Open Access Article
Open Access ArticleThe impact of temperature and dissolved oxygen (DO) on the partial nitrification of immobilized fillers, and application in municipal wastewater
Jiawei Wanga,
Hong Yang *a,
Xuyan Liua,
Jiawei Wangb and
Jiang Changb
*a,
Xuyan Liua,
Jiawei Wangb and
Jiang Changb
aKey Laboratory of Beijing for Water Quality Science and Water Environment Recovery Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: yhong@bjut.edu.cn
bBeijing Drainage Group Co. Ltd, Beijing 100022, China
First published on 7th October 2020
Abstract
To achieve the stable partial nitrification of municipal wastewater, activated sludge with high ammonia-oxidizing bacteria (AOB) content and low nitrite-oxidizing bacteria (NOB) content were immobilized in a polyvinyl alcohol filler. The effects of different levels of dissolved oxygen (DO) on the activity of AOB and NOB in the filler with temperature changes at the initial ammonia concentration of approximately 100 mg L−1 were investigated. At 25 °C, when the DO concentration was greater than 5 mg L−1, the O2-limiting condition inside the filler was destroyed as the demand for oxygen in AOB was certain, and resulted in enhanced NOB activity. At 15 °C, the DO concentration was not a key factor in determining the NOB activity due to the negative effect of temperature on NOB activity. The immobilized filler reactor of municipal wastewater achieved a nitrite accumulation rate (NAR) of >86.7 and >82% at 24–26 °C and 14–16 °C, respectively. Low temperatures did not deteriorate the stable partial nitrification performance. The total nitrogen (TN) removal efficiency of the immobilized filler reactor was 21.7–26.1% and 10.3–15.3% at 24–26 °C and 14–16 °C, respectively. The TN removal efficiency and NAR in municipal wastewater were higher as compared to simulated wastewater, indicating that the organic carbon in municipal wastewater enhanced nitrate reduction by denitrification. High-throughput sequencing analysis showed that denitrifying bacteria and nitrifying bacteria were identified as the predominant bacteria genera, while the dominant species of NOB was Nitrobacter. This study is a viable approach to promoting partial nitrification in municipal WWTPs.
1. Introduction
Compared to the traditional biological nitrogen removal processes in wastewater treatment plants (WWTPs), the partial nitrification process has the advantages of low oxygen demand for nitrification, producing less sludge and reducing the chemical oxygen demand (COD) depletion for denitrification.1,2 Partial nitrification is achieved by controlling the operational parameters such as dissolved oxygen (DO),3,4 free ammonia (FA),5,6 free nitrous acid (FNA),7 solids retention time (SRT)8 and temperature.9 Achieving the stable nitrite accumulation via controlling these parameters lead to a stable inhibition or washout of nitrite-oxidizing bacteria (NOB) while retaining ammonium-oxidizing bacteria (AOB). For example, partial nitrification is achieved in the SHARON (single reactor high activity ammonia nitrogen removal nitrite) process at high ammonia nitrogen concentrations (500–1500 mg L−1) mainly through high pH, high FA and high temperature.9 In the activated sludge system of municipal WWTPs, stable suppression of NOB was achieved at low ammonia concentrations (40–60 mg L−1) via low DO control or the FA/FNA treatment pathway to achieve high nitrite accumulation.10–12 However, the partial nitrification of low ammonia concentrations in the activated sludge systems is currently achieved by controlling the DO concentration, causing a negative impact on the ammonia oxidation efficiency.13,14 In summary, achieving partial nitrification in the activated sludge systems has the disadvantages of complicated processes and low ammonia oxidation efficiency.In this context, the immobilized filler can fix bacteria in the filler to ensure that the bacterial density of the immobilized filler system is higher than that of the activated sludge system.15 Isaka et al.16 achieved partial nitrification using high temperatures in the immobilized filler reactor at the influent ammonia concentrations of 710–1340 mg L−1. Rongsayamanont et al.17 achieved partial nitrification in the immobilized filler reactor at room temperature (24–26 °C) with influent total ammonia nitrogen concentrations of 625 mg L−1 by controlling DO and FA concentrations. These studies highlighted that the partial nitrification of immobilized cells was achieved by controlling DO and high temperatures at high influent ammonia concentrations. However, there have been few reports on the partial nitrification of municipal wastewater with immobilized fillers at different temperatures.
