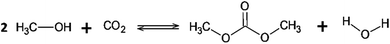Open Access Article
Open Access ArticleDehydrating agent effect on the synthesis of dimethyl carbonate (DMC) directly from methanol and carbon dioxide
Douglas José Fariaa,
Leonardo Moreira dos Santosb,
Franciele Longaray Bernardb,
Ingrid Selbacch Pintob,
Maria Angélica Carmona da Motta Resendec and
Sandra Einloft *ab
*ab
aPost-Graduation Program in Materials Engineering and Technology, Pontifical Catholic University of Rio Grande do Sul – PUCRS, Brazil. E-mail: einloft@pucrs.br
bSchool of Technology, Pontifical Catholic University of Rio Grande do Sul PUCRS, Brazil
cCENPES/Petrobras, Brazil
First published on 21st September 2020
Abstract
CO2 emissions and global warming have increased with the growth of the world economy and industrialization. Direct synthesis of dimethyl carbonate (DMC) from CO2 and methanol (CH3OH) has been considered a promising route from a green chemistry point of view due to global warming mitigation by CO2 emission reduction. However, DMC yield, when obtained by direct synthesis, is limited due to unfavorable thermodynamics and catalyst deactivation by water formation in the reaction process. This problem motivated us to investigate the effect of dehydration on DMC production by direct synthesis. Herein, different dehydrating agents (2,2-dimethoxypropane, sodium sulfate, magnesium oxide and butylene oxide) were combined with molecular sieves to remove the water and minimize the reverse reaction. A new reactor presenting a compartment to accommodate molecular sieves in the gas phase was developed as well. The chemical/product analysis was carried out by gas chromatography and the results were used to calculate methanol conversion and DMC selectivity. The highest methanol conversion value was found for the combination of molecular sieves in the gas phase with 2,2-dimethoxypropane in the reaction liquid phase (methanol conversion = 48.6% and 88% selectivity). The results showed that dehydration systems may promote increased yield in direct DMC synthesis under mild conditions. The dehydration systems tested in this work exhibited excellent conversion and yield as compared to other reported studies.
1. Introduction
The growth of the world economy allied to human and industrial activities is the main origin of the increase in CO2 emission in the atmosphere.1 CO2 emission reached 24 Gt in 1995, growing about 1% a year from 1990 to 1999 and arriving at 35 Gt in 2015. The CO2 level is expected to reach 40 Gt in 2020, growing 2.0% by year over the decade.2 This scenario corroborates the need for exploring new ways of using CO2 as a raw material in innovative industrial processes obtaining high added value products and reducing the environmental impact.3 Direct synthesis reactions from CO2 and alcohol producing organic carbonates, such as dimethylcarbonate (DMC) are being investigated.4 Alkyl carbonate production allows the use of excess CO2 generating low toxicity, biodegradable and non-corrosive products.5DMC is an important carbonate being used in several niches, such as intermediate in polycarbonate production for building utilization, medical devices, automobiles and electrolytic solvent in lithium batteries due to the high dielectric constant. Yet, due to the high oxygen composition (53%) and octane number (105) dimethyl carbonate has been described as an excellent diesel additive reducing the emission of soot particles and, consequently, the environmental impact.6 Methyl tert-butyl ether (MTBE) is used as additive in fuels, however, several countries are banning its use due to the generated environmental impacts. Therefore, DMC comes as a substitute, since, in the concentration of 40% (v/v) in relation to the MTBE concentration achieves the same effectiveness as MTBE reducing waste generation by 50%.7–10
Industrial production of DMC is environmentally friendly, however the Gibbs energy for DMC production by direct reaction is greater than zero. Reaction equilibrium constant around 10−5 indicates that the formation reaction does not occur spontaneously. DMC synthesis has traditionally occurred through three routes. In the first, DMC is synthesized from phosgene and methanol (phosgene method) having a high environmental impact.11 The second route uses methanol, oxygen and carbon monoxide (oxidative carboxylation) presenting an inherent health and safety risk by using CO as reagent in addition to the high process and feedstock cost.12–14 The third route uses dimethyl sulfate and sodium carbonate (transesterification method), products from the oil refinery, presenting the highest total cost per DMC produced.13,15 In addition to the traditional methods, DMC can be obtained by direct synthesis using carbon dioxide and methanol. This route provides the lowest total cost per DMC produced and safer chemicals besides a favorable carbon balance. The main drawback of DMC direct synthesis is the low yield and selectivity.13,14 Therefore, to improve yield and selectivity in DMC direct synthesis modelled reactors, changes in pressure and temperature and the use of techniques for directing the equilibrium constant for product formation must be evaluated.16 In addition, DMC direct synthesis from methanol and CO2 produces water as a by-product (see Fig. 1), decreasing yield by directing the reaction balance towards reagents.17,18
This scenario evidences the need of assessing efficient dehydrating agents for the removal of water produced during reaction, consequently improving DMC yield. Several dehydrating agents have been investigated for DMC synthesis.11,12 Among investigated dehydrating agents molecular sieves can be highlighted due to their large surface area, thermal stability and excellent adsorption.13 Organic and inorganic dehydrating agents, such as 2,2-dimethoxypropane (DMP) and magnesium oxide, are also described as efficient water withdrawn agents during DMC synthesis increasing product yield.14,15
The main goal of this work is to evaluate a new dehydration system for direct DMC synthesis using CH3I as promoter, CH3OK as catalyst. In this new designed system, molecular sieves were tested in the reaction liquid phase and in the gas phase. Yet, a combination of organic and inorganic dehydrating agents (2,2-dimethoxypropane, sodium sulfate, magnesium oxide and butylene oxide) in liquid phase and molecular sieve in the gas phase were tested as well.
