drug Metab. Dispos., 2003, 31, 1499–1506 CrossRef CAS PubMed.
- J. Zhang, X. Wang, V. Vikash, Q. Ye, D. Wu, Y. Liu and W. Dong, ROS and ROS-Mediated Cellular Signaling, Oxid. Med. Cell. Longevity, 2016, 2016, 1–18 CAS.
- Z. J. Wang, J. H. Xie, S. P. Nie and M. Y. Xie, Review on cell models to evaluate the potential antioxidant activity of polysaccharides, Food Funct., 2017, 8, 915–926 RSC.
- Z. Wang, J. Xie, Y. Yang, F. Zhang, S. Wang, T. Wu, M. Shen and M. Xie, Sulfated Cyclocarya paliurus polysaccharides markedly attenuates inflammation and oxidative damage in lipopolysaccharide-treated macrophage cells and mice, Sci. Rep., 2017, 7, 40402–40412 CrossRef CAS PubMed.
- X. Tong, J. Xu, F. Lian, X. Yu, Y. Zhao, L. Xu, M. Zhang, X. Zhao, J. Shen, S. Wu, X. Pang, J. Tian, C. Zhang, Q. Zhou, L. Wang, B. Pang, F. Chen, Z. Peng, J. Wang, Z. Zhen, C. Fang, M. Li, L. Chen and L. Zhao, Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial, mBio, 2018, 9, e02392-17 CrossRef PubMed.
- J. H. Wang, S. Bose, S. K. Lim, A. Ansari, Y. W. Chin, H. S. Choi and H. Kim, Houttuynia cordata Facilitates Metformin on Ameliorating Insulin Resistance Associated with Gut Microbiota Alteration in OLETF Rats, Genes, 2017, 8, 239–258 CrossRef PubMed.
- G. R. Gibson and R. Fuller, Aspects of in vitro and in vivo research approaches directed toward identifying probiotics and prebiotics for human use, J. Nutr., 2000, 130, 391S–395S CrossRef CAS PubMed.
- L. Montagne, J. R. Pluske and D. J. Hampson, A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals, Anim. Feed Sci. Technol., 2003, 108, 95–117 CrossRef.
- A. Salonen and W. M. de Vos, Impact of diet on human intestinal microbiota and health, Annu. Rev. Food Sci. Technol., 2014, 5, 239–262 CrossRef CAS PubMed.
- H. Yang, Y. Xiao, G. Gui, J. Li, J. Wang and D. Li, Microbial community and short-chain fatty acid profile in gastrointestinal tract of goose, Poult. Sci., 2018, 97, 1420–1428 CrossRef CAS PubMed.
- H. Yang, Y. Xiao, J. Wang, Y. Xiang, Y. Gong, X. Wen and D. Li, Core gut microbiota in jinhua pigs and its correlation with strain, farm and weaning age, J. Microbiol., 2018, 56, 346–355 CrossRef CAS PubMed.
- X. Wang, X. Wang, H. Jiang, C. Cai, G. Li, J. Hao and G. Yu, Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: an overview, Carbohydr. Polym., 2018, 195, 601–612 CrossRef CAS PubMed.
- Y. Wang, Y. Fei, L. Liu, Y. Xiao, Y. Pang, J. Kang and Z. Wang, Polygonatum odoratum Polysaccharides Modulate Gut Microbiota and Mitigate Experimentally Induced Obesity in Rats, Int. J. Mol. Sci., 2018, 19, 3587 CrossRef PubMed.
- M. A. Mahowald, F. E. Rey, H. Seedorf, P. J. Turnbaugh, R. S. Fulton, A. Wollam and J. I. Gordon, Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 5859–5864 CrossRef CAS PubMed.
- S. Hooda, B. M. V. Boler, M. C. R. Serao, J. M. Brulc, M. A. Staeger, T. W. Boileau and K. S. Swanson, Pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber, J. Nutr., 2012, 142(7), 1259–1265 CrossRef CAS PubMed.
- K. P. Scott, J. C. Martin, S. H. Duncan and H. J. Flint, Prebiotic stimulation of human colonic butyrate-producing bacteria and bifidobacteria, in vitro, FEMS Microbiol. Ecol., 2014, 87(1), 30–40 CrossRef CAS PubMed.
