Open Access Article
Open Access ArticleTwo 3D Mn-based coordination polymers: synthesis, structure and magnetocaloric effect†
Ning-Fang
Li
,
Ye-Min
Han
,
Jia-Nian
Li
,
Jia-Peng
Cao
,
Ze-Yu
Du
and
Yan
Xu
 *
*
College of Chemical Engineering, State Key Laboratory of Materials-Oriented Chemical Engineering, Nanjing Tech University, Nanjing 211816, P. R. China. E-mail: yanxu@njtech.edu.cn
First published on 10th September 2020
Abstract
Two three-dimensional (3D) coordination polymers, namely MnII6(CH3COO)2(HCOO)2(IN)8(C4H8O)2(H2O) and MnIII6MnII12(μ3-O)6(CH3COO)12(IN)18(H2O)7.5 (abbreviated as MnII6 and MnII12MnIII6 respectively; HIN = isonicotinic acid), were synthesized by the reaction of Mn(CH3COO)2·4H2O and isonicotinic acid under solvothermal conditions. Magnetic studies revealed that antiferromagnetic interactions may be present in compounds MnII6 and MnII12MnIII6. Moreover, the values of −ΔSm (26.27 (MnII6) and 37.69 (MnII12MnIII6) J kg−1 K−1 at ΔH = 7 T) are relatively larger than those of the reported Mn-based coordination polymers. This work provides a great scope in the magnetocaloric effect (MCE) of pure 3d-type systems.
Introduction
Manganese (Mn) ions owing to their variety in valency and coordination numbers make the Mn-based compounds exhibit interesting structures, such as chain-like1 and wheel-like,2,3 as well promising applications in the proton exchange membrane,4,5 catalysis,6–8 adsorption and so on.3,9–15 Moreover, since the discovery of the first case of single-molecule magnet behavior (SMM) in the compound MnIII8MnIV4 in 1980,16 Mn-based compounds have played an active role in the field of magnetism.17–21 For example, MnX (X = 7, 12, 16, 19, 26, 30, 32, 44, 70, and 84) demonstrate the single-molecule magnet (SMM) behaviour,9,17,22–30 and MnX (X = 1, 4, 10, 14, and 17) demonstrate the magnetocaloric effect (MCE),31–34 which can replace expensive and scarce 3He.35–40Undoubtedly, all molecular magnetic complexes can exhibit MCE to a certain extent.35–40 However, only some GdIII-based compounds displayed large magnetic entropy change (ΔSm), which is an important criterion to evaluate MCE.37,45–47 Studies manifest that the neglected magnetic anisotropy, large spin ground state (S) and low-lying excited spin states are beneficial to the MCE.38,46 Although lanthanide (4f)-type compounds have made a breakthrough in magnetic cooling materials, the search for highly efficient MCE materials without lanthanide remains a crucial issue owing to the expensive and rare 4f compounds.41–44 In 2014, [Mn(glc)2(H2O)] with ΔSm = 60.3 J kg−1 K−1, the value of which is larger than that of the majority of pure Gd-style and 3d-Gd compounds, was prepared by Tong et al.31 This example inspires us to study the MCE of 3d-type systems. Thus, we aim at developing a procedure regarding the pure 3d-type systems with MCE.
According to the literature, using HIN as a ligand is a better choice to prepare metal coordination polymers.48 The coordination sites (one N- and two O-donors) of HIN can connect at least one metal ion; thus, HIN ligands are beneficial to form high-nuclear metal clusters accompanied with large MCE, such as Gd52Ni52 with −ΔSm = 35.6 J kg−1 K−1.38
Herein, two six/eighteen-nuclear manganese coordination polymers MnII6(CH3COO)2(HCOO)2(IN)8(C4H8O)2(H2O) and MnIII6MnII12(μ3-O)6(CH3COO)12(IN)18(H2O)7.5 (abbreviated as MnII6 and MnII12MnIII6, respectively), were successfully obtained by the reaction of Mn(CH3COO)2·4H2O and HIN under solvothermal conditions. The magnetic studies reveal that antiferromagnetic interactions are present in MnII6 and MnII12MnIII6, and both show excellent MCE properties with −ΔSm = 26.27 (MnII6) and 37.69 (MnII12MnIII6) J kg−1 K−1. The −ΔSm values obtained in this work are relatively larger than those of the existing 3d-based compounds.32,33 In 2018, [MnII3]6, with an interesting wheel structure and 18 metal MnII ions, was synthesized by Qin et al.3 In our study, comparing MnII12MnIII6 with [MnII3]6, we found that (i) the valencies of Mn ions in MnII12MnIII6 are +2 and +3, as determined by X-ray photoelectron spectroscopy (XPS); (ii) [MnII3]6 was mainly applied to sorption; however, we deeply studied the MCE for MnII12MnIII6. Moreover, among the current pure Mn-type compounds, −ΔSm (37.69 J kg−1 K−1) for MnII12MnIII6 is nearly the largest. Our pure Mn-type materials are highly promising applications in MCE.
