Open Access Article
Open Access ArticleSynthesis of biodiesel from waste cooking oil catalyzed by β-cyclodextrin modified Mg–Al–La composite oxide
Guanhao Liu ,
Jingyi Yang* and
Xinru Xu
,
Jingyi Yang* and
Xinru Xu
Research Institute of Petroleum Processing, East China University of Science and Technology, Meilong Road 130, Shanghai, 20023, P. R. China. E-mail: 10110567@mail.ecust.edu.cn; Fax: +86 21 64252160; Tel: +86 21 64252160
First published on 14th July 2020
Abstract
In this study, Mg–Al–La composite oxide loaded with ionic liquid [Bmim]OH was used as a catalyst for the synthesis of fatty acid isobutyl ester (FAIBE) via transesterification between waste cooking oil and isobutanol. Mg–Al–La composite oxide was synthesized from the β-cyclodextrin (β-CD) intercalation modification of Mg–Al–La layered double hydroxides. The structure of the catalyst was characterized via XRD, BET and EDS. The results showed that the interlayer space of the catalyst was increased due to β-CD intercalation modification. The IL/CD–Mg–Al–La catalyst exhibited significant catalytic activity and regeneration performance in transesterification due to large interlayer space and strongly alkaline ionic liquid. The yield of FAIBE achieved was 98.3% under the optimum reaction condition and 95.2% after regeneration for six times. The viscosity–temperature curve of FAIBE was determined and the phase transition temperature was −1 °C. The pour point of FAIBE was only −10 °C, which exhibited excellent low temperature fluidity.
1. Introduction
Fatty acid esters obtained via esterification are the main components of biodiesel in chemical industry.1,2 However, the high pour point of biodiesel prepared from methanol often limits its application. The esterification between isobutanol and waste cooking oil can obtain biodiesel with a low pour point, and herein, the biological synthesis method of isobutanol has been reported.3 The energy pressure in the world can be greatly relieved and the green production of fuel can be achieved by this method. Since the solid base catalysts for esterification are less corrosive and easy to separate from the product, hydrotalcites (HTs) have been considered as catalysts or carriers.Hydrotalcites and hydrotalcite-like compounds are double-layered hydroxide materials.4 The composite metal oxides obtained by the calcination of HTs can be used as catalysts for transesterification. The catalytic properties of composite oxides were affected remarkably by elemental composition,5,6 spatial structures and so on. In order to improve the catalytic properties, some strong alkaline substances such as KF (ref. 7) and ionic liquids8 have been immobilized on HTs. The influence of the spatial structure was relatively less reported. The different interlayer spacing was obtained when terephthalic acid was intercalated in HTs at different drying temperatures.9 The maximum interlayer spacing was obtained, and the terephthalic acid was vertically arranged between the layers when the temperature was 25 °C. The interlayer spacing decreased at 100 °C because the molecular plane of terephthalic acid was parallel to the layers. Prevot10 reported that HTs with different interlayer spacing modified by tartaric acid were synthesized at different temperatures because of the configuration transformation of interlayer tartaric acid anions with temperature. In this study, β-cyclodextrin was used to extend the interlayer of HTs.
β-Cyclodextrin is an oligomerized cyclic molecule by 7 glucopyranose units with a hydrophilic external wall and a hydrophobic internal wall.11 It can act as a selective envelope for a variety of organic and inorganic molecules to form inclusion or molecular assembly systems. Mohanambe12 synthesized hydrophobic nanoscale inclusion complexes by intercalating β-cyclodextrin into Mg–Al HTs for including anthracene molecules. Carolina13 studied the adsorption behavior of gold nanorods in cyclodextrin inclusion complexes and pointed out that cyclodextrin or cyclodextrin inclusion complexes can be used for the storage of gold nanorods.
In this study, functionalized basic IL-supported solid base catalysts modified by β-cyclodextrin were synthesized to improve the efficiencies of catalysts. The obtained catalysts were characterized via X-ray diffraction (XRD), N2 adsorption (BET) and Edax Falcon energy dispersive spectroscopy (EDS). The activity of catalysts and the low temperature fluidity of FAIBE were investigated.