2. Methodology
2.1 Materials
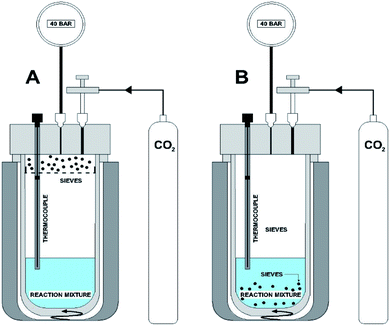
Methanol (>99.9% – EMSURE®), potassium methoxide (>95% – ALDRICH), iodomethane (>99.5% – ALDRICH), diethyl ether (>99.9% – EMSURE®), magnesium oxide (98% – ALDRICH), 2,2-dimethoxypropane – DMP (98% – ALDRICH), sodium sulfate (>98% – ALDRICH), dimethylcarbonate – DMC (>99.5% – ALDRICH), pearl-shaped molecular sieves (3A – ALDRICH), butylene oxide (99% – ALDRICH) and CO2 (99,8% – White Martins).2.2 DMC synthesis
Two 120 ml reactors (made of titanium alloy) with constant magnetic stirring were used to perform the experiments (see Fig. 2). Temperature was controlled by means of a thermocouple connected to a temperature controller and kept constant using a resistive thermal band in both reactors. A reactor was equipped with a compartment metallic support in the gas phase filled with molecular sieves (see Fig. 2A). For a typical reaction 213 mmol of methanol, 10 mmol of CH3OK, 20 mmol of CH3I, the desired dehydrating agent and 40 bar of CO2 were used. Reactions were carried out using different dehydrating agents (molecular sieve, 2,2-dimethoxypropane, sodium sulfate, magnesium oxide and butylene oxide) added direct to the liquid phase or by combining a molecular sieve placed in the gas phase with dehydrating agent added in the liquid phase. The reactor was pressurized with CO2 at 40 bar and heated at 80 °C. Reaction time ranged from 6 h to 30 h. At the end of the reaction, the reactor was cooled to room temperature and slowly depressurized. The new reactor designed in this work (Fig. 2) contains an apparatus in the upper part to supporting the molecular sieves (A) capturing water in the gaseous phase. As a way of comparison of the results for the DMC synthesis using the new reactor and the already described process the sieves were also tested in the liquid part (B). Yet, the use of molecular sieves in the gaseous phase combined with different dehydrating agents in the liquid phase in the DMC obtainment reaction by direct synthesis will also be tested using the system described in Fig. 2A.In order to compare the results a reaction without dehydrating agent was also performed. All tests were performed in triplicate. The products of catalytic tests were analyzed by gas chromatography (GC) in order to determine yield, conversion and selectivity. Gas Chromatograph Shimadzu GC-2014 equipped with SH-Rtx-5 column using a heating ramp of 31 °C for 0.5 minutes, 10 °C min−1 to 50 °C for 1 minute, 20 °C min−1 to 100 °C for 2 minutes and 50 °C min−1 to 220 °C for 2 minutes. Samples were diluted with a concentration of 4% (v/v) in ethyl ether and the peak area of DMC (2.4–2.7 minutes) was used to define the concentration through the calibration curve of pure DMC following procedures described by Valente, Riedo and Augusto (2003).23 Conversion and selectivity were performed as described by Chen et al. (2012).20
Methanol conversion was calculated using eqn (1).
| Methanol conversion (%) = ((Methanol reacted)/(Methanol total)) × 100 | (1) |
DMC selectivity was obtained by eqn (2).
| DMC selectivity (%) = ((DMC)/(DMC + (by-products))) × 100 | (2) |
DMC yield was determined using eqn (3).
| DMC yield (%) = ((Methanol conversion (%)) × (DMC selectivity (%)))/100 | (3) |
In addition, water content was determined (in triplicate) using the Karl Fischer test by digital automatic titration equipment with the Karl Fischer solution.