Experimental section
X-ray crystallography
A Bruker Apex II CCD detector was applied to collect the data of single-crystal X-ray diffraction (SCXRD) analyses under 296 K and 50 kV as well 30 mA with a sealed tube X-ray source (Mo-Kα radiation, λ = 0.71 Å). The structures of MnII6 and MnII12MnIII6 were resolved based on direct methods, and were refined based on full-matrix least-squares refinement using the SHELXL-2018/3 program package.Synthesis of compounds
Results and discussion
Synthesis
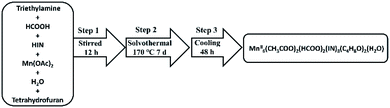
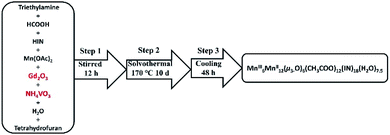
Developing a synthetic process for desired products with higher yields is much harder for the following reasons. The growth of target composition is sensitive for the initial reaction circumstances and conditions, such as the ratio of reactants, the type and ratio of solvents and the temperature.36,49–56 In this work, HCOOH, tetrahydrofuran, ethanediamine, Mn·(CH3COO)2·4H2O, and HIN were chosen as ideal reactants. In addition, we also tried out other organic solvents (ethyl alcohol, methyl alcohol, acetonitrile and N,N-dimethyl formamide) in the synthetic process, but no products could be synthesized. Ethanediamine was not observed in the final structure, but played an indispensable role in the construction of 3D Mn-clusters. Besides, HCOOH and ethanediamine must be measured at first. Interestingly, while adding NH4VO3 and Gd2O3 as the reactants, a beautiful structure with 18 Mn ions was obtained. We also optimized the reaction, and MnII12MnIII6 was successfully synthesized in the presence of NH4VO3 and Gd2O3. During the cooling process, the yield and morphology of products were unproductive for a cooling time of less than 48 h. Consequently, on cooling for over 48 h, two compounds were obtained with yields of 68% and 38%. In order to study the related properties regarding the two compounds, purification is an important step. The products were washed with CH3CH2OH and then filtrated to obtain target products (Table 1).| a R 1 = Σ||Fo| − |Fc||/Σ|Fo|. b wR2 = Σ[w(Fo2 − Fc2)2]/Σ[w(Fo2)2]1/2. | ||
|---|---|---|
| Compound | Mn II 12 Mn III 6 | Mn II 6 |
| Empirical formula | C132H123Mn18N18O73 | C62H58Mn6N8O27 |
| Formula weight | 4118.40 | 1676.80 |
| Crystal system | Trigonal | Monoclinic |
| Space group |
P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
C2/c |
| a (Å) | 24.293(8) | 15.513(2) |
| b (Å) | 24.293(8) | 10.486(2) |
| c (Å) | 9.537(5) | 22.374(4) |
| α (deg) | 90 | 90 |
| β (deg) | 90 | 107.311(3) |
| γ (deg) | 120 | 90 |
| Volume (Å3) | 4874(4) | 3474.8(11) |
| Z | 1 | 2 |
| Dc (Mg m−3) | 1.403 | 1.603 |
| μ (mm−1) | 1.204 | 1.146 |