2. Experimental
2.1 Catalyst preparation
CD–Mg–Al–La composite oxides were prepared via coprecipitation. Mg(NO3)2·6H2O, Al(NO3)3·9H2O and La(NO3)3·6H2O were dissolved in 100 mL deionized water to obtain the solution A according to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 molar ratio of Mg, Al and La. NaOH and Na2CO3 were mixed with a certain amount of β-cyclodextrin to form the solution B. The quantity of NaOH was determined according to the 2
0.4 molar ratio of Mg, Al and La. NaOH and Na2CO3 were mixed with a certain amount of β-cyclodextrin to form the solution B. The quantity of NaOH was determined according to the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of NaOH and the sum of Mg and Al. The molar ratio of NaOH and Na2CO3 was 4
1 molar ratio of NaOH and the sum of Mg and Al. The molar ratio of NaOH and Na2CO3 was 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Some deionized water was added into a 500 mL round bottom flask with three necks, then heated up to 65 °C, and stirred constantly with a magnetic stirrer. A and B were transferred to two constant pressure funnels, and then a double titration reaction occurred by controlling the drop rate A and B solution in the constant pressure funnels. The titration lasted for about 1 hour and the pH of the whole reaction system was kept at about 10 using the pH meter. The reaction system was vigorously stirred for 0.5 h after the end of titration. The reaction products of the round bottom flask were transferred to a 500 mL conical flask and then aged for 24 h in the thermostat-controlled water-bath at 70 °C. Mg–Al–La hydrotalcite modified by β-cyclodextrin could be obtained after washing, filtering and drying. The CD–Mg–Al–La composite oxides were obtained by calcining CD–Mg–Al–La hydrotalcites at 500 °C for 6 h to dehydrate and decarburize.
1. Some deionized water was added into a 500 mL round bottom flask with three necks, then heated up to 65 °C, and stirred constantly with a magnetic stirrer. A and B were transferred to two constant pressure funnels, and then a double titration reaction occurred by controlling the drop rate A and B solution in the constant pressure funnels. The titration lasted for about 1 hour and the pH of the whole reaction system was kept at about 10 using the pH meter. The reaction system was vigorously stirred for 0.5 h after the end of titration. The reaction products of the round bottom flask were transferred to a 500 mL conical flask and then aged for 24 h in the thermostat-controlled water-bath at 70 °C. Mg–Al–La hydrotalcite modified by β-cyclodextrin could be obtained after washing, filtering and drying. The CD–Mg–Al–La composite oxides were obtained by calcining CD–Mg–Al–La hydrotalcites at 500 °C for 6 h to dehydrate and decarburize.
The IL/CD–Mg–Al–La composite oxides were prepared via ionic liquid immobilization. The IL [Bmim]OH were obtained from hydrotalcite precursors according to our previous research.14 [Bmim]OH was loaded on the CD–Mg–Al–La composite oxide by a grafting method. The ionic liquid and carriers were uniformly dispersed into anhydrous toluene and refluxed for 24 h under the atmosphere of nitrogen at 80 °C. The IL/CD–Mg–Al–La catalyst was obtained after being cooled, filtered, washed with ethyl acetate and dried for 12 h.