2.3 Statistical analysis
Minitab 18 Statistical Software-ANOVA was used to perform statistical analysis in order to assess the standard deviation of tests performed in triplicate and analyze the Tukey test with 95% reliability, where equal letters show statistical equality between the samples averages.3. Results and discussion
3.1 Direct synthesis of DMC using molecular sieve (3A) and combination of dehydrating agents
Thermodynamic limitations and/or catalyst deactivation due to water formation during DMC direct synthesis makes this reaction unfavorable resulting in low DMC yield.5,24,25 Aiming to evaluate the effect of dehydrating agents (chemical or physical) on the yield of DMC obtained by direct synthesis reaction CH3OK was used as catalyst and CH3I as promoter. Table 1 presents methanol conversion, DMC selectivity and DMC yield values, reaction parameters and Tukey test results used to evaluate statistical equality between samples (see letters a to g in conversion column, Table 1).| Entry | Sieve | Dehydrating agent | Time (h) | Temperature (°C) | Pressure (bar) | DMC selectivity (%)* | Methanol conversion (%)** | DMC yield (%)*** | Water (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Phase | (g) | |||||||||
| a Fixed parameters → methanol: 213 mmol; catalyst (CH3OK): 10 mmol; promoter (CH3I): 20 mmol. *: eqn (1); **: eqn (2); ***eqn (3) described in Methodology section. | ||||||||||
| 1 | — | 0 | — | 24 | 80 | 40 | 100 | 7.0 ± 1.6f,g | 7.0 ± 1.6 | 7.1 ± 0.1 |
| 2 | Liquid | 1 | Sieve | 24 | 80 | 40 | 100 | 13.6 ± 1.9d | 13.6 ± 1.9 | 3.7 ± 0.1 |
| 3 | Liquid | 2 | Sieve | 24 | 80 | 40 | 100 | 17.2 ± 1.1c,e | 17.2 ± 1.1 | 1.6 ± 0.1 |
| 4 | Liquid | 3 | Sieve | 24 | 80 | 40 | 100 | 9.3 ± 0.7f | 9.3 ± 0.7 | 1.5 ± 0.3 |
| 5 | Gas | 1 | Sieve | 24 | 80 | 40 | 100 | 7.0 ± 0.7g | 7.0 ± 0.7 | 3.3 ± 0.2 |
| 6 | Gas | 2 | Sieve | 24 | 80 | 40 | 100 | 30.5 ± 2.3a | 30.5 ± 2.3 | 0.5 ± 0.1 |
| 7 | Gas | 3 | Sieve | 24 | 80 | 40 | 100 | 8.7 ± 0.5f | 8.7 ± 0.5 | 4.5 ± 0.1 |
| 8 | Gas | 2 | Sieve | 6 | 80 | 40 | 100 | 9.7 ± 1.2f | 9.7 ± 1.2 | 2.5 ± 0.1 |
| 9 | Gas | 2 | Sieve | 12 | 80 | 40 | 100 | 14.3 ± 0.7d | 14.3 ± 0.7 | 2.6 ± 0.2 |
| 10 | Gas | 2 | Sieve | 18 | 80 | 40 | 100 | 16.1 ± 0.4c | 16.1 ± 0.4 | 3.9 ± 0.8 |
| 11 | Gas | 2 | Sieve | 30 | 80 | 40 | 100 | 26.2 ± 0.8b | 26.2 ± 0.8 | 6.1 ± 1.2 |
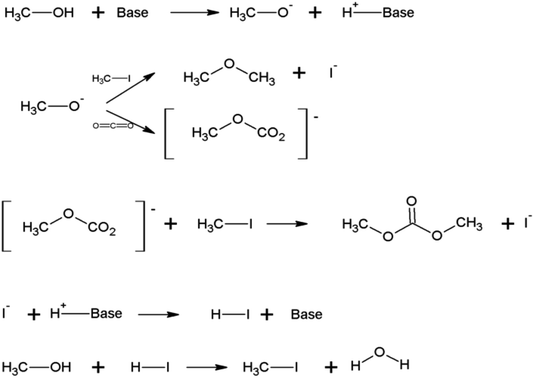
One of the acceptable mechanisms for DMC direct synthesis from CO2 and methanol (CH3OH) using CH3OK as catalyst and iodomethane CH3I (Table 1, entry 1) is shown in Fig. 3. CH3I can act as reaction promoter or as a reagent.26 In this mechanism the hydrogen of the methanol hydroxyl is abstracted by the catalyst producing a methoxy anion, which reacts with CH3I or CO2, forming dimethyl ether or methyl carbonate anion which successively reacts with CH3I to form DMC. The iodine anion formed during the reaction steps reacts with the proton recycling the catalyst. Produced HI reacts with methanol recovering the CH3I.26
Results obtained in this work suggest that CH3I may be acting as a promoter as no selectivity reduction was observed by gas chromatography when only CH3I and CH3OK were used (Table 1, entry 1).