- J. M. Leiro, R. Castro, J. A. Arranz and J. Lamas, Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh, Int. Immunopharmacol., 2007, 7, 879–888 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL g−1, extraction time of 4.3 h, extraction temperature of 60 °C. Under the optimal conditions, the extraction yield and the sugar content of the AOP were 5.56% and 52.65%. Gel permeation chromatography coupled to multi-angle laser light scattering, a refractive index detection system and ion-exchange chromatography were used to determine the characterization of AOP. These results indicated that AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), had narrow polydispersity and rod conformations, and was composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid with molar ratio of 6.87
1 mL g−1, extraction time of 4.3 h, extraction temperature of 60 °C. Under the optimal conditions, the extraction yield and the sugar content of the AOP were 5.56% and 52.65%. Gel permeation chromatography coupled to multi-angle laser light scattering, a refractive index detection system and ion-exchange chromatography were used to determine the characterization of AOP. These results indicated that AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), had narrow polydispersity and rod conformations, and was composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid with molar ratio of 6.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.67
10.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.13
54.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.49
2.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.37
18.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.83
4.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64
2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64. In addition, AOP exerted antioxidant ability in vitro and in vivo (rats). Moreover, AOP significantly modulated the composition of cecal microbiota population. Therefore, AOP is expected to be a functional ingredient for health improvement through improving antioxidant ability and modulating gut health.
2.64. In addition, AOP exerted antioxidant ability in vitro and in vivo (rats). Moreover, AOP significantly modulated the composition of cecal microbiota population. Therefore, AOP is expected to be a functional ingredient for health improvement through improving antioxidant ability and modulating gut health.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) for precipitation of polysaccharide for 48 h at 4 °C. The sediment was collected by centrifugation (12
1, v/v) for precipitation of polysaccharide for 48 h at 4 °C. The sediment was collected by centrifugation (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 15 min) and washed successively with petroleum ether, acetone, and ethanol. Then the sediment was dissolved in water and deproteinated twice with Sevag reagent (n-butyl alcohol
000 × g, 15 min) and washed successively with petroleum ether, acetone, and ethanol. Then the sediment was dissolved in water and deproteinated twice with Sevag reagent (n-butyl alcohol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform = 1
chloroform = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Afterward, the supernatant was collected and dialyzed using a biological semipermeable membrane (molecular weight cutoff: 500 Da, Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) against distilled water at 4 °C for 48 h, with changing the distilled water every 12 h. The resulting solution was lyophilized by a vacuum evaporate to prepare the powder, and stored at −20 °C until use. The total carbohydrate content was measured by using the phenol–sulfuric acid method using glucose as the standard.13 The protein content was determined by Coomassie brilliant blue method using bovine serum albumin as the standard.14 The polyphenols content was accomplished by Folin–Ciocalteu reagent assay using gallic acid as the standard.15 The uronic acid content was evaluated using glucuronic acid as a standard.16
4). Afterward, the supernatant was collected and dialyzed using a biological semipermeable membrane (molecular weight cutoff: 500 Da, Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) against distilled water at 4 °C for 48 h, with changing the distilled water every 12 h. The resulting solution was lyophilized by a vacuum evaporate to prepare the powder, and stored at −20 °C until use. The total carbohydrate content was measured by using the phenol–sulfuric acid method using glucose as the standard.13 The protein content was determined by Coomassie brilliant blue method using bovine serum albumin as the standard.14 The polyphenols content was accomplished by Folin–Ciocalteu reagent assay using gallic acid as the standard.15 The uronic acid content was evaluated using glucuronic acid as a standard.16
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 10 min to collect the supernatant. Gel permeation chromatography (GPC) coupled to multi-angle laser light scattering (MALLS; Heleos, Wyatt Technology Corp., Santa Barbara, CA, USA), and a refractive index (RI; model RI-150; Thermo Electron Corp., Yokohama, Japan) detection system (GPC-MALLS-RI system) was used to determine the average molecular weight of AOP. The mobile phase of the GPC-MALLS-RI was 0.1 M NaNO3 with 0.02% (w/v) NaN3 at a flow rate of 0.4 mL min−1 and column oven temperature was set at 60 °C. The value of refractive index increment (dn/dc) was 0.14 mL g−1. The injection volume was 20 μL. The Mw, polydispersity (Mw/Mn) and root mean square (RMS) radius were calculated.