To achieve stable partial nitrification of municipal wastewater at different temperatures, activated sludge with high AOB content and low NOB content was immobilized in a polyvinyl alcohol carrier. Through experiments at different DO concentrations, we investigated the effects of DO levels on the activities of AOB and NOB in immobilized filler at different temperatures. Subsequently, we investigated the partial nitrification characteristics of the immobilized filler at the synthetic wastewater and municipal wastewater. The removal of total nitrogen (TN) and COD in the immobilized filler reactor was evaluated. High-throughput sequencing was used to investigate the changes in the microbial population in the immobilized filler after the partial nitrification of municipal wastewater.
2. Materials and methods
2.1. Preparation of inoculated sludge
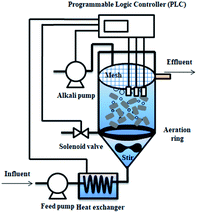
A continuous-flow reactor (Fig. 1(a)) with a working volume of 95 L was used to cultivate activated sludge extracted from the return sludge in the Gao Bei Dian WWTP in Beijing, China. The inoculated sludge with high AOB content and low NOB content was cultivated through continuous sludge discharge and a FA inhibition strategy based on our previous research.18 The pH was adjusted to 7.6–7.8 using a Programmable Logic Controller (PLC) equipped with a pH probe and Na2CO3 (pH buffer, alkalinity source). Continuous aeration was supplied through a perforated tube using an air pump. The DO concentration was at 0.8–1.0 mg L−1 through a DO probe by a solenoid valve OFF/ON through PLC control. The temperature was controlled at 24–26 °C using a heat exchanger to heat the water via PLC control. Hydraulic residence time (HRT) was maintained at 4.0 h.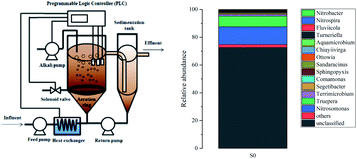 | ||
| Fig. 1 Schematic diagram of (a) the cultivation reactor and (b) bacterial community distribution in the inoculated sludge. | ||
The inoculated sludge oxidized 87% of oxidized ammonia, which was converted to nitrite without further oxidation to nitrate. The number of active NOB cells in the inoculum determined the partial nitrification enhancement in the immobilized filler. In this study, Nitrosomonas belonging to AOB in the inoculated sludge was 12.48%, while Nitrospira and Nitrobacter affiliated with NOB in the inoculated sludge were not detected because the copy number of the sample was lower than the detection limit (Fig. 1(b)).
2.2. Preparation of immobilized inoculated sludge
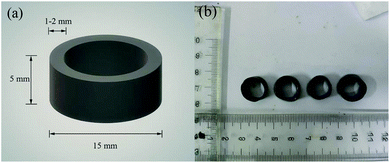
The materials used to prepare the immobilized fillers included calcium carbonate (CaCO3), powdered activated carbon (less than 120 mesh), anhydrous sodium sulfate (Na2SO4), boric acid (H3BO4), and PVA (degree of polymerization 2200, degree of alcoholysis 20–99%). All the above materials were of analytical purity. The PVA powder was dissolved in water at a temperature of 95 °C and mixed to form a 20% (w/w) PVA solution. After cooling to 37 °C, the 0.5 L inoculated sludge after centrifugation was mixed with the 0.5 L PVA solution to a 1 L mixture. The sludge concentration (dry weight) was 5% (w/w) of the total mixture. Subsequently, CaCO3 (18.32 g L−1) and powdered activated carbon (38.30 g L−1) were added.19 The encapsulating solution was evenly coated on the stainless steel cylindrical carrier (length 500 mm, diameter 12 mm) and then placed in a saturated boric acid solution to complete the cross-linking. Finally, the filler was removed from the stainless steel carrier and cut into small cylinders with a growth degree of 5 mm. The barrel-shaped immobilized filler (Fig. 2) with a diameter of 15 mm, a height of 5 mm, and a thickness of 1–2 mm can be more widely applied with the small ball-shaped filler, used in most studies.16,17,20–222.3. Experimental setup for the immobilized filler
The reactor (Fig. 3) with an effective volume of 15 L was operated at the filling rate of 6% (v/v). The temperature was controlled at 24–26 °C and 14–16 °C using a heat exchanger to heat the water via PLC control. The immobilized filler tests were carried out in synthetic wastewater experiments and municipal wastewater experiments.Continuous flow tests were conducted to investigate the partial nitrification of the immobilized filler in synthetic wastewater; synthetic wastewater with 40–60 mg L−1 NH4+–N was used as the influent. The DO concentration was controlled at the optimum DO level determined from batch tests using PLC equipped with a DO probe and an ON/OFF air blower. The pH was adjusted to 7.6–7.8 using a PLC equipped with a pH probe and pH buffer. The tests were run for 40 days. The temperature was 24–26 °C with HRT of 1.0 h in Phase I, and the temperature was 14–16 °C with HRT of 2.0 h Phase II. The reactor was fed with stock solutions containing NH4Cl (nitrogen source), KH2PO4 (phosphorus source) and trace element stock solution consisting of 1.8 μg L−1 MgSO4·7H2O, 1.8 μg L−1 FeSO4·7H2O, 0.5 μg L−1 ZnSO4·7H2O, 0.5 μg L−1 MnCl2·4H2O, 0.6 μg L−1 CoCl2·6H2O, 0.6 μg L−1 CuSO4·5H2O and 0.2 μg L−1 NiCl2·6H2O.