| F(000) | 2075 | 1704 |
| Crystal size (mm3) | 0.160 × 0.130 × 0.130 | 0.150 × 0.100 × 0.100 |
| Radiation type | Mo Kα | Mo Kα |
| 2θ range for data collection (°) | 0.968–25.484 | 1.907–25.099 |
| Limiting indices | −29 ≤ h ≤ 27 | −17 ≤ h ≤ 18 |
| −28 ≤ k ≤ 29 | −12 ≤ k ≤ 12 | |
| −11 ≤ l ≤ 11 | −26 ≤ l ≤ 26 | |
| Reflections collected | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170 170 |
| R int | 0.0763 | 0.0321 |
| Data/restraints/parameters | 6053/66/388 | 3100/19/252 |
| Goodness-of-fit on F2 | 1.081 | 1.037 |
| Final R indices, R1a, wR2b | R 1 = 0.0365 | R 1 = 0.0268 |
| [I > 2σ(I)] | wR2 = 0.1071 | wR2 = 0.0670 |
| R indices (all data) | R 1 = 0.0459 | R 1 = 0.0308 |
| wR2 = 0.1114 | wR2 = 0.0692 | |
Structure
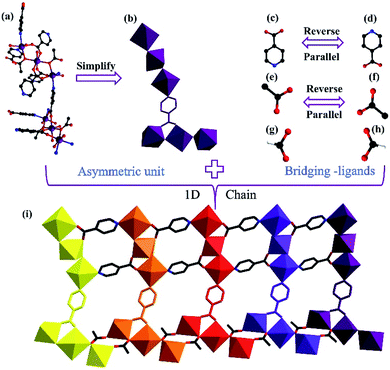
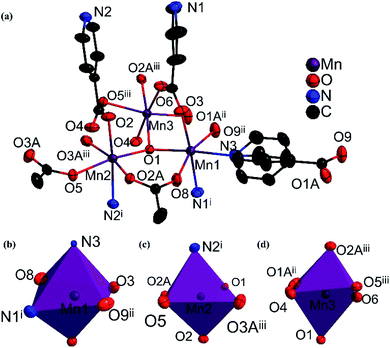
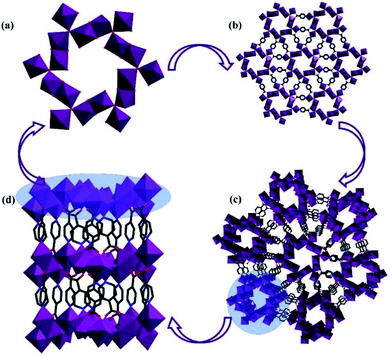
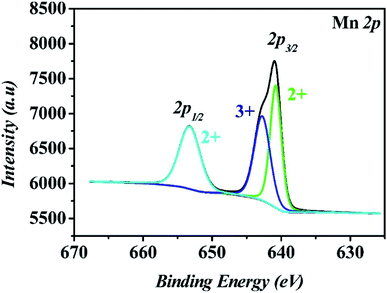
Single-crystal X-ray analysis was performed, which made it clear that MnII6 and MnII12MnIII6 crystallize in the monoclinic space group C2/c and the trigonal space group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) , respectively. As shown in Fig. 1a and b, the asymmetric unit of MnII6 contains six MnII ions, two CH3COO− groups, two HCOO− groups and eight IN− ligands, one free H2O and two free tetrahydrofuran. Moreover, the asymmetric unit of MnII12MnIII6 contains twelve MnII ions, six MnIII ions (determined by XPS, Fig. 6), twelve CH3COO−, eighteen IN− ligands, six μ3-O2− and 7.5 free water molecules (Fig. 2a). All Mn ions in MnII6 and MnII12MnIII6 are hexa-coordinated, displaying a near-octahedral geometry. As shown in Fig. S1,† Mn1 of MnII6 is coordinated to two N atoms (from two HIN) and four O atoms (from one HCOO− and one CH3COO− and two IN− groups); Mn2 of MnII6 is coordinated to six O atoms (from one HCOO− and one CH3COO− and four IN− groups). For MnII12MnIII6 (Fig. 2b–d and S2†), Mn1 is coordinated to two N atoms (from two IN− ligands) and four O atoms (from one μ3-O2− group, two IN− ligands and one CH3COO− group); Mn2 is coordinated to one N atom (from one IN− ligand) and five O atoms (from one μ3-O2− group), two Mn3 is coordinated to six O-donors (from one μ3-O2− group, three HIN ligands and two CH3COO− groups). The Mn–O bond lengths are between 2.131(3) and 2.277(2) Å as well as the Mn–N bond lengths are between 2.312(3) and 2.391(3) Å, which are approximate to the data obtained in the previously reported Mn-based compounds.3,31–34 However, in MnII6 and MnII12MnIII6, the coordination modes of IN− and CH3COO− ligands have only one mode, as indicated in Fig. S3,† which are both coordinated with three Mn ions.