2.2 Characterization method of the catalyst
The crystallite sizes and morphologies of the solid catalyst were characterized via X-ray diffraction (XRD) on Rigaku D/max2550VB/PC with CuKα radiation (λ = 0.154 nm, 100 mA, 45 kV). The scanning range was 10–80° (2θ) with a rate of 0.02° s−1. Surface elements were measured using an Edax Falcon energy dispersive spectrometer (EDS). The pore size distribution and surface area of catalysts were obtained via the Brunauer–Emmett–Teller (BET) method on Micromeritrics ASAP-2400.142.3 Transesterification procedures
The raw oil used in the experiment was WCO, which was vegetable oil after repeatedly frying at a high temperature. The properties and composition of WCO are shown in Table 1. A certain amount of waste cooking oil, alcohol and catalysts were added to the reactor. The catalyst was filtered out and the filtrate was stratified in a separating funnel after the reaction. The upper layer of the liquid was crude methyl ester, while the lower layer was crude glycerol. The upper liquid was washed by hot water at 80 °C and then separated from water. Fatty acid ester can be obtained from the supernatant liquid after vacuum drying at 60 °C for 2 hours. Fatty acid ester in products was determined via gas chromatography.7| Properties | Component% | ||
|---|---|---|---|
| Iodine value (g (I2)/100 g) | 125 | C16:0 | 15.0 |
| Acid value (mg (KOH) per g) | 4.88 | C18:0 | 6.80 |
| Saponification value (mg (KOH) per g) | 196.3 | C18:1 | 26.6 |
| Average molar mass (g mol−1) | 883.6 | C18:2 | 46.3 |
| Water content (mg kg−1) | 400 | Others | 5.30 |
2.4 Characteristics determination of FAIBE
The ASTM D7346, ASTM D7689 and ASTM D6371 standard were used for determining pour point, cloud point and cold filter point of FAIBE, respectively. The viscosity of FAIBE was determined using an HAAKE Viscotester E viscometer produced by Thermo Fisher Scientific. Different sizes of rotors and different speeds of the rotary viscometer can be used to obtain different viscosity measurement ranges. The crystallization temperature was determined by a DSC-200PC differential scanning calorimeter and the crystallization behavior of FAIBE at a low temperature was analyzed.3. Results and discussion
3.1 Catalyst characterization
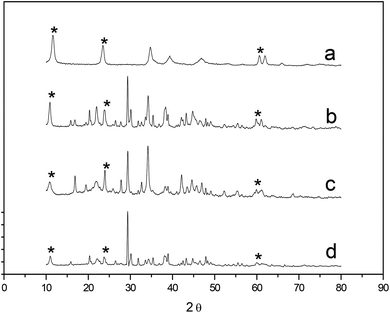
The XRD patterns of C0 (Mg–Al–La) and Mg–Al–La hydrotalcite modified by β-CD are shown in Fig. 1. The hydrotalcite peaks of the samples appeared at the place, where 2θ were 11.0°, 22.3°, 62.3° in the patterns of C1 (0.01CD–Mg–Al–La), C2 (0.02CD–Mg–Al–La) and C3 (0.03CD–Mg–Al–La) hydrotalcite synthesized according to the adding amount of β-CD, which were 0.01 mol L−1, 0.02 mol L−1 and 0.03 mol L−1, respectively.The lattice parameters of Mg–Al–La hydrotalcite modified by β-CD are presented in Table 2. d003 was the interlayer space of the hydrotalcites. The d003 value of a, b, c and d were 0.810, 1.621, 1.630 and 1.635, respectively, which showed that the interlayer space of the hydrotalcites will be significantly increased with the increase in the amount of β-CD in the preparation process. This indicated that β-CD was inserted into the interlayer in the crystallization of the hydrotalcite and expanded the interlayer space. The lattice parameter A of hydrotalcite was the atomic arrangement density of the d(003) diffraction surface, which was only related to the radius and composition ratio of Mg/Al/La atoms. The parameter A of 0.01CD–Mg–Al–La hydrotalcite and 0.02CD–Mg–Al–La hydrotalcite was the same as Mg–Al–La hydrotalcite. However, the value of A changed when the added amount of β-CD was 0.03 mol L−1, which indicated that the composition ratio of Mg/Al/La in the 0.03CD–Mg–Al–La hydrotalcite was affected by the intercalation of β-CD.