| Items | Range | Average |
|---|---|---|
| COD/mg L−1 | 102.32–177.50 | 143.58 |
| TN/mg L−1 | 42.75–56.79 | 48.23 |
| NH4+–N/mg L−1 | 35.27–50.81 | 42.70 |
| NO2−–N/mg L−1 | 0.15–0.36 | 0.31 |
| NO3−–N/mg L−1 | 0.75–3.15 | 1.75 |
| pH | 7.6–8.2 | 7.8 |
2.4. Analytical methods
In each batch experiment, samples were collected from the reactor every 20 minutes. NH4+–N, NO2−–N, NO3−–N, TN were determined according to standard methods.23 The COD was measured with a 5B-3F type COD rapid measuring instrument (Lianhua technology).The nitrite accumulation ratio (NAR) in the continuous flow experiments was calculated according to eqn (1):
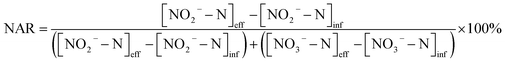 | (1) |
The ammonia removal rate (ARR) was calculated according to eqn (2):
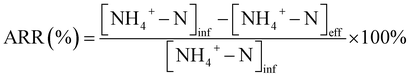 | (2) |
2.5. High-throughput 16S rRNA gene sequencing
Sample 1 and sample 2 were obtained from the immobilized filler reactor of municipal wastewater experiments on day 1 and day 55, respectively. Two samples were analyzed using high-throughput sequencing to investigate the microbial population changes inside the filler after long-term partial nitrification (SinoGenoMaxCorp, China). Samples were prepared for DNA extraction and the extracted DNA was supplied for PCR amplification of 16S rRNA in the V3–V4 region. The bacterial 16S rRNA gene sequences were aligned using the National Center for Biotechnology Information (NCBI) database. For the sequencing data, the MEGAN software was used to analyze the 16S rRNA genes of environmental microorganisms, and the obtained sequences were classified according to certain thresholds to obtain multiple sequence clustering into operational taxonomic units (OTUs). Diversity analysis was performed according to the OTUs. The results of the analysis were visualized to make the information easier to interpret.3. Results and discussion
3.1. Effects of DO levels on the activities of AOB and NOB in immobilized filler at different temperatures
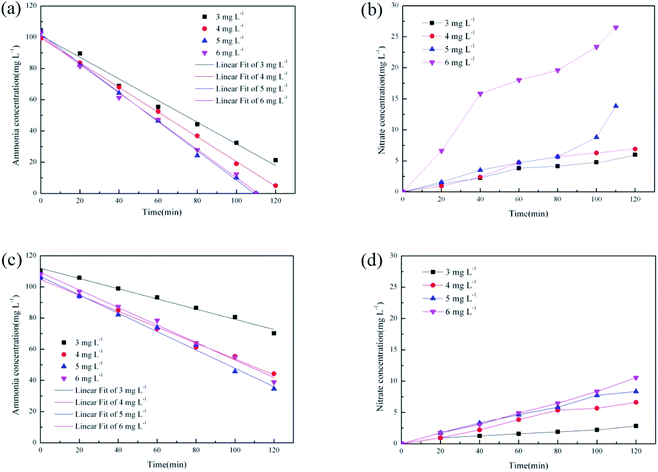
Batch tests were performed on the samples at different temperatures and DO levels to investigate the effect of DO concentration on partial nitrification. The bacterial density in the filler was significantly larger than that in the activated sludge, according to the Monod equation, the DO consumption in the filler was significantly greater than that of the bacteria in the activated sludge. Furthermore, a concentration gradient existed in the immobilized filler due to DO entering the immobilized filler via molecular diffusion. Consequently, the dissolved oxygen concentration in the solution was kept high to ensure that the dissolved oxygen in the filler enabled nitrification. The DO concentrations in the batch tests were 3, 4, 5, and 6 mg L−1.Fig. 4 shows the variation in the partial nitrification performance in samples at different DO levels at 15 °C and 25 °C. As shown in Fig. 4(a), the ammonia oxidation ability increased at 25 °C with an increase in DO concentration. Previous studies demonstrated that the ammonia-oxidizing activity increased at high bulk DO concentrations.24 However, the ammonia oxidation efficiency did not vary significantly