, respectively. As shown in Fig. 1a and b, the asymmetric unit of MnII6 contains six MnII ions, two CH3COO− groups, two HCOO− groups and eight IN− ligands, one free H2O and two free tetrahydrofuran. Moreover, the asymmetric unit of MnII12MnIII6 contains twelve MnII ions, six MnIII ions (determined by XPS, Fig. 6), twelve CH3COO−, eighteen IN− ligands, six μ3-O2− and 7.5 free water molecules (Fig. 2a). All Mn ions in MnII6 and MnII12MnIII6 are hexa-coordinated, displaying a near-octahedral geometry. As shown in Fig. S1,† Mn1 of MnII6 is coordinated to two N atoms (from two HIN) and four O atoms (from one HCOO− and one CH3COO− and two IN− groups); Mn2 of MnII6 is coordinated to six O atoms (from one HCOO− and one CH3COO− and four IN− groups). For MnII12MnIII6 (Fig. 2b–d and S2†), Mn1 is coordinated to two N atoms (from two IN− ligands) and four O atoms (from one μ3-O2− group, two IN− ligands and one CH3COO− group); Mn2 is coordinated to one N atom (from one IN− ligand) and five O atoms (from one μ3-O2− group), two Mn3 is coordinated to six O-donors (from one μ3-O2− group, three HIN ligands and two CH3COO− groups). The Mn–O bond lengths are between 2.131(3) and 2.277(2) Å as well as the Mn–N bond lengths are between 2.312(3) and 2.391(3) Å, which are approximate to the data obtained in the previously reported Mn-based compounds.3,31–34 However, in MnII6 and MnII12MnIII6, the coordination modes of IN− and CH3COO− ligands have only one mode, as indicated in Fig. S3,† which are both coordinated with three Mn ions.
As shown in Fig. 1f, the adjacent MnII6 units are linked by one CH3COO−, one HCOO− and two IN− ligands, forming a 1D chain structure. Besides, the three kinds of bridge-ligands are all parallel and reverse (Fig. 1c–f). The adjacent 1D chains are interconnected to form a 2D layer based on IN− ligands only (Fig. 3a–e). The neighbouring 2D layers are further connected by IN−, HCOO− and CH3COO− ligands, forming a 3D structure (Fig. 3f).
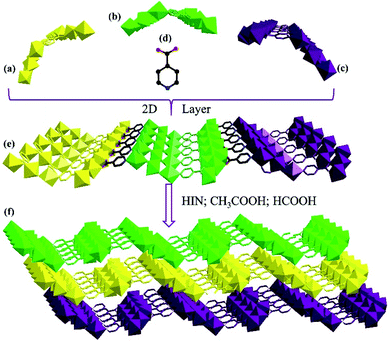 | ||
| Fig. 3 The 1D chain structure of MnII6 (a–c); the ball and stick of HIN (d); the 2D-layer of MnII6 (e); the 3D structure of MnII6 (f). | ||
As shown in the Fig. 4b and S8† [MnII2MnIII(μ3-O)(CH3COO)2]3+ unit (the first building unit; abbreviated as MnII2MnIII) consists of three Mn ions and two CH3COO− and one μ3-O2− group. As we can see in Fig. 4, the adjacent MnII2MnIII units are lined by acetic acid, forming a [MnII12MnIII6(μ3-O)6(CH3COO)12]18+ (the second building unit; abbreviated as the MnII12MnIII6) unit. In addition, Mn2 and Mn3 from adjacent MnII2MnIII units are linked by two CH3COO− groups to form a screwy wheel. As shown in Fig. 4d, 12CH3COO− groups evenly distribute on both sides of the wheel. The wheel of MnII12MnIII6 fragments are nearly planar (Fig. 4f). As indicated in Fig. 5b, the adjacent MnII12MnIII6 are interconnected only by the IN− ligands, forming a porous plane. Meanwhile, the HIN ligands act as bonds furtherly linked to adjacent MnII12MnIII6 wheels, resulting in a 3D structure (Fig. 5c). Besides, adjacent IN− ligands are all orderly arranged in the opposite direction (Fig. 5d).
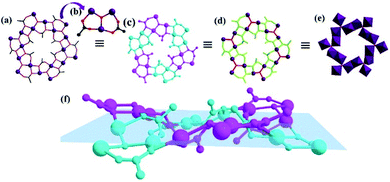 | ||
| Fig. 4 The different expressional patterns of the MnII12MnIII6 the coordination polymer (a) and (c–f); Ball and stick plot of MnII2MnIII unit (b). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MnIII) is 2
MnIII) is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is consistent with the result obtained from single-crystal X-ray diffractometry.
1, which is consistent with the result obtained from single-crystal X-ray diffractometry.