000 × g for 10 min to collect the supernatant. Gel permeation chromatography (GPC) coupled to multi-angle laser light scattering (MALLS; Heleos, Wyatt Technology Corp., Santa Barbara, CA, USA), and a refractive index (RI; model RI-150; Thermo Electron Corp., Yokohama, Japan) detection system (GPC-MALLS-RI system) was used to determine the average molecular weight of AOP. The mobile phase of the GPC-MALLS-RI was 0.1 M NaNO3 with 0.02% (w/v) NaN3 at a flow rate of 0.4 mL min−1 and column oven temperature was set at 60 °C. The value of refractive index increment (dn/dc) was 0.14 mL g−1. The injection volume was 20 μL. The Mw, polydispersity (Mw/Mn) and root mean square (RMS) radius were calculated.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 1A), temperature of 60 °C (Fig. 1B) and extraction time of 4 h (Fig. 1C). Of note, in the present study, the extraction yield of AOP significantly increased with the increase of extraction temperature from 20 to 60 °C and significantly decreased when the extraction temperature increased from 60 to 100 °C. An increasing extraction temperature could increase the molecular movement, resulting in accelerating the transfer of intracellular substances from the cells.21 However, higher temperature can cause the degradation of some thermo-sensitive materials, such as polysaccharide, resulting in declined yield.22 In addition, DPPH radical scavenging ability (%) was higher under this condition as well (Fig. 1A–C). Therefore, a liquid–solid ratio of 10
1 (Fig. 1A), temperature of 60 °C (Fig. 1B) and extraction time of 4 h (Fig. 1C). Of note, in the present study, the extraction yield of AOP significantly increased with the increase of extraction temperature from 20 to 60 °C and significantly decreased when the extraction temperature increased from 60 to 100 °C. An increasing extraction temperature could increase the molecular movement, resulting in accelerating the transfer of intracellular substances from the cells.21 However, higher temperature can cause the degradation of some thermo-sensitive materials, such as polysaccharide, resulting in declined yield.22 In addition, DPPH radical scavenging ability (%) was higher under this condition as well (Fig. 1A–C). Therefore, a liquid–solid ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–20
1–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a temperature range between 40–80 °C and an extraction time of 2–6 h was selected for the BBD (Table 2). The experimental scheme was arranged by BBD and experimental results were shown in Table 3. The response surface plots and contour plots between the factors and AOP yield were shown in Fig. 2A–F, which depicted the interactions between two variables by keeping the other variables at their zero levels for AOP yield. The shapes of the contour plots, circular or elliptical, indicate whether the mutual interactions between variables are significant or not.23 In addition, the analysis of variance was conducted and detailed in Table 4. From Fig. 2 and Table 4, it could be seen that two interaction coefficients (A and C) and all quadratic term coefficients (A2, B2 and C2) were significant with low P values (P < 0.05). Moreover, the model was highly significant (Table 4), which indicated that the predicted model more highly illustrated the pattern of the interactions between variables.24
1, a temperature range between 40–80 °C and an extraction time of 2–6 h was selected for the BBD (Table 2). The experimental scheme was arranged by BBD and experimental results were shown in Table 3. The response surface plots and contour plots between the factors and AOP yield were shown in Fig. 2A–F, which depicted the interactions between two variables by keeping the other variables at their zero levels for AOP yield. The shapes of the contour plots, circular or elliptical, indicate whether the mutual interactions between variables are significant or not.23 In addition, the analysis of variance was conducted and detailed in Table 4. From Fig. 2 and Table 4, it could be seen that two interaction coefficients (A and C) and all quadratic term coefficients (A2, B2 and C2) were significant with low P values (P < 0.05). Moreover, the model was highly significant (Table 4), which indicated that the predicted model more highly illustrated the pattern of the interactions between variables.24

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; extraction time of 4.28 h and extraction temperature of 59.96 °C. The prediction yield of AOP was estimated to be up to 5.84%. The extraction experiment was repeated three times under the optimal extraction condition (4.3 h, 60 °C, and liquid–solid ratio of 15.4
1; extraction time of 4.28 h and extraction temperature of 59.96 °C. The prediction yield of AOP was estimated to be up to 5.84%. The extraction experiment was repeated three times under the optimal extraction condition (4.3 h, 60 °C, and liquid–solid ratio of 15.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to verify the reliability of the results, and the extractive rate of AOP was 5.56% (Table 5), which was very close to the predicted value. It was suggested that the optimum conditions for AOP extraction could be effectively performed in practice. The carbohydrate content, protein content, uronic acid content and phenolic content for AOP were 52.65%, 2.39%, 4.27% and 0.11% (Table 5).
1) to verify the reliability of the results, and the extractive rate of AOP was 5.56% (Table 5), which was very close to the predicted value. It was suggested that the optimum conditions for AOP extraction could be effectively performed in practice. The carbohydrate content, protein content, uronic acid content and phenolic content for AOP were 52.65%, 2.39%, 4.27% and 0.11% (Table 5).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rg–log
Rg–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mw indicated the rod conformations was adopted for AOP with a slope of 0.78 (Fig. 3B). The value of the line slope of the line when plotting log
Mw indicated the rod conformations was adopted for AOP with a slope of 0.78 (Fig. 3B). The value of the line slope of the line when plotting log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rg versus log
Rg versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M can be used to obtain conformational information of polymers in solution, e.g., the value of v is approximately 0.3 for hard sphere conformation, approximately 0.5 for random coil, and approximately 1 for rigid rod.29,30 The conformation plot of log
M can be used to obtain conformational information of polymers in solution, e.g., the value of v is approximately 0.3 for hard sphere conformation, approximately 0.5 for random coil, and approximately 1 for rigid rod.29,30 The conformation plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rg–log
Rg–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mw indicated the rigid rod conformations were adopted for AOP.