when DO increased from 5 mg L−1 to 6 mg L−1. Similarly, the ammonia oxidation efficiency did not increase at 15 °C as the DO concentration increased from 4 mg L−1 to 6 mg L−1 (Fig. 4(c)). These findings indicate that AOB inside the entrapped filler had a limited demand for oxygen at a limited substrate concentration. When the DO concentration was greater than 4 mg L−1, compared with 25 °C, the ammonia oxidation efficiency did not increase significantly with increasing DO concentration at 15 °C. This indicates that when the temperature was reduced, the demand for DO was reduced due to the reduced AOB activity inside the filler. Isaka et al.21 found that AOB cultivated in a gel carrier had a low affinity for ammonium, leading to incomplete nitrification at 10 °C.
As shown in Fig. 4(b), at 25 °C, the amount of nitrite formation was 26.5 mg L−1 at the DO concentration of 6 mg L−1, which was larger than the amount of nitrate formed at the DO concentrations of 3, 4 and 5 mg L−1 (4.7 mg L−1, 6.3 mg L−1 and 13.8 mg L−1). The DO concentration inside the immobilized filler was determined by the DO transfers via molecular diffusion and the internal oxygen consumption. When the filler contains a large number of AOB, the consumption of DO due to ammonia oxidation reaction leads to an O2-limiting condition inside the filler. The O2-limiting condition inhibits the activity of NOB inside the filler. According to Fig. 4(a), as the concentration of DO increased, due to the demand for oxygen in AOB, the O2-limiting condition inside the filler was destroyed. However, the nitrate concentration increased from 8.8 mg L−1 to 13.8 mg L−1 during the last 10 min of the batch experiment (25 °C and DO = 5 mg L−1), which was different from the first 100 min. Consequently, the ammonia concentration was reduced to a low level in the last 10 minutes, causing AOB to have less oxygen demand than in the first 100 minutes, which resulted in more DO for NOB. As shown in Fig. 4(d), under DO concentrations of 3, 4, 5, and 6 mg L−1, the nitrate concentration at 15 °C increased by 2.8 mg L−1, 5.9 mg L−1, 8.3 mg L−1, and 10.5 mg L−1 without any sudden increase in the nitrate concentration. This indicated that DO was not a key factor in determining the NOB activity due to the negative effect of temperature on NOB activity at low temperatures. AOB activity was more affected by temperature than NOB activity, but the ammonia oxidation efficiency was approximately 4 times the nitrate oxidation efficiency at 15 °C. This indicated that the immobilized filler achieved partial nitrification at low temperatures.
Rongsayamanont et al.22 suggested that the number of active NOB cells and bulk DO concentration are the key decisive factors for enhancing partial nitrification by immobilized cells. However, by increasing the AOB content of the immobilized filler to increase the internal oxygen consumption of the filler, the O2-limiting condition inside the filler can be achieved so that NOB can be suppressed at low ammonia concentrations. On the other hand, high ammonia oxidation efficiency due to high AOB content was important for maintaining partial nitrification at low temperatures.