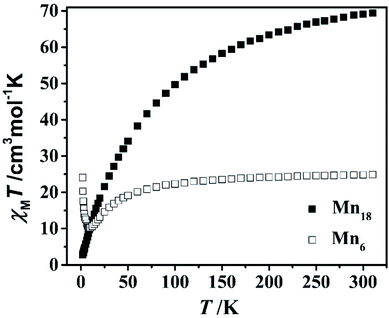
The M–H of compounds MnII6 and MnII12MnIII6 was studied under T = 1.8–10 K and H = 0–7 T (Fig. S23 and S24†). Along with the increase in H, M (MnII6) slowly increased, gradually saturated and reached 10.78 NμB at 7 T. For MnII12MnIII6, M slowly increased and reached 17.57 NμB at 7 T with the increasing H.
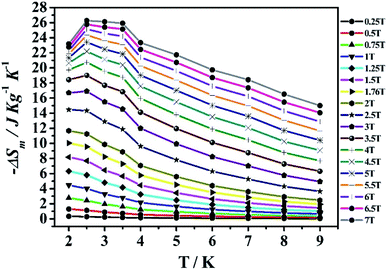
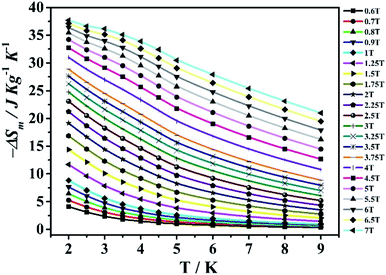
The magnetization data of compounds MnII6 and MnII12MnIII6 were analysed in the range of 2–9 K, based on the Maxwell relation in equation ΔSm(T) = ∫[∂M(T, H)/∂T]HdH.25 The compound MnII6 is discussed first. The consequential maximum value of −ΔSm is 26.27 J kg−1 K−1 (MnII6) at approximately 2.5 K and ΔH = 7 T (Fig. 8), which was slightly smaller than the theoretical calculated value [53.17 J kg−1 K−1, which was obtained on R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1)].33 The discrepancy could be due to the antiferromagnetic magnetic interaction among the metal ions.18 Besides, the maximum value of −ΔSm was 37.69 J kg−1 K−1 (MnII12MnIII6) at 2 K and ΔH = 7 T (Fig. 9), which was slightly smaller than the theoretically calculated value [62.71 J kg−1 K−1, which was gained by R
ln(2S + 1)].33 The discrepancy could be due to the antiferromagnetic magnetic interaction among the metal ions.18 Besides, the maximum value of −ΔSm was 37.69 J kg−1 K−1 (MnII12MnIII6) at 2 K and ΔH = 7 T (Fig. 9), which was slightly smaller than the theoretically calculated value [62.71 J kg−1 K−1, which was gained by R![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1)].55,56 The discrepancy could be due to the antiferromagnetic magnetic interaction among metal ions.33 The maximum values of −ΔSm for compounds MnII6 and MnII12MnIII6 are large in the pure 3d- or 4f-type and 3d–4f systems (Table 2). In addition, the −ΔSm of MnII12MnIII6 is largest in the pure Mn-type compounds, except for [Mn(glc)2(H2O)2]n.31
ln(2S + 1)].55,56 The discrepancy could be due to the antiferromagnetic magnetic interaction among metal ions.33 The maximum values of −ΔSm for compounds MnII6 and MnII12MnIII6 are large in the pure 3d- or 4f-type and 3d–4f systems (Table 2). In addition, the −ΔSm of MnII12MnIII6 is largest in the pure Mn-type compounds, except for [Mn(glc)2(H2O)2]n.31
| Compound | −ΔSmaxm | ΔH (T) | Ref. |
|---|---|---|---|
| [Mn(glc)2(H2O)2]n | 60.30 | 7.0 | 31 |
| Mn II 12 Mn III 6 | 37.69 | 7.0 | This work |
| Mn II 6 | 26.27 | 7.0 | This work |
| MnIII6MnII8 | 25.00 | 7.0 | 33 |
| FeIII14 | 20.30 | 7.0 | 51 |
| MnII4 | 19.30 | 7.0 | 32 |
| MnIII6MnII4 | 17.00 | 7.0 | 33 |
| Mn2Gd | 50.10 | 7.0 | 21 |
| Mn2Gd2 | 37.90 | 9.0 | 52 |
| Gd104 | 46.9 | 7.0 | 53 |
| Gd38 | 37.9 | 7.0 | 54 |
| Gd18 | 25.9 | 5.0 | 55 |
Conclusions
In summary, two 3D complexes (MnII6 and MnII12MnIII6) were successfully synthesized based on solvothermal conditions. In terms of their synthesis, MnII12MnIII6 was obtained by adding Gd2O3 and NH4VO3 in the synthesis of MnII6, which is a valid synthesis process and may contribute to synthesizing numerous coordination polymers. Magnetic studies revealed that MnII6 and MnII12MnIII6 are potential magnetic materials with −ΔSm = 26.27 and 37.69 J kg−1 K−1 respectively, which are much larger than the previously synthesized Mn-based coordination polymers. Moreover, the −ΔSm value of MnII12MnIII6 is almost the largest among pure Mn-type coordination polymers, making it a potential polymer in the MCE of pure 3d-type systems.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant 21571103), Jiangsu Province (BK20191359), and the Major Natural Science Projects of the Jiangsu Higher Education Institution (Grant 16KJA150005).Notes and references
- N. E. Chakov, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2004, 43, 5919–5930 CrossRef CAS PubMed.