| Cell parameter (nm) | C0 | C1 | C2 | C3 |
|---|---|---|---|---|
| d003 | 0.810 | 1.621 | 1.630 | 1.635 |
| d006 | 0.375 | 0.713 | 0.722 | 0.724 |
| d009 | 0.263 | 0.501 | 0.510 | 0.521 |
| d110 | 0.153 | 0.153 | 0.153 | 0.151 |
| A | 0.306 | 0.306 | 0.306 | 0.302 |
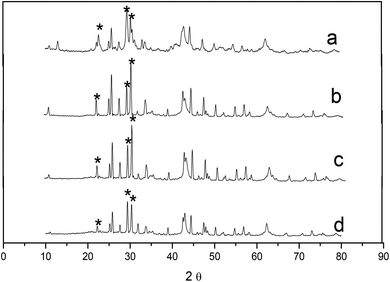
The XRD patterns of the β-CD modified composite oxides can be seen from Fig. 2. In the patterns of four kinds of composite oxides, the La2O3 peak of samples appeared at the place where 2θ was 22.0°, 29.1°, 30.0° and got gradually sharp and regular with the increase in the added amount of β-CD in the coprecipitation. The strength of the La2O3 peak weakened when the dosage of β-CD was 0.03 mol L−1, which indicated that the intercalation of β-CD in hydrotalcite had affected the La2O3 crystalline phase of Mg–Al–La after calcination. Thus, the appropriate amount of β-CD was 0.02 mol L−1 in the preparation process. The trivalent cation La3+ increased the concentration of O2−, and thus modified the oxide's exhibited higher activity.15
The specific surface area and pore size of IL/Mg–Al–La before and after modification by β-cyclodextrin are shown in Table 3. The results showed that the insertion of β-cyclodextrin on the interlayer of hydrotalcite increased the average pore size and the specific surface area of IL/Mg–Al–La. After adding 0.02 mol L−1 β-CD in the preparation of hydrotalcite, the specific surface area of IL/CD–Mg–Al–La increased from 30.2 m2 g−1 to 48.7 m2 g−1 and the average pore size increased from 13.3 nm to 20.1 nm.
| Catalyst | Specific surface area (m2 g−1) | Pore size (nm) |
|---|---|---|
| IL/Mg–Al–La | 30.2 | 13.3 |
| IL/CD–Mg–Al–La | 48.7 | 20.1 |
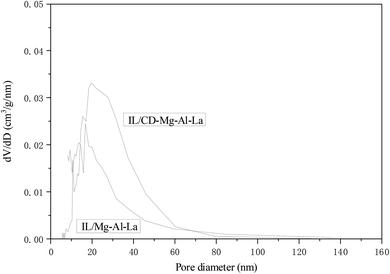
Fig. 3 was the pore size distribution curve of the IL/Mg–Al–La composite oxide catalyst before and after the modification by β-cyclodextrin. The size of most pores in the composite oxide supported by ionic liquid was about 19 nm, while the pore size of composite oxide modified by β-cyclodextrin was distributed mainly on 27 nm. Obviously, the number of pores whose diameter was larger than 20 nm in IL/CD–Mg–Al–La was more than that in IL/Mg–Al–La, as shown in Fig. 3. β-Cyclodextrin with a large molecular volume can effectively increase the lamellar spacing after intercalation in the hydrotalcites, which further leads to the increase in the pore size of the composite oxide. The excellent catalytic effect for the transesterification of isobutanol also proved this point. However, the pore size distribution of IL/CD–Mg–Al–La became more dispersed.
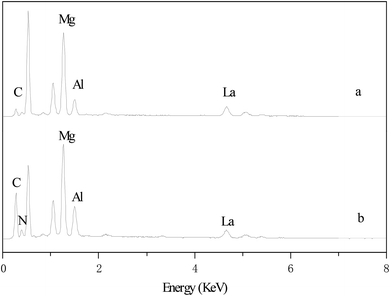
Fig. 4 reflected the EDS analysis results for CD–Mg–Al–La and IL/CD–Mg–Al–La composite oxides. The results showed that the content of main elements on the surface of Mg–Al–La modified by β-CD were 7.25% C, 23.8% Mg, 5.39% Al and 25.1% La. The main elements on the surface of IL/CD–Mg–Al–La were 16.7% C, 18.5% Mg, 4.35% Al, 17.0% La and 5.12% N. The loading of ionic liquid resulted in the change of surface elements on composite oxides. The reason for the appearance of the N element was that the imidazolium cation in [Bmim]OH contained nitrogen. The presence of carbon element in the ionic liquid brought about the rise of the carbon content. The increase in the total element content led to the relative decrease in the Mg, Al and La element. All above indicated that the ionic liquid had been immobilized on the Mg–Al–La composite oxide successfully.