The catalytic system employed by Cai et al. 2005 (ref. 27) using CH3OK (catalyst) and CH3I (promoter) in the direct synthesis of DMC from CO2 was selected to evaluate the efficiency of dehydrating agents. In this work, catalyst content and reactor size were different from that used by Cai et al. (2005).27 The reaction described in literature obtained a yield of ∼9.7% with 100% of selectivity using reaction conditions of 80 °C, 40 bar and 6 h of reaction time. Different catalyst content and promoter combined with the use of a smaller reactor may explain the lower yield (see entry 1, Table 1) when using the same catalyst system reported by Cai et al. (2005).27 The use of different amounts (1.0–3.0 g) of molecular sieves in the liquid phase demonstrated to be effective when compared to the reaction carried out without the presence of dehydrating agent (entry 1, Table 1: yield = 7.0%), as statistically proved by the Tukey test represented in Table 1 by unequal letters. The highest DMC yield was obtained using 2.0 g of molecular sieves (entry 3, Table 1: yield = 17.2%) followed by reaction yields obtained with 1.0 g (entry 2, Table 1: yield = 13.6%) and 3.0 g (entry 4, Table 1: yield = 9.3%) of molecular sieve respectively. The use of molecular sieve reduced the water content from 7.1% to 3.7% (entry 2, Table 1: 1.0 g of molecular sieve), 1.6% (entry 3, Table 1: 2.0 g of molecular sieve) and 1.5% (entry 4, Table 1: 3.0 g of molecular sieve). These results indicate that increasing the amount of molecular sieve in the liquid phase from 2.0 to 3.0 g can hinder the interaction of reagents with the catalyst active sites reducing catalytic activity. The use of 1.0 g of molecular sieve may not be sufficient to remove the formed water and achieve optimal yield values.
Surprisingly tests performed with molecular sieve in the gas phase showed an increase of more than 300% when using 2.0 g of molecular sieve (entry 6, Table 1: yield = 30.5%), 22%, 3.0 g (entry 7, Table 1: yield = 8.7%) and 0%, 1.0 g (entry 5, Table 1: yield = 7.0%) when compared to the reaction without molecular sieve addition (entry 1, Table 1, yield = 7.0%). Increasing molecular sieve content from 1.0 g (entry 5, Table 1: water = 3.3%) to 2.0 g (entry 6, Table 1: water = 0.5%) significantly reduced the water content present in the reaction medium. However, increasing molecular sieve content from 2.0 g (entry 6, Table 1: water = 0.5%) to 3.0 g (entry 6, Table 1: water = 4.5%) increased the water content present in the reaction medium. This result indicates that the increase in molecular sieve content is probably reducing the contact surface of molecular sieves with water vapor due to the sieves agglomeration in the reactor metallic support. These results are corroborated by the Tukey test. It should also be emphasized that the use of molecular sieves in the gas phase facilitates the dehydrating agent separating process from the reaction mixture reducing cost and steps in DMC production process.
Effect of reaction time on DMC yield when molecular sieves are used in the gaseous phase was also evaluated. In 6 hours of reaction time a yield of around 9.7% (entry 8, Table 1) was achieved. This result is similar to Cai et al. (2005)27 founds using the same reaction conditions. However, after 6 hours an increase in DMC production was observed. Yield of 14.3% (entry 9, Table 1) was achieved in 12 h, 16.1% (entry 10, Table 1) 18 hours and the highest yield value of 30.5% (entry 6, Table 1) in 24 hours. After the 24 hour period, a small decrease in the amount of DMC yield was observed reaching 26.2% (entry 11, Table 1). According to literature,28 water withdrawal from the reaction medium has great interference in the DMC synthesis optimization time since smaller amount of water allows greater catalytic activity and catalyst durability. From these results one can infer that the molecular sieve porosity improved catalytic activity and increased DMC production by withdrawing the water produced during the reaction. Therefore, probably after 24 hours of reaction time the molecular sieve become less effective decreasing reaction yield. The use of 2.0 g of molecular sieve in the gas phase for 24 hours was the best result for the experimental conditions tested. Therefore, it was used in combination with other dehydrating agents in the liquid phase (DMP, Na2SO4, MgO and butylene oxide) aiming further yield improvement as shown in Table 2.
| Entry | Sieve | Dehydrating agent | Temperature (°C) | Pressure (bar) | DMC selectivity (%)* | Methanol conversion (%)** | DMC yield (%)*** | Water (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Phase | (g) | ||||||||
| a Fixed parameters → methanol: 213 mmol; catalyst (CH3OK): 10 mmol; promoter (CH3I): 20 mmol; dehydrating agent: 10 mmol; time: 24 hours. ND = not detected. *: eqn (1); **: eqn (2); ***: eqn (3) described in Methodology section. | |||||||||
| 1 | — | — | DMP | 80 | 40 | 55 | 6.4 ± 0.9c | 3.5 ± 0.9 | 0.6 ± 0.1 |
| 2 | — | — | Na2SO4 | 80 | 40 | 100 | 5.0 ± 1.4c,d | 5.0 ± 1.4 | 1.1 ± 0.3 |
| 3 | — | — | Butylene oxide | 80 | 40 | ND | NDb | ND | Traces |
| 4 | — | — | MgO | 80 | 40 | ND | NDb | ND | Traces |
| 5 | Combined-gas | 2 | Sieve/DMP | 80 | 40 | 88 | 48.6 ± 1.9a | 42.8 ± 1.9 | 0.6 ± 0.1 |
| 6 | Combined-gas | 2 | Sieve/Na2SO4 | 80 | 40 | 100 | 14.8 ± 2.0e | 14.8 ± 2.0 | 2.3 ± 0.2 |
| 7 | Combined-gas | 2 | Sieve/butylene oxide | 80 | 40 | 15 | 2.5 ± 1.4c,d | 0.3 ± 1.4 | 2.3 ± 0.8 |
| 8 | Combined-gas | 2 | Sieve/MgO | 80 | 40 | 100 | 4.5 ± 0.9c | 4.5 ± 0.9 | 0.7 ± 0.1 |
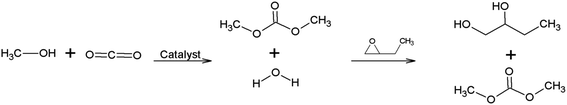
DMP was evaluated as dehydrating agent (Table 2, entry 1) due to its quick action by chemical hydrolysis making it easy to use in dehydration processes. In addition DMP is stable during storage.29 As seen in Fig. 4 acetone is produced as by-product decreasing reaction selectivity.21,22
Butylene oxide is an effective dehydrating agent promoting an increase in product conversion due to the excellent dehydration capacity into the reaction medium.30 When reacting with water, produces ethylene glycol decreasing DMC selectivity due to by-product formation,31 as show in Fig. 5.