3.2. Nitrite pathway by synthetic wastewater
The DO concentration for the continuous flow experiment was maintained at 4.5–5.0 mg L−1, which was the optimum concentration at different temperatures by the batch experiment results. During the overall operation period, the immobilized filler reactor achieved stable partial nitrification of synthetic wastewater. As shown in Fig. 5(a), in Phases I and II, the influent ammonia concentration was 40–60 mg L−1 and effluent ammonia concentration was <4 mg L−1, while ARR was >92.7%, indicating that the immobilized filler achieved the effective removal of ammonia. The NAR of the immobilized filler reactor was 88.1–90.5% at 24–26 °C, which was slightly higher than the ratio at 14–16 °C (80.2–83.2%). This phenomenon indicated that the ammonia oxidation rate was more sensitive than the nitrite oxidation rate at lower temperatures.25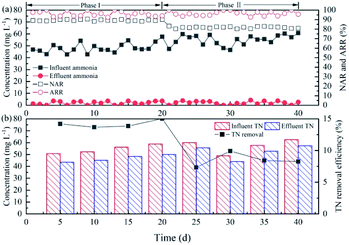 | ||
| Fig. 5 The performance of the immobilized filler in simulated wastewater: (a) partial nitrification characteristics; (b) TN removal. | ||
Fig. 5(b) shows the TN concentration of the influent and effluent every 5 days. The TN removal efficiencies of the immobilized filler reactor were 13.7–15.0% and 7.3–9.9% at 24–26 °C and 14–16 °C, respectively, which indicated that the denitrification reaction appeared in the reactor. NO2−–N was found to be produced in a denitrification upflow sludge bed.26 This partial denitrification (NO3−–N → NO2−–N) is a possible way to promote partial nitrification in the immobilized filler. Rongsayamanont et al.22 observed that the TN loss rate was 9–11% in the partial nitrification by the entrapped-cell-based reactor. Under substrate limiting conditions as within the filler, soluble microbial products were produced substantially and can become the source of organic carbon for the denitrification reaction.27 On the other hand, the DO gradient from the outside to the interior of the immobilized filler was conducive for the formation of aerobic, anoxic, or anaerobic microenvironments, and provided suitable environmental conditions for the proliferation of the denitrifying bacteria.28
3.3. Nitrite pathway by municipal wastewater
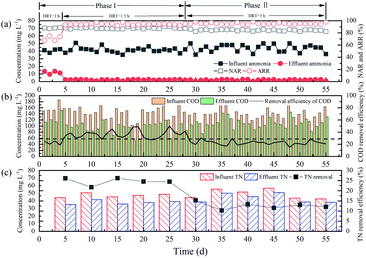 | ||
| Fig. 6 The performance of the immobilized filler in municipal wastewater: (a) partial nitrification characteristics; (b) COD removal; (c) TN removal. | ||
Fig. 6(a) shows the partial nitrification performance during the operation period, including HRT, NAR and ARR. Partial nitrification was achieved at both high and low temperatures, the ARR was higher than 93.3%, and the NAR was higher than 82.3%. Although the complex wastewater environment affected the biochemistry reaction inside the immobilized filler, the ammonium oxidation process was still capable of being completed in a shorter time as compared to other studies on partial nitrification by the activated sludge.23,29,30 High ammonia oxidation efficiency was achieved due to the high AOB content in the filler (Fig. 7). The key to achieving the partial nitrification of municipal wastewater at low temperature was maintaining high ammonia oxidation efficiency. Moreover, the NAR was >82.3% for the entire operation period in municipal wastewater, which was slightly higher than the ratio in synthetic wastewater (>80.2%). The reason for the higher NAR in the municipal wastewater was that the organic carbon in municipal wastewater enhanced nitrate reduction by denitrification (more details can be found in Section 3.4).
During municipal wastewater experiments, the TN removal efficiencies of the immobilized filler reactor were 21.7–26.1% and 10.3–15.3% at 24–26 °C and 14–16 °C, respectively, which were higher than the efficiency in synthetic wastewater. This indicated that the organic carbon in municipal wastewater promoted denitrification in the immobilized filler, consistent with the results obtained by other authors.32,33 Sabba34 proposed that higher bulk ammonia concentrations, higher nitrite concentrations, lower dissolved oxygen, and greater biofilm thicknesses result in higher N2O emissions in nitrifying biofilms. However, in this study, N2O emissions were not the main reason for TN removal due to the characteristics of the immobilized filler and the low influent ammonia concentration. The high content of denitrifying bacteria in the filler (Fig. 7) also proved that TN removal was mainly through denitrification. Significantly, when the temperature decreased from 25 to 15 °C, the COD removal efficiency and TN removal efficiency decreased while the ARR did not decrease. The possible reason for this phenomenon was that the heterotrophic bacteria were more sensitive to temperature reduction than the autotrophic bacteria.