3.2 Transesterification
Methanol and isobutanol were used as raw materials for transesterification respectively in order to investigate the effect of increasing layer spacing in composite oxides by the intercalation of β-cyclodextrin. The catalytic effects of IL/CD–Mg–Al–La and IL/Mg–Al–La in the transesterification of WCO are shown in Fig. 5. The transesterification was carried out under 65 °C at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio of alcohol and oil with 3 wt% catalyst dosage at an ordinary pressure.
1 mole ratio of alcohol and oil with 3 wt% catalyst dosage at an ordinary pressure.
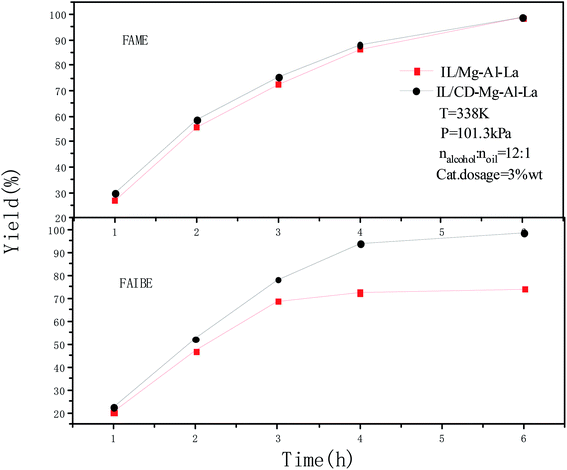 | ||
| Fig. 5 Transesterification of WCO with methanol and isobutanol catalyzed by IL/Mg–Al–La and IL/CD–Mg–Al–La. | ||
A strong basic catalyst was favorable for methanol to form methoxy anion attacking carbon in the carbonyl. The generated tetrahedral intermediate was dissociated into fatty acid methyl ester and glycerol. The loading of the ionic liquid strengthened the basicity of the composite oxide further and thus improved the efficiency of the catalytic reaction. The yield of the fatty acid methyl ester (FAME) catalyzed by IL/Mg–Al–La increased from 26.9% to 98.5% from time 1 h to 6 h, while the yield of the FAME catalyzed by IL/CD–Mg–Al–La rose from 29.9% to 98.6% from 1 h to 6 h. The yields of FAME were almost the same after the WCO was transesterified with methanol for 6 h and was catalyzed by IL/Mg–Al–La and IL/CD–Mg–Al–La because the molecular volume of methanol was smaller and thus the steric hindrance was negligible. However, the yields of FAIBE were 73.9% and 98.3% after the WCO was transesterified with isobutanol for 6 h and was catalyzed by IL/Mg–Al–La and IL/CD–Mg–Al–La, respectively. The catalytic effect of IL/CD–Mg–Al–La was better than that of IL/Mg–Al–La due to the intercalation of β-cyclodextrin. The BET analysis demonstrated that the modification by β-CD increased the specific surface area and pore size of the catalyst, which was beneficial to the collision and adsorption between the bulky alcohol molecule and the alkaline center of the catalyst surface. In addition, the strong alkaline ionic liquid also increased the reaction activity of catalyst in the transesterification reaction.
The Forcite module of the material studio software was used to calculate the molecular size of methyl oleate and isobutyl oleate. The molecular models are shown in Fig. 6. The results showed that the volume of methyl oleate was 363.59 Å3, while the volume of isobutyl oleate was 413.18 Å3. The isobutanol molecules are larger than the methanol molecules and thus the effect of β-cyclodextrin is more obvious for isobutanol. The hydrotalcite needed to be expanded for isobutanol (with a larger molecular size) although hydrotalcite had excellent catalytic performance for the esterification of methanol. However, the supported ionic liquid with strong alkalinity increased the activity of the catalyst.
