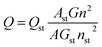Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of N, P-doped carbon dots from maize starch via a solvothermal approach for the highly sensitive detection of Fe3+†
Guohua Dong a,
Kun Langa,
He Ouyangc,
Wenzhi Zhang
a,
Kun Langa,
He Ouyangc,
Wenzhi Zhang *a,
Liming Baia,
Shijie Chena,
Zhuanfang Zhang
*a,
Liming Baia,
Shijie Chena,
Zhuanfang Zhang a,
Yueyue Gao*b,
Zhonghua Mua and
Xiaodan Zhaoa
a,
Yueyue Gao*b,
Zhonghua Mua and
Xiaodan Zhaoa
aCollege of Chemistry and Chemical Engineering, Heilongjiang Provincial Key Laboratory of Catalytic Synthesis for Fine Chemicals, Qiqihar University, Qiqihar 160006, P. R. China. E-mail: wenzzhang1968@163.com
bKey Laboratory of Photovoltaic Materials, Henan University, Qiqihar 151001, P. R. China
cCollege of Environmental and Chemical Engineering, Hefei University of Technology, Hefei 230009, P. R. China
First published on 10th September 2020
Abstract
Nitrogen/phosphorus-doped carbon dots (N, P-CDs) with a quantum yield as high as 76.5% were synthesized by carbonizing maize starch via a facile ethanol solvothermal approach. Transmission electron microscopy (TEM) measurement shows that the as-prepared N, P-CDs displayed a quasi-spherical shape with a mean size of ca. 2.5 nm. Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy disclosed the presence of –OH, –NH2, –COOH, and –CO functional groups over the surface of N, P-CDs. On the basis of excellent fluorescent properties with strong blue fluorescence emission at 445 nm upon excitation at 340 nm, these N, P-CDs were adopted as a fluorescent probe towards the effective detection of Fe3+ ions in water. The limit of detection (LOD) was as low as 0.1 μmol L−1 and showed a better linear relationship in the range of 0.1 ∼ 50 μmol L−1. In conclusion, these synthesized N, P-CDs can be efficiently used as a promising candidate for the detection of Fe3+ ions in some practical samples.
Introduction
Carbon dots (CDs) are a category of promising nanomaterials with sizes below 10 nm. They have displayed tremendous research value for chemosensors, energy-storage electrocatalysis, photocatalysis and live cell imaging due to their intrinsic properties, such as tunable fluorescence emission, biocompatibility, low-cost synthesis and prominently low cytotoxicity. Therefore, CDs are regarded as a better replacement for heavy metal based quantum dots.1–4 Conventionally, there are two main strategies for the preparation of CDs.5,6 The first method, also named the top-down method, is conducted through breaking down carbon precursor materials with larger molecular structures, such as graphite, carbon nanotubes, and graphite oxide via laser ablation, electrochemical oxidation, ultrasonication, etc.1,7 The other method is called the bottom-up route, which is performed through combustion, hydrothermal, microwave pyrolysis, microwave and so on.1,8 Regardless of the techniques applied for the synthesis of CDs, the selection of the starting carbon source materials is one of the prerequisites.For the green synthesis of CDs, one of the cost-effective and eco-friendly carbon sources is the natural biomass materials, such as various fruit juices, grass, plant leaves, chitosan and sweat pepper, kitchen wastes, etc.3,8–12 However, for most of the synthesis of CDs, this type of green materials commonly suffers from serious bottleneck such as time consuming process, toxic organic solvents, and especially the low fluorescence quantum yield of the final CDs, etc.3,13,14 Therefore, for improving the fluorescence properties, many efforts have been devoted to the modulation and optimization of the preparation routes of CDs derived from the natural biomass materials, such as doping with other functional materials and surface modification and passivation. Among them, heteroatoms doping is a facile and effective strategy for further modulating the fluorescence properties of the biomass-based CDs. For example, N doped CDs can be prepared by utilizing both of the folic acid and Chinese yam as the source of carbon and nitrogen, respectively.15 Meanwhile, the fluorescence quantum yield as high as 25% can be confirmed for the co-doped CDs with nitrogen and sulfur (abbreviated as N, S/C-dots).16