3.4. High-throughput sequencing for microbial diversity analysis
The abundance estimators ACE and Chao1 were used to reflect the microbial abundance, while the Shannon and Simpson indices characterized the diversity of microbial components. The values for Chao1, Shannon, Simpson and ACE in the sludge and filler are shown in Table 2. Higher values of ACE and Chao1 indicate that the bacteria in the immobilized filler after the operation were abundant. The high Shannon index and low Simpson index in sample 2 indicate that the abundance and diversity of the bacteria in the immobilized filler increased after municipal wastewater experiments due to the changes in the wastewater characteristics.| Sample | ACE | Chao1 | Shannon | Simpson |
|---|---|---|---|---|
| 1 | 540![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 623.06 623.06 |
135![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 611.12 611.12 |
3.90 | 0.15 |
| 2 | 556![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 426.77 426.77 |
164![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 238.52 238.52 |
4.04 | 0.11 |
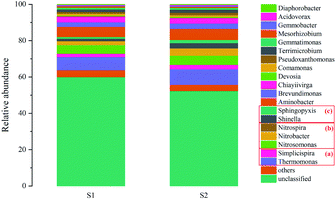
The bacterial community was studied at the genus level to determine the effects of microbial changes on the partial nitrification characteristics after the operation of municipal wastewater. As evident in Fig. 7, Nitrosomonas affiliated with AOB is the main genus in municipal WWTPs, though Nitrospira and Nitrobacter affiliated with NOB are also found in WWTPs.10 After a 55 d operation of municipal wastewater, the Nitrosomonas content increased from 4.78 to 5.03%, indicating that the AOB population could be maintained. The contents of Nitrospira and Nitrobacter increased from below the detection limit and 2.15% to 0.01 and 4.01%, respectively. NOB grew inside because the bacteria were fixed in the filler, leading to NOB competition within the filler. Nitrospira had a significantly lower proliferation rate and a better substrate affinity.35 In contrast, Nitrobacter had a poor affinity due to the substrate, which can form a competitive advantage through rapid growth with a high concentration of substrate.36 The proportion of Nitrobacter in the filler was higher than that of Nitrospira, indicating that the Nitrobacter in the filler grew faster than Nitrospira. The growth characteristics of Nitrobacter also explain why the nitrate production did not increase proportionally despite an increase in the proportion of Nitrobacter.
Thermomonas and Simplicispira are two kinds of typical denitrificans that can be found in the wastewater treatment bioreactor.37,38 Thermomonas was the main denitrifying bacteria in the immobilized filler. The organic carbon in the municipal wastewater promoted the growth of denitrifying bacteria and the contents of Thermomonas and Simplicispira increased from 7.24 and 1.78% to 8.54 and 2.42%, respectively. Simon et al.39 reported that Thermomonas mainly utilizes nitrate as an electron acceptor under acetate conditions. It should be noted that the growth of Thermomonas due to organic carbon in municipal wastewater was mainly responsible for the higher NAR in municipal wastewater as compared to synthetic wastewater. Shinella and Sphingopyxis are heterotrophic bacteria with the ability to degrade organic carbon.40,41 The Shinella and Sphingopyxis contents were only 2.89 and 1.75% after the operation, indicating that heterotrophic bacteria in the filler were not the main degraders of organic carbon.