When combining molecular sieve with dehydrating agents different yield values were obtained (DMP 42.8% (entry 5, Table 2), Na2SO4 14.8% (entry 2, Table 2), MgO, 4.5% (entry 4, Table 2) and 0.3% butylene oxide (entry 3, Table 2)). Although the addition of solid dehydrating agent (Na2SO4 or MgO) in the liquid phase promotes the reduction of the amount of water formed in the reaction medium the presence of these compounds may be reducing the interaction between the reactants and the catalyst active sites. Yet, the use of DMP decreases selectivity due to the ketone production in the reaction medium, consequently decreasing yield. However, even with this setback the yield is superior when using this system. Ketone removal step, by distillation, can be added to the process in order to improve selectivity increasing it to 100%.19,32
Butylene oxide decreased DMC selectivity by 15%, reaching a methanol conversion of 2.5% being inefficient. So, it is clear from results that the best performance was obtained by combining the molecular sieve and DMP resulting in a yield increase of almost 50% when compared to the reaction performed using only molecular sieve in the gaseous phase (entry 6, Table 1). The dehydrating agents DMP (entry 1, Table 2), Na2SO4 (entry 2, Table 2), butylene oxide (entry 3, Table 2) and MgO (entry 4, Table 2) were also evaluated. Obtained yield values were lower/similar when compared to the reaction carried without dehydrating agent (entry 1, Table 1: yield = 7.0%).
DMP (entry 1, Table 2) showed a methanol conversion value statistically equal when compared to the reaction carried without dehydrating agent (entry 1, Table 1). However, a reduction in selectivity of 55% was observed and attributed to acetone formation as a by-product. When using sodium sulfate a lower yield was achieved when comparing with the reaction without dehydrating agent addition. Sodium sulfate is an effective dehydrating agent for use at room temperature and is considered a slow-acting dehydrating agent. These features can influence the process of water removing from the reaction medium.33,34
When using butylene oxide (entry 3, Table 2) and MgO (entry 4, Table 2) as dehydrating agent no methanol conversion was detected under the tested reaction conditions. When using butylene oxide as dehydrating agent and more drastic reaction conditions (150 °C and pressure of 90 bar) a methanol conversion of 11.7% was reached as described elsewhere.31 Magnesium oxide suffers a large dilation when retaining water. This feature can reduce the contact of reagents and catalyst disfavoring DMC synthesis.35,36
Temperature and pressure range used for DMC synthesis described in literature (see Table 3) were varied from 80 °C to 140 °C and 6 bar up to 200 bar respectively. Our results, using mild conditions (80 °C, 40 bar) and molecular sieves placed in the gas phase or the combination of molecular sieve placed in the gas phase/DMP placed in the liquid phase were superior to results described in literature (see Table 3, entries 1 and 2). Molecular sieves effect on DMC yield obtained by direct synthesis was described elsewhere obtaining a yield up to 31% using dibutyltin dimethoxide (catalyst), high pressure (300 bar) and 180 °C of temperature (see Table 3, entry 10) using a facility that allow to place the dehydrating agent at room temperature and circulate the reaction mixture through the dehydration tube by a high-pressure circulation pump.25 DMC synthesis using CH3OK and CH3I, without the presence of dehydrating agents, at a pressure of 73 bar and 80 °C of temperature was described achieving a conversion of 16.2% (ref. 37) (see Table 3, entry 7). Our results evidenced that the use of molecular sieves in the gas phase allied to DMP in the liquid phase increases DMC yield up to 42.8%. This yield value is superior when compared to current literature as seen in Table 3. Yet, a DMC yield of 30.5% was obtained when placing molecular sieves in the gas phase using mild temperature and pressure conditions (80 °C and 40 bar) as seen in Table 3, entry 1. The placement of the molecular sieves in the gas phase is somehow facilitating the water withdrawing besides being very important in terms of easiness of molecular sieve separation.