- M. Murugesu, J. Raftery, W. Wernsdorfer, G. Christou and E. K. Brechin, Inorg. Chem., 2004, 43, 4203–4209 CrossRef CAS PubMed.
- L. Zhou, B. Zhou, S. Cao, Z. Cui, B. Qin, W. Li, X. Zhang and J. Zhang, Chem.–Eur. J., 2018, 24, 19152–19155 CrossRef CAS PubMed.
- S.-L. Li and Q. Xu, Energy Environ. Sci., 2013, 6, 1656–1683 RSC.
- S. Liu, Y. Deng and F. Xu, Chem. Commun., 2020, 54, 6066–6069 RSC.
- Y.-F. Li and Z.-P. Liu, J. Am. Chem. Soc., 2018, 140, 1783–1792 CrossRef CAS PubMed.
- A. I. Nguyen, D. L. M. Suess, L. E. Darago, P. H. Oyala, D. S. Levine, M. S. Ziegler, R. D. Britt and T. D. Tilley, J. Am. Chem. Soc., 2017, 139, 5579–5587 CrossRef CAS PubMed.
- T. Ghosh and G. Maayan, Angew. Chem., Int. Ed., 2019, 58, 2785–2790 CrossRef CAS PubMed.
- A. J. Tasiopoulos, A. Vinslava, W. Wernsdorfer, K. A. Abboud and G. Christou, Angew. Chem., Int. Ed., 2004, 43, 2117–2121 CrossRef CAS PubMed.
- U. S. Sadana, P. Sharma, N. C. Ortiz, D. Samal and N. Claassen, J. Plant Nutr. Soil Sci., 2005, 168, 581–589 CrossRef CAS.
- J. Liang, Z. Liu, L. Qiu, Z. Hawash, L. Meng, Z. Wu, Y. Jiang, L. K. Ono and Y. Qi, Adv. Energy Mater., 2018, 8, 1800504–1800511 CrossRef.
- G. E. Kostakis, A. M. Ako and A. K. Powell, Chem. Soc. Rev., 2010, 39, 2238–2271 RSC.
- M. Manoli, S. Alexandrou, L. Pham, G. Lorusso, W. Wernsdorfer, M. Evangelisti, G. Christou and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2016, 55, 679–684 CrossRef CAS PubMed.
- M. U. Anwar, L. N. Dawe, M. S. Alam and L. K. Thompson, Inorg. Chem., 2012, 51, 11241–11250 CrossRef CAS PubMed.
- C. J. Milios, S. Piligkosb and E. K. Brechin, Dalton Trans., 2008, 1809–1817 RSC.
- T. Lis, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2042–2046 CrossRef.
- A. M. Ako, I. J. Hewitt, V. Mereacre, R. Clrac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2006, 118, 5048–5051 CrossRef.
- V. Mereacre, A. M. Ako, R. Clérac, W. Wernsdorfer, I. J. Hewitt, C. E. Anson and A. K. Powell, Chem.–Eur. J., 2008, 14, 3577–3584 CrossRef CAS PubMed.
- B. Zhao, H.-L. Gao, X.-Y. Chen, P. Cheng, W. Shi, D.-Z. Liao, S.-P. Yan and Z.-H. Jiang, Chem.–Eur. J., 2006, 12, 149–158 CrossRef CAS PubMed.