3.5. Implications to municipal WWTPs
This study proposes a promising approach that maintains stable partial nitrification in municipal wastewater. While the strategy is demonstrated in a laboratory-scale reactor, the economic efficiency in actual municipal WWTPs need to be further improved. The recent studies proposed the use of non-woven fabric made by waste plastic bottles as packing material for both up-flow anaerobic sludge bed (UASB) and downflow hanging non-woven fabric (DHNW) reactors to treat organic matter in sewage water with a good removal effect.42,43 The proposed packing material with low cost has a promising capacity as an efficient material that could be used for the treatment of wastewater. Therefore, choosing reusable or waste-regenerated immobilized materials can promote the application of this strategy in municipal WWTPs.4. Conclusions
The O2-limiting condition inside the filler and high ammonia oxidation efficiency are the keys to the partial nitrification of low concentrations of ammonia at different temperatures. Under different temperature conditions (13–15 °C and 24–26 °C), the immobilized filler reactor achieved the ARR of >93.3% and the NAR of >82.3% in municipal wastewater with COD and TN removal. The DO gradient from the outside to the interior of the immobilized filler promoted the diversity of microorganisms. In addition, the organic carbon in municipal wastewater enhanced nitrate reduction by denitrification. This study investigated the characteristics of the immobilized filler for the partial nitrification of municipal wastewater at different temperatures, which might be practically helpful for achieving the partial nitrification of municipal WWTPs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Beijing Municipal Commission of Education owned by the municipal government of Beijing under the Program “Research on reinforcement and stability of nitrogen removal performance in wastewater treatment based on the new landmark conditions” (Z161100004516015).Notes and references
- Y. Z. Peng, Y. Chen, C. Y. Peng, M. Liu, S. Y. Wang, X. Q. Song and Y. W. Cui, Water Sci. Technol., 2004, 50, 35–43 CrossRef CAS.
- A. Pollice, V. Tandoi and C. Lestingi, Water Res., 2002, 36, 2541–2546 CrossRef CAS.
- C. Picioreanu, M. C. M. van-Loosdrecht and J. J. Heijnen, Water Sci. Technol., 1997, 36, 147–156 CrossRef CAS.
- R. Blackburne, Z. Yuan and J. Keller, Biodegradation, 2008, 19, 303–312 CrossRef CAS.
- D. J. Kim, D. I. Lee and J. Keller, Bioresour. Technol., 2006, 97, 459–468 CrossRef CAS.
- F. Zhang, H. Yang, J. W. Wang, Z. Q. Liu and Q. K. Guan, RSC Adv., 2018, 8, 31987–31995 RSC.
- S. W. H. Van-Hulle, H. J. P. Van-deweyer, B. D. Meesschaert, P. A. Vanrolleghem, P. Dejans and A. Dumoulin, Chem. Eng. J., 2010, 162, 1–20 CrossRef CAS.
- Q. Yuan and J. A. Oleszkiewicz, Water Sci. Technol., 2011, 63, 2802–2807 CrossRef CAS.
- W. H. Hulle-Stijn, I. P. Volcke-Eveline, L. Teruel-Josefa, B. Donckels, C. M. van-LoosdrechtMark and P. A. Van-rolleghem, J. Chem. Technol. Biotechnol., 2007, 82, 471–480 CrossRef.
- S. Ge, S. Wang, X. Yang, S. Qiu, B. Li and Y. Peng, Chemosphere, 2015, 140, 85–98 CrossRef CAS.
- H. R. Duan, Q. L. Wang, V. E. Dirk, L. Ye and Z. G. Yuan, Sci. Total Environ., 2018, 644, 360–370 CrossRef CAS.
- Q. L. Wang, L. Ye, G. M. Jiang, S. H. Hu and Z. G. Yuan, Water Res., 2014, 55, 245–255 CrossRef CAS.
- B. Ma, B. Peng, W. Yan, G. B. Zhu, Z. G. Yuan and Y. Z. Peng, Sci. Rep., 2015, 5, 13048 CrossRef.
- P. Regmi, R. Bunce, M. Miller, H. Park, K. Chandran, B. Wett, S. Murthy and C. B. Bott, Biotechnol. Bioeng., 2015, 112, 2060–2067 CrossRef CAS.
- A. K. Pour, D. Karamanev and A. Margaritis, Water Res., 2005, 39, 3704–3714 CrossRef CAS.
- K. Isaka, H. Itokawa, Y. Kimura, K. Noto and T. Murakami, Bioresour. Technol., 2011, 102, 7720–7726 CrossRef CAS.
- C. Rongsayamanont, E. Khan and T. Limpiyakorn, J. Environ. Manage., 2019, 251, 1–7 Search PubMed.
- M. Y. Yu, Y. Liu, Y. B. Tian, H. Shi, F. Xu and H. Yang, Environ. Sci., 2017, 38, 2925–2930 Search PubMed.
- H. Yang and Q. K. Guan, Water Sci. Technol., 2016, 74, 1773–1778 CrossRef CAS.