Mw indicated the rigid rod conformations were adopted for AOP.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.67
10.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.13
54.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.49
2.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.37
18.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.83
4.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64
2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64 (Fig. 4B). It has been reported that polysaccharide could modulate the composition and abundance of beneficial microbiota through altering fermentation behavior, which is greatly influenced by their monosaccharide compositions (see following sections).31
2.64 (Fig. 4B). It has been reported that polysaccharide could modulate the composition and abundance of beneficial microbiota through altering fermentation behavior, which is greatly influenced by their monosaccharide compositions (see following sections).31


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F. However, the effect of AOP on the ADFI was not detected (P > 0.05). Our previous study indicated that Artemisia argyi aqueous extract and Artemisia annua L. aqueous extract could improve growth performance of broilers.35,36 The present study was further confirmed the growth prompting effect of polysaccharide as the main component in the plant aqueous extract.
F. However, the effect of AOP on the ADFI was not detected (P > 0.05). Our previous study indicated that Artemisia argyi aqueous extract and Artemisia annua L. aqueous extract could improve growth performance of broilers.35,36 The present study was further confirmed the growth prompting effect of polysaccharide as the main component in the plant aqueous extract.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) F, g g−1
F, g g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 056 sequences were gained with an average length of 432 bp. A total of 555 OTUs were generated via clustering analysis for high-quality sequences at a 97% similarity cutoff. The rarefaction and Shannon index curves that were generated from the OTUs revealed that the data had covered most of the diversity and new phylotypes (Fig. 6), suggesting that high sampling coverage was captured with the sequencing depth, such that further increasing the sequencing depth was unlikely to achieve more gut microbiota diversity. Furthermore, the diets played a key role in shaping phylogenetic diversity based on the α-diversity. Thereinto, Shannon index was significantly increased (p < 0.05) after AOP treatment (Table 8). The principal component analysis (PCA) was used to analyze the composition changes in the gut microbiota. The results revealed that AOP diet caused significant separation of fecal microbiota for CON and AOP diet groups (Fig. 7).
056 sequences were gained with an average length of 432 bp. A total of 555 OTUs were generated via clustering analysis for high-quality sequences at a 97% similarity cutoff. The rarefaction and Shannon index curves that were generated from the OTUs revealed that the data had covered most of the diversity and new phylotypes (Fig. 6), suggesting that high sampling coverage was captured with the sequencing depth, such that further increasing the sequencing depth was unlikely to achieve more gut microbiota diversity. Furthermore, the diets played a key role in shaping phylogenetic diversity based on the α-diversity. Thereinto, Shannon index was significantly increased (p < 0.05) after AOP treatment (Table 8). The principal component analysis (PCA) was used to analyze the composition changes in the gut microbiota. The results revealed that AOP diet caused significant separation of fecal microbiota for CON and AOP diet groups (Fig. 7).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mL g−1, extraction time of 4.3 h, and extraction temperature of 60 °C. AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), has narrow polydispersity and rod conformations, and is composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid monohydrate with molar ratio of 6.87
1 mL g−1, extraction time of 4.3 h, and extraction temperature of 60 °C. AOP, with a molecular weight of 2.1 kDa (62.6%) and 1.5 kDa (37.4%), has narrow polydispersity and rod conformations, and is composed of arabinose, galactose, glucose, xylose, mannose, galacturonic acid and glucuronic acid monohydrate with molar ratio of 6.87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10.67
10.67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54.13
54.13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.49
2.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18.37
18.37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.83
4.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64
2.64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.64. In addition, AOP could exert the ability to scavenge radicals in vitro. Furthermore, it was found that the AOP enhanced the activities of SOD, CAT, and GPx in the liver and serum of rats, and up-regulated the corresponding gene expressions. Moreover, AOP significantly modulated the composition of cecal bacterial populations. Therefore, AOP is expected to be a functional ingredient for health improvement through modulating the gut health.
2.64. In addition, AOP could exert the ability to scavenge radicals in vitro. Furthermore, it was found that the AOP enhanced the activities of SOD, CAT, and GPx in the liver and serum of rats, and up-regulated the corresponding gene expressions. Moreover, AOP significantly modulated the composition of cecal bacterial populations. Therefore, AOP is expected to be a functional ingredient for health improvement through modulating the gut health.