| Entry | Catalyst | Promoter/dehydrating agent | Temperature (°C) | Pressure (bar) | DMC yield (%) | Literature |
|---|---|---|---|---|---|---|
| 1 | CH3OK | CH3I/sieves (gas phase) | 80 | 40 | 30.5 | This work |
| 2 | CH3OK | CH3I/sieves (gas phase) + DMP (liquid phase) | 80 | 40 | 42.8 | This work |
| 3 | Cu/Ce | — | 140 | 50 | 1.6 | Marciniak et al. (2019)38 |
| 4 | Chitosan/IL | — | 100 | 75 | 16.7 | Tamboli et al. (2016)39 |
| 5 | Cu–Ni/graphene | — | 110 | 30 | 13.0 | Deerattraku et al. (2020)40 |
| 6 | K2CO3 + EmimBr | CH3I | 80 | 73 | 5.7 | Kabra et al. (2016)41 |
| 7 | CH3OK | CH3I | 80 | 73 | 16.2 | Fang and Fujimoto (1996)37 |
| 8 | K2CO3 | CH3I, DMP | 140 | 200 | 12.0 | O'Neil, Clayton and Mayeda (1969)42 |
| 9 | CeO2 | Molecular sieves (4A) | 120 | 6 | 3.2 | Zhang et al. (2015)43 |
| 10 | Dibutyltin dimethoxide | Molecular sieves (3A) | 180 | 300 | 31 | Choi et al. (2002)25 |
| 11 | Dibutyltin dimethoxide | — | 180 | 300 | 3 | Choi et al. (2002)25 |
| 12 | Cu/Ce | 2-Cyanopyridine | 140 | 50 | 5.0 | Marciniak et al. (2019)38 |
| 13 | Cu/Ce | Methyl trichloroacetate | 140 | 50 | 12 | Marciniak et al. (2019)38 |
4. Conclusions
Different dehydrating agents (molecular sieve, 2,2-dimethoxypropane, sodium sulfate, magnesium oxide and butylene oxide) were evaluated in the DMC direct synthesis. Yet, a new system for dehydration under mild conditions was proposed. The best results were obtained when 2.0 g of molecular sieve was placed in the gas phase (yield = 30.5%) or when 2.0 g of molecular sieve was combined in the gas phase with 10 mmol DMP in the liquid phase (yield = 42.8%). In the dehydrating system proposed in this work the sieves can be easily separated, since they are deposited in the gas phase and the acetone formed from the DMP can be easily separated from the reaction medium using the distillation technique. This catalytic system also achieved higher yield values when compared to other systems reported in literature for direct synthesis of DMC. Thus, the proposed dehydration system in this work may be an effective way to promote increase in DMC direct synthesis yield whereas can be used with different catalysts.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Douglas Faria thanks CAPES; Sandra Einloft thanks CNPq for research scholarship. This study was financed in party by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. The authors thank Petrobras for financial support.References
- M. K. Kim and J.-H. Choi, Can increased outdoor CO2 concentrations impact on the ventilation and energy in buildings? A case study in Shanghai, China, Atmos. Environ., 2019, 210, 220–230, DOI:10.1016/j.atmosenv.2019.04.015.
- I. Chang, M. Lee and G. C. Cho, Global CO2 emission-related geotechnical engineering hazards and the mission for sustainable geotechnical engineering, Energies, 2019, 12(13), 2567, DOI:10.3390/en12132567.
- A. Bansode and A. Urakawa, Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products, J. Catal., 2014, 309, 66–70, DOI:10.1016/j.jcat.2013.09.005.
- Z. Fu, Y. Zhong and Y. Yu, et al., TiO2-Doped CeO2 nanorod catalyst for direct conversion of CO2 and CH3OH to dimethyl carbonate: catalytic performance and kinetic study, ACS Omega, 2018, 3(1), 198–207, DOI:10.1021/acsomega.7b01475.
- X. Hu, H. Cheng, X. Kang, L. Chen, X. Yuan and Z. Qi, Analysis of direct synthesis of dimethyl carbonate from methanol and CO2 intensified by in-situ hydration-assisted reactive distillation with side reactor, Chem. Eng. Process., 2018, 129, 109–117, DOI:10.1016/j.cep.2018.05.007.
- A. Sánchez, L. M. Gil and M. Martín, Sustainable DMC production from CO2 and renewable ammonia and methanol, J. CO2 Util., 2019, 33, 521–531, DOI:10.1016/j.jcou.2019.08.010.
- G. Fiorani, A. Perosa and M. Selva, Dimethyl carbonate: a versatile reagent for a sustainable valorization of renewables, Green Chem., 2018, 20(2), 288–322, 10.1039/c7gc02118f.
- H.-Z. Tan, Z.-Q. Wang and Z.-N. Xu, et al., Review on the synthesis of dimethyl carbonate, Catal. Today, 2018, 316, 2–12, DOI:10.1016/j.cattod.2018.02.021.
- R. Porcar, P. Lozano, M. Isabel Burguete and S. V. L. Eduardo Garcia-Verdugo, Dimethyl carbonate as a non-innocent benign solvent for the multistep continuous flow synthesis of amino alcohols, React. Chem. Eng., 2018, 4, 572, 10.1039/c8re00097b.
- S. K. Kabra, M. Huuhtanen, R. L. Keiski and G. D. Yadav, Selectivity engineering of O-methylation of hydroxybenzenes with dimethyl carbonate using ionic liquid as catalyst, React. Chem. Eng., 2016,(3), 330–339, 10.1039/c6re00016a.