- W. M. Rostron, D. C. Stuckey and A. A. Young, Water Res., 2001, 35, 1169–1178 CrossRef CAS.
- K. Isaka, S. Yoshie, T. Sumino, Y. Inamori and S. Tsuneda, Biochem. Eng. J., 2007, 37, 49–55 CrossRef CAS.
- C. Rongsayamanont, T. Limpiyakorn and E. Khan, Bioresour. Technol., 2014, 164, 254–263 CrossRef CAS.
- APHA, Standard Methods for the examination of water and wastewater, 20th edn, American Public Health Association, Washington D.C. US, 1998 Search PubMed.
- H. D. Park and D. R. Noguera, Water Res., 2004, 38, 3275–3286 CrossRef CAS.
- J. H. Guo, Y. Z. Peng, H. J. Huang, S. Y. Wang, S. J. Ge, J. R. Zhang and Z. W. Wang, J. Hazard. Mater., 2010, 179, 471–479 CrossRef CAS.
- S. B. Cao, B. K. Li, R. Du, N. Q. Ren and Y. Z. Peng, Water Res., 2016, 90, 309–316 CrossRef CAS.
- C. Rongsayamanont, T. Limpiyakorn, B. Law and E. Khan, Enzym. Microb. Technol., 2010, 46, 229–236 CrossRef CAS.
- X. Y. Xu, Z. X. Jin, B. Wang, C. P. Lv, B. B. Hu and D. Z. Shi, Process Biochem., 2017, 63, 214–220 CrossRef CAS.
- H. R. Duan, L. Ye, X. Y. Lu and Z. G. Yuan, Environ. Sci. Technol., 2019, 53, 1937–1946 CrossRef CAS.
- Q. L. Wang, H. R. Duan, W. Wei, B. J. Ni, A. Laloo and Z. G. Yuan, Environ. Sci. Technol., 2017, 51, 9800–9807 CrossRef CAS.
- H. Yang and S. Su, Chin. J. Environ. Eng., 2019, 13, 765–772 Search PubMed.
- L. G. Hong, J. Li and Y. Liu, Process Biochem., 2019, 87, 151–156 CrossRef.
- G. M. Cao, Q. X. Zhao, X. B. Sun and T. Zhang, Enzym. Microb. Technol., 2002, 30, 49–55 CrossRef CAS.
- F. Sabba, A. Terada, G. Wells, B. F. Smets and R. Nerenberg, Appl. Microbiol. Biotechnol., 2018, 102, 9815–9829 CrossRef CAS.
- H. Fujitani, Y. Aoi and S. Tsuneda, Microb. Environ., 2013, 28, 236–243 CrossRef.
- R. Blackburne, V. M. Vadivelu, Z. G. Yuan and K. Jürg, Water Res., 2007, 41, 3042 CrossRef.
- C. Peng, Y. L. Gao, X. Fan, P. C. Peng, H. Huang, X. X. Zhang and H. Q. Ren, Bioresour. Technol., 2019, 287, 121387 CrossRef CAS.
- D. P. Wang, T. Li, K. L. Huang, X. W. He and X. X. Zhang, Sci. Total Environ., 2019, 655, 1355–1363 CrossRef CAS.
- S. J. McIlroy, A. Starnawska, P. Starnawski, A. M. Saunders, M. Nierychlo, P. H. Nielsen and J. L. Nielsen, Environ. Microbiol., 2016, 18, 50–64 CrossRef CAS.
- H. L. Tian, L. A. Fotidis, E. Mancini, L. Treu, A. Mahdy, M. Ballesteros, C. González-Fernández and L. Angelidaki, Bioresour. Technol., 2018, 247, 616–623 CrossRef CAS.
- Z. Mavriou, L. Alexandropoulou, P. Melidis, D. G. Karpouzas and S. Ntougias, Environ. Sci. Pollut. Res., 2020, 5, 1–13 Search PubMed.
- M. A. El-Khateeb, M. A. Saad, H. I. Abdel-Shafy, F. A. Samhan and M. F. Shaaban, Desalin. Water Treat., 2018, 111, 94–100 CrossRef CAS.
- M. A. El-Khateeb, W. M. Emam, W. A. Darweesh and E. S. Abd El-Sayed, Desalin. Water Treat., 2019, 164, 48–55 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2020 |