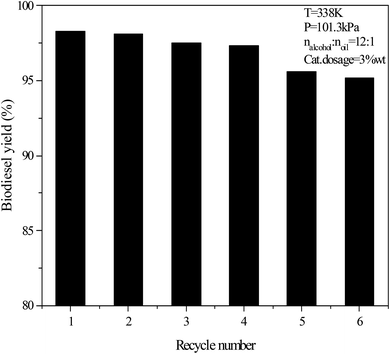
The used catalyst was washed with methanol and vacuum dried for 1 hour at 70 °C. The solid catalyst was collected for testing the regeneration effect after calcining at 550 °C for 2 hours. The regeneration effect of IL/CD–Mg–Al–La in the transesterification is shown in Fig. 7. The transesterification conditions were as follows: 65 °C, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar mass ratio of isobutanol and WCO, 3 wt% catalyst usage, and 6 h reaction time. The results showed that there was no obvious deactivation and the yield of FAIBE was 95.2% after regenerating six times.
1 molar mass ratio of isobutanol and WCO, 3 wt% catalyst usage, and 6 h reaction time. The results showed that there was no obvious deactivation and the yield of FAIBE was 95.2% after regenerating six times.
3.3 Property of FAIBE
The pour point, cold filter point and cloud point of FAIBE from the transesterification catalyzed by IL/CD–Mg–Al–La were determined in this study. The experimental results are shown in Table 4. The FAIBE obtained in the transesterification of WCO and isobutanol met the standard of −10# diesel. Its pour point, cold filter point, and cloud point were −10 °C, −7 °C, and −5 °C, respectively. Isobutyl reduced the pour point effectively because the isomerization of fatty acid ester could increase the steric hindrance and prevent the process of nucleation and crystallization of different molecules.| Cloud point | −5 °C |
| Cold filter point | −7 °C |
| Pour point | −10 °C |
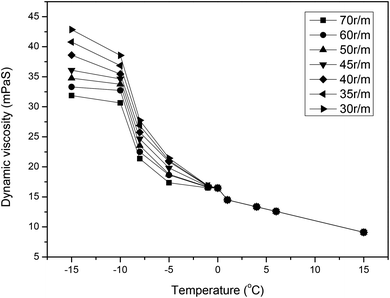
The viscosity–temperature curve of FAIBE is shown in Fig. 8. FAIBE was a liquid phase system at 15–1 °C and its viscosity changed only with the temperature, which indicated that FAIBE was the Newtonian fluid. The appearance of the wax crystal phase in the system led to the slope change of the viscosity temperature curve at 1 °C, and thus, the wax precipitation temperature of FAIBE was 1 °C.
The viscosity of FAIBE changed with the temperature and shear rate below −1 °C, which showed that it was transformed into the non-Newtonian fluid. The liquid was in a continuous phase and the wax crystal was in a dispersed phase at this moment. The phase transition temperature of FAIBE was −1 °C.
The viscosity of FAIBE changed with the shear rate obviously after the temperature got lower. When the temperature was lower than the condensation point −10 °C, the viscosity temperature curve tended to be parallel at different shear rates, which indicated that the wax crystallization slowed down at the temperature below the condensation point and FAIBE can flow only when the shear force was strong enough. The wax crystal became a continuous phase while the liquid was surrounded by wax crystals and lost the flowability.
4. Conclusions
The interlayer of the catalyst needed to be expanded due to the larger volume of isobutanol. The results showed that the IL/CD–Mg–Al–La catalyst had a high catalytic activity and regeneration performance in transesterification. The XRD and BET characterizations indicated that the intercalation of β-cyclodextrin improved the specific surface area and pore size of the immobilized composite oxide catalyst. The yield of FAIBE reached 98.3% after transesterification for 6 h with 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of alcohol and oil and 3 wt% catalyst amount at 65 °C. The yield can still reach 95% after regenerating six times. The rheological properties of FAIBE changed obviously from a Newtonian fluid to a pseudoplastic fluid due to the different wax crystal morphology when the temperature changed from high to low. The viscosity–temperature curve indicated that FAIBE had excellent low temperature fluidity.