Similar with other metal ions such as Na+, K+, Ca2+, Zn2+ and Cu2+, Fe3+ ion is one of the most necessary elements in human body and other biological systems.17,18 Its excess or deficiency will result in different biological disorders, such as the eonian loss of motor skills and Parkinson's and Alzheimer's diseases, etc.17,19 Thus, it is extremely necessary for periodically monitoring the Fe3+ or other metal ions to investigate the physiological functions or diagnose and prevent diseases. In the past few years, many effective techniques have been reported for recognizing the ions with low concentrations, for instance gold nanoparticles, electrochemical methods, photonic crystals, luminescence, holography, etc.17,20 However, these techniques commonly suffer from many undesired disadvantages, such as high-cost of the raw materials, complexity of the material fabrication, inflexible use and detailed data analysis and so on. Actually, the above-mentioned CDs with many fascinating properties such as bio-compatibility, low-cytotoxity, good dispersibility in any solvents and photostability can be the perfect candidate for the selective and sensitive detection of the Fe3+.21–23
Maize is the third most important worldwide agricultural crop species for satisfying the human and animal nutrition requirements. Thus, there are enough raw materials for the exploration of the CDs together with its potential application. Herein, we developed a scalable and facile synthesis of nitrogen and phosphorus co-doped CDs (N, P-CDs) via the alcohol solvothermal method from widespread maize starch. TEM measurement demonstrated that the synthesized N, P-CDs showed quasi-spherical shape with mean size of ca. 2.5 nm. Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy revealed the presence of –OH, –NH2, –COOH, and –CO functional groups over the surface of N, P-CDs. Furthermore, the N, P-CDs displayed excellent fluorescent properties with strong blue fluorescence emission at 445 nm upon excitation at 340 nm. Finally, the N, P-CDs were adopted as a fluorescent probe towards the effective detection of Fe3+ ions in water by fluorescence spectroscopy. The limit of detection (LOD) was as low as 0.1 μmol L−1, which is far lower than that of the concentration value proposed by World Health Organization (WHO).
Experimental
Chemicals
Disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O), sodium dihydrogen phosphate hydroxide (NaH2PO4·2H2O), corn starch, urea, CuSO4·5H2O, NiSO4·6H2O, Al2(SO4)3·18H2O, K2SO4, FeCl3·6H2O, CaCl2, BaCl2, Hg(NO3)2, Cd(NO3)2·4H2O and ZnSO4·7H2O were purchased from Tianjin Kaitong Chemistry Co., Ltd., China. MnCl2·4H2O were bought from Fuchen Chemical Reagent Co., Ltd. (Tianjin, China). Dialysis bags (molecular weight cut off = 3500) were purchased from the Chemical Reagent Co., Ltd., China. Note that all the chemicals used in this work were analytical grade without further post-treatment and the used water is Mili-Q ultra-pure water processed in our lab.Synthesis of N, P-CDs
Corn starch (1.5 g), Na2HPO4·12H2O (0.35 g), NaH2PO4·2H2O (0.35 g) and urea (0.7 g) were dispersed into absolute ethyl alcohol (18 mL) and energetically stirred for 10 min in a Teflon-lined stainless-steel autoclave. After heating at 190 °C for 12 hours and then cooling down to the room temperature, the resulting dark brown solution was collected with a centrifugal process at a speed of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The final supernatant was dialyzed through a dialysis membrane (molecular weight cutoff is 3500) against Mili-Q ultra-pure water for 2 days with changing the solvents every 12 h. At last, the brown N, P-CDs liquid was obtained. For characterization, the N, P-CDs powder was obtained via evaporating the obtained solution with a rotary evaporator. The detailed synthetic procedure was schematically presented in Fig. 1.
000 rpm. The final supernatant was dialyzed through a dialysis membrane (molecular weight cutoff is 3500) against Mili-Q ultra-pure water for 2 days with changing the solvents every 12 h. At last, the brown N, P-CDs liquid was obtained. For characterization, the N, P-CDs powder was obtained via evaporating the obtained solution with a rotary evaporator. The detailed synthetic procedure was schematically presented in Fig. 1.