- T. C. Stamatatos, S. J. Teat, W. Wernsdorfer and G. Christou, Angew. Chem., Int. Ed., 2009, 48, 521–524 CrossRef CAS PubMed.
- F.-S. Guo, Y.-C. Chen, J.-L. Liu, J.-D. Leng, Z.-S. Meng, P. Vrábel, M. Orendáč and M.-L. Tong, Chem. Commun., 2012, 48, 12219–12221 RSC.
- C. J. Milios, I. A. Gass, A. Vinslava, L. Budd, S. Parsons, W. Wernsdorfer, S. P. Perlepes, G. Christou and E. K. Brechin, Inorg. Chem., 2007, 46, 6215–6217 CrossRef CAS PubMed.
- X. Ma, D. Zhao, L.-F. Lin, S.-J. Qin, W.-X. Zheng, Y.-J. Qi, X.-X. Li and S.-T. Zheng, Inorg. Chem., 2016, 55, 11311–11315 CrossRef CAS PubMed.
- M. Riaz, R. K. Gupta, H.-F. Su, Z. Jagličić, M. Kurmoo, C.-H. Tung, D. Sun and L.-S. Zheng, Inorg. Chem., 2019, 58, 14331–14337 CrossRef CAS PubMed.
- T. C. Stamatatos, V. Nastopoulos, A. J. Tasiopoulos, E. E. Moushi, W. Wernsdorfer, G. Christou and S. P. Perlepes, Inorg. Chem., 2008, 47, 10081–10089 CrossRef CAS PubMed.
- M. Soler, W. Wernsdorfer, K. Folting, M. Pink and G. Christou, J. Am. Chem. Soc., 2004, 126, 2156–2165 CrossRef CAS PubMed.
- E. E. Moushi, C. Lampropoulos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, J. Am. Chem. Soc., 2010, 132, 16146–16155 CrossRef CAS PubMed.
- M. Manoli, R. Inglis, M. J. Manos, V. Nastopoulos, W. Wernsdorfer, E. K. Brechin and A. J. Tasiopoulos, Angew. Chem., Int. Ed., 2011, 50, 4441–4444 CrossRef CAS PubMed.
- A. Vinslava, A. J. Tasiopoulos, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2016, 55, 3419–3430 CrossRef CAS PubMed.
- T. Shiga, H. Nojiri and H. Oshio, Inorg. Chem., 2020, 59, 4163–4166 CrossRef CAS PubMed.
- Y.-C. Chen, F.-S. Guo, J.-L. Liu, J.-D. Leng, P. Vrábel, M. Orendáč, J. Prokleška, V. Sechovský and M.-L. Tong, Chem.–Eur. J., 2014, 20, 3029–3035 CrossRef CAS PubMed.
- J.-P. Zhao, R. Zhao, Q. Yang, B.-W. Hu, F.-C. Liu and X.-H. Bu, Dalton Trans., 2013, 42, 14509–14515 RSC.
- M. Manoli, A. Collins, S. Parsons, A. Candini, M. Evangelisti and E. K. Brechin, J. Am. Chem. Soc., 2008, 130, 11129–11139 CrossRef CAS PubMed.
- S. Nayak, M. Evangelisti, A. K. Powell and J. Reedijk, Chem.–Eur. J., 2010, 16, 12865–12872 CrossRef CAS PubMed.
- F. Shao, J.-J. Zhuang, M.-G. Chen, N. Wang, H.-Y. Shi, J.-P. Tong, G. Luo, J. Tao and L.-S. Zheng, Dalton Trans., 2018, 47, 16850–16854 RSC.
- N.-F. Li, Q.-F. Lin, X.-M. Luo, J.-P. Cao and Y. Xu, Inorg. Chem., 2019, 58, 10883–10889 CrossRef CAS PubMed.
- Y. Yu, X. Pan, C. Cui, X. Luo, N. Li, H. Mei and Y. Xu, Inorg. Chem., 2020, 59, 5593–5599 CrossRef CAS PubMed.
- Q. Lin, J. Li, Y. Dong, G. Zhou, Y. Song and Y. Xu, Dalton Trans., 2017, 46, 9745–9749 RSC.
- Q. Lin, Y. Zhang, W. Cheng, Y. Liu and Y. Xu, Dalton Trans., 2017, 46, 643–646 RSC.
- J.-L. Liu, W.-Q. Lin, Y.-C. Chen, J.-D. Leng, F.-S. Guo and M.-L. Tong, Inorg. Chem., 2013, 52, 457–463 CrossRef CAS PubMed.
- J.-B. Peng, Q.-C. Zhang, X.-J. Kong, Y.-Z. Zheng, Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2012, 134, 3314–3317 CrossRef CAS PubMed.
- J.-B. Peng, Q.-C. Zhang, X.-J. Kong, Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng and Z. Zheng, Angew. Chem., Int. Ed., 2011, 50, 10649–10652 CrossRef CAS PubMed.
- Y.-Z. Zheng, M. Evangelisti and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2011, 123, 3776–3779 CrossRef.
- W.-P. Chen, J. Singleton, L. Qin, A. Camón, L. Engelhardt, F. Luis, R. E. P. Winpenny and Y.-Z. Zheng, Nat. Commun., 2018, 9, 1–6 CrossRef PubMed.
- D. I. Alexandropoulos, L. Cunha-Silva, J. Tang and T. C. Stamatatos, Dalton Trans., 2018, 47, 11934–11941 RSC.
- J.-D. Leng, J.-L. Liu and M.-L. Tong, Chem. Commun., 2012, 48, 5286–5288 RSC.
- L. Sun, H. Chen, C. Ma and C. Chen, Dalton Trans., 2015, 44, 20964–20971 RSC.
- L. Wang, R. Zhao, L.-Y. Xu, T. Liu, J.-P. Zhao, S.-M. Wang and F.-C. Liu, CrystEngComm, 2014, 16, 2070–2077 RSC.
- S. Jeong, X. Song, S. Jeong, M. Oh, X. Liu, D. Kim, D. Moon and M. S. Lah, Inorg. Chem., 2011, 50, 12133–12140 CrossRef CAS PubMed.
- X.-Y. Li, H.-F. Su, Q.-W. Li, R. Feng, H.-Y. Bai, H.-Y. Chen, J. Xu and X.-H. Bu, Angew. Chem., Int. Ed., 2019, 58, 10184–10188 CrossRef CAS PubMed.
- R. Shaw, R. H. Laye, L. F. Jones, D. M. Low, C. Talbot-Eeckelaers, Q. Wei, C. J. Milios, S. Teat, M. Helliwell, J. Raftery, M. Evangelisti, M. Affronte, D. Collison, E. K. Brechin and E. J. L. McInnes, Inorg. Chem., 2007, 46, 4968–4978 CrossRef CAS PubMed.
- T. Rajeshkumar, R. Jose, P. R. Remya and G. Rajaraman, Inorg. Chem., 2019, 58, 11927–11940 CrossRef CAS PubMed.
- J.-B. Peng, X.-J. Kong, Q.-C. Zhang, M. Orendáč, J. Prokleška, Y.-P. Ren, L.-S. Long, Z. Zheng and L.-S. Zheng, J. Am. Chem. Soc., 2014, 136, 17938–17941 CrossRef CAS PubMed.
- F.-S. Guo, Y.-C. Chen, L.-L. Mao, W.-Q. Lin, J.-D. Leng, R. Tarasenko, M. Orendáč, J. Prokleška, V. Sechovský and M.-L. Tong, Chem.–Eur. J., 2013, 19, 14876–14885 CrossRef CAS PubMed.
- K. Wang, Z.-L. Chen, H.-H. Zou, K. Hu, H.-Y. Li, Z. Zhang, W.-Y. Sun and F.-P. Liang, Chem. Commun., 2016, 52, 8297–8300 RSC.
- X.-M. Luo, Z.-B. Hu, Q.-f. Lin, W. Cheng, J.-P. Cao, C.-H. Cui, H. Mei, Y. Song and Y. Xu, J. Am. Chem. Soc., 2018, 140, 11219–11222 CrossRef CAS PubMed.
- S. L. Xiong, J. S. Chen, X. W. Lou and H. C. Zeng, Adv. Funct. Mater., 2012, 22, 861–871 CrossRef CAS.
- X. Li, Y. Zhu, X. Zhang, J. Liang and Y. Qian, RSC Adv., 2013, 3, 10001–10006 RSC.
- B. J. Tan, K. J. Klabunde and P. M. A. Sherwood, J. Am. Chem. Soc., 1991, 113, 855–861 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Physical measurements, crystal synthesis, additional structural pictures, XPS, PXRD, FT-IR spectra, TG analysis, magnetic properties, as well as selected bond lengths and angles. Tables S1, S2, and Fig. S1–S24. CCDC 2004735 and 2004736 for compounds MnII6 and MnII12MnII6. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra05926a |
| This journal is © The Royal Society of Chemistry 2020 |