- C.-M. Hsu, S.-J. Wang, Y.-T. Chen and D. S.-H. Wong, Novel separation process design for non-phosgene dimethylhexane-1,6-dicarbamate synthesis by reacting dimethyl carbonate with 1,6-hexanediamine, J. Taiwan Inst. Chem. Eng., 2019, 97, 54–65, DOI:10.1016/j.jtice.2019.02.004.
- J.-M. Woo, J. Y. Seo and H. Kim, et al., CuY zeolite catalysts prepared by ultrasonication-assisted ion-exchange for oxidative carbonylation of methanol to dimethyl carbonate, Ultrason. Sonochem., 2018, 44, 146–151, DOI:10.1016/j.ultsonch.2018.02.013.
- R. Guil-López, N. Mota and J. Llorente, et al., Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis, Materials, 2019, 12(23), 3902, DOI:10.3390/ma12233902.
- M. Zhang, Y. Xu and B. L. Williams, et al., Catalytic Materials for Direct Synthesis of Dimethyl Carbonate (DMC) from CO2, J. Cleaner Prod., 2020, 123344, DOI:10.1016/j.jclepro.2020.123344.
- H. Li, G. Zhang, Y. Wang and S. Zhang, Transesterification of ethylene carbonate with methanol over Zn-La mixed oxide catalysts, J. Fuel Chem. Technol., 2018, 46(8), 977–984, DOI:10.1016/s1872-5813(18)30041-0.
- B. Y. Yu, M. K. Chen and I. L. Chien, Assessment on CO2 Utilization through Rigorous Simulation: Converting CO2 to Dimethyl Carbonate, Ind. Eng. Chem. Res., 2018, 57(2), 639–652, DOI:10.1021/acs.iecr.7b02923.
- S. Chaemchuen, O. V. Semyonov and J. Dingemans, et al., Progress on Catalyst Development for Direct Synthesis of Dimethyl Carbonate from CO2 and Methanol, Chem. Afr., 2019, 2(4), 533–549, DOI:10.1007/s42250-019-00082-x.
- P. Unnikrishnan, P. Varhadi and D. Srinivas, Efficient, direct synthesis of dimethyl carbonate from CO2 using a solid, calcined zirconium phenylphosphonate phosphite catalyst, RSC Adv., 2013, 3(46), 23993–23996, 10.1039/c3ra44368j.
- J.-C. Choi, K. Kohno, Y. Ohshima, H. Yasuda and T. Sakakura, Tin- or titanium-catalyzed dimethyl carbonate synthesis from carbon dioxide and methanol: large promotion by a small amount of triflate salts, Catal. Commun., 2008, 9(7), 1630–1633, DOI:10.1016/j.catcom.2008.01.013.
- H. Chen, S. Wang, M. Xiao, D. Han, Y. Lu and Y. Meng, Direct Synthesis of Dimethyl Carbonate from CO2 and CH3OH Using 0.4 nm Molecular Sieve Supported Cu-Ni Bimetal Catalyst, Chin. J. Chem. Eng., 2012, 20(5), 906–913, DOI:10.1016/s1004-9541(12)60417-0.
- D.-H. Lee, J. You and J.-M. Woo, et al., Influence of dehydrating agents on the oxidative carbonylation of methanol for dimethyl carbonate synthesis over a Cu/Y-zeolite catalyst, Chin. J. Chem. Eng., 2018, 26(5), 1059–1063, DOI:10.1016/j.cjche.2018.02.018.
- K. Tomishige and K. Kunimori, Catalytic and direct synthesis of dimethyl carbonate starting from carbon dioxide using CeO2-ZrO2 solid solution heterogeneous catalyst: effect of H2O removal from the reaction system, Appl. Catal., A, 2002, 237(1–2), 103–109, DOI:10.1016/s0926-860x(02)00322-8.
- A. L. P. Valente, C. R. F. Riedo and F. Augusto, Análise quantitativa por cromatografia, Revista Chemkeys, 2018, 10, 1–10, DOI:10.20396/chemkeys.v0i10.9602.
- Y. Wen, R. Zhang and Y. Cang, et al., Direct synthesis of dimethyl carbonate and propylene glycol using potassium bicarbonate as catalyst in supercritical CO2, Pol. J. Chem. Technol., 2015, 17(1), 62–65, DOI:10.1515/pjct-2015-0010.
- J. C. Choi, L. N. He, H. Yasuda and T. Sakakura, Selective and high yield synthesis of dimethyl carbonate directly from carbon dioxide and methanol, Green Chem., 2002, 4(3), 230–234, 10.1039/b200623p.
- S. Fujita, B. M. Bhanage, M. Arai and Y. Ikushima, Synthesis of dimethyl carbonate from carbon dioxide and methanol in the presence of methyl iodide and base catalysts under mild conditions: effect of reaction conditions and reaction mechanism, Green Chem., 2001, 3(2), 87–91, 10.1039/b100363l.
- Q. Cai, C. Jin, B. Lu, H. Tangbo and Y. Shan, Synthesis of Dimethyl Carbonate from Methanol and Carbon dioxide using Potassium Methoxide as Catalyst under Mild Conditions, Catal. Lett., 2005, 103(3–4), 225–228, DOI:10.1007/s10562-005-7158-2.
- Y. Chen, Y. Yang and S. Tian, et al., Highly effective synthesis of dimethyl carbonate over CuNi alloy nanoparticles@porous organic polymers composite, Appl. Catal., A, 2019, 587, 117275, DOI:10.1016/j.apcata.2019.117275.
- J. R. Thorpe and D. M. R. Harvey, Optimization and investigation of the use of 2,2-dimethoxypropane as a dehydration agent for plant tissues in transmission electron microscopy, J. Ultrastruct. Res., 1979, 68(2), 186–194, DOI:10.1016/s0022-5320(79)90153-9.
- E. Leino, P. Mäki-Arvela and V. Eta, et al., The influence of various synthesis methods on the catalytic activity of cerium oxide in one-pot synthesis of diethyl carbonate starting from CO2, ethanol and butylene oxide, Catal. Today, 2013, 210, 47–54, DOI:10.1016/j.cattod.2013.02.011.
- V. Eta, P. Mäki-Arvela, J. Wärn, T. Salmi, J. P. Mikkola and D. Y. Murzin, Kinetics of dimethyl carbonate synthesis from methanol and carbon dioxide over ZrO2-MgO catalyst in the presence of butylene oxide as additive, Appl. Catal., A, 2011, 404(1–2), 39–46, DOI:10.1016/j.apcata.2011.07.004.
- K. Kohno, J.-C. Choi, Y. Ohshima, A. Yili, H. Yasuda and T. Sakakura, Reaction of dibutyltin oxide with methanol under CO2 pressure relevant to catalytic dimethyl carbonate synthesis, J. Organomet. Chem., 2008, 693(7), 1389–1392, DOI:10.1016/j.jorganchem.2008.01.028.
- D. B. G. Williams and M. Lawton, Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants, J. Org. Chem., 2010, 75(24), 8351–8354, DOI:10.1021/jo101589h.
- D. R. Burfield, K.-H. Lee and R. H. Smithers, Desiccant efficiency in solvent drying. A reappraisal by application of a novel method for solvent water assay, J. Org. Chem., 1977, 42(18), 3060–3065, DOI:10.1021/jo00438a024.
- R. Salomão and V. C. Pandolfelli, Hidratação e desidratação de óxido de magnésio em concretos refratários (Magnesia sinter hydration-dehydration behavior in refractory castables), Cerâmica, 2008, 54, 145–151 CrossRef.
- M. V. Dover and J. W. Marden, A Comparison of the Efficiency of Some Common Desiccants, J. Am. Chem. Soc., 1917, 39(8), 1609–1614, DOI:10.1021/ja02253a009.
- S. Fang and K. Fujimoto, Direct synthesis of dimethyl carbonate from carbon dioxide and methanol catalyzed by base, Appl. Catal., A, 1996, 142(1), L1–L3, DOI:10.1016/0926-860x(96)00081-6.
- A. A. Marciniak, O. C. Alves, L. G. Appel and C. J. A. Mota, Synthesis of dimethyl carbonate from CO2 and methanol over CeO2: role of copper as dopant and the use of methyl trichloroacetate as dehydrating agent, J. Catal., 2019, 371, 88–95, DOI:10.1016/j.jcat.2019.01.035.
- A. H. Tamboli, A. A. Chaugule and H. Kim, Highly selective and multifunctional chitosan/ionic liquids catalyst for conversion of CO2 and methanol to dimethyl carbonates at mild reaction conditions, Fuel, 2016, 166, 495–501, DOI:10.1016/j.fuel.2015.11.023.
- V. Deerattrakul, A. Panitprasert, P. Puengampholsrisook and P. Kongkachuichay, Enhancing the Dispersion of Cu-Ni Metals on the Graphene Aerogel Support for Use as a Catalyst in the Direct Synthesis of Dimethyl Carbonate from Carbon Dioxide and Methanol, ACS Omega, 2020, 5(21), 12391–12397, DOI:10.1021/acsomega.0c01143.
- S. K. Kabra, E. Turpeinen, R. L. Keiski and G. D. Yadav, Direct synthesis of dimethyl carbonate from methanol and carbon dioxide: a thermodynamic and experimental study, J. Supercrit. Fluids, 2016, 117, 98–107, DOI:10.1016/j.supflu.2016.05.039.
- J. R. O'Neil, R. N. Clayton and T. K. Mayeda, Oxygen Isotope Fractionation in Divalent Metal Carbonates, J. Chem. Phys., 1969, 51(12), 5547–5558, DOI:10.1063/1.1671982.
- M. Zhang, M. Xiao, S. Wang, D. Han, Y. Lu and Y. Meng, Cerium oxide-based catalysts made by template-precipitation for the dimethyl carbonate synthesis from carbon dioxide and methanol, J. Cleaner Prod., 2015, 103, 847–853, DOI:10.1016/j.jclepro.2014.09.024.
| This journal is © The Royal Society of Chemistry 2020 |