1 molar ratio of alcohol and oil and 3 wt% catalyst amount at 65 °C. The yield can still reach 95% after regenerating six times. The rheological properties of FAIBE changed obviously from a Newtonian fluid to a pseudoplastic fluid due to the different wax crystal morphology when the temperature changed from high to low. The viscosity–temperature curve indicated that FAIBE had excellent low temperature fluidity.
Conflicts of interest
There are no conflicts to declare.References
- Y. Liu, E. Lotero and X. Mo, Transesterification of poultry fat with methanol using Mg-Al hydrotalcite derived catalysts, Appl. Catal., A, 2007, 331, 138–148 CrossRef CAS.
- M. Ghasemi and A. M. Dehkordi, Transesterification of Waste Cooking Oil to Biodiesel Using KOH/γ-Al2O3 Catalyst in a New Two-Impinging-Jets Reactor, Ind. Eng. Chem. Res., 2014, 53, 12238–12248 CrossRef CAS.
- S. Li, J. Wen and X. Jia, Engineering Bacillus subtilis for isobutanol production by heterologous Ehrlich pathway construction and the biosynthetic 2-ketoisovalerate precursor pathway overexpression, Appl. Microbiol. Biotechnol., 2011, 91, 577–589 CrossRef CAS PubMed.
- F. Cavvani, F. Trifirò and A. Vaccari, Hydrotalcite-type anionic clays: Preparation, properties and applications, Catal. Today, 1991, 11, 173–301 CrossRef.
- C. C. C. M. Silva, N. F. P. Riheiro, M. M. V. M. Souza and D. A. G. Aranda, Biodiesel production from soybean oil and methanol using hydrotalcites as catalyst, Fuel Process. Technol., 2010, 91, 205–210 CrossRef CAS.
- D. G. Cantrell, L. J. Gillie, A. F. Lee and K. Wilson, Structure-reactivity correlation in MgAl hydrotalcite catalysts for biodiesel synthesis, Appl. Catal., A, 2005, 287, 183–190 CrossRef CAS.
- J. Sun, J. Yang, S. Li and X. Xu, Preparation and characterization of fluorine modified oxides for transesterification, Catal. Commun., 2015, 59, 88–91 CrossRef CAS.
- B. S. Caldas, C. S. Nunes and P. R. Souza, Supercritical ethanolysis for biodiesel production from edible oil waste using ionic liquid [HMim][HSO4] as catalyst, Appl. Catal., B, 2016, 181, 289–297 CrossRef CAS.
- F. Kooli, I. C. Chisem and M. Vucelic, Synthesis and properties of terephthalate and benzoate intercalates of Mg-A1 layered double hydroxides possessing varying layer Charge, Chem. Mater., 1996, 8(8), 1969–1977 CrossRef CAS.
- V. Prevot, C. Forano and J. P. Besse, Syntheses and thermal and chemical behaviors of tartrate and succinate intercalated Zn3Al and Zn2Cr layered double hydroxides, Inorg. Chem., 1998, 37(17), 4293–4301 CrossRef CAS PubMed.
- V. T. Karathanos, I. Mourtzinos and K. Yannakopoulou, Study of the solubility, antioxidant activity and structure inclusion complex of vanillin with β-cyclodextrin, Food Chem., 2007, 101, 652–658 CrossRef CAS.
- L. Mohanambe and S. Vasudevan, Orientational dynamics of anthracene in a cyclodextrin functionalized layered solid, J. Phys. Chem. B, 2005, 109, 11865–11869 CrossRef CAS PubMed.
- H. Bárbara, A. Carolina and Y. Nicolás, Selective nanodecoration of modified cyclodextrin crystals with gold nanorods, J. Colloid Interface Sci., 2013, 389(1), 42–45 CrossRef PubMed.
- J. Sun, J. Yang, S. Li and X. Xu, Basic ionic liquid immobilized oxides as heterogeneous catalyst for biodiesel synthesis from waste cooking oil, Catal. Commun., 2016, 83, 35–38 CrossRef CAS.
- J. Sun, J. Yang, S. Li and X. Xu, Basicity-FAME yield correlations in metal cation modified MgAl mixed oxides for biodiesel synthesis, Catal. Commun., 2014, 52, 1–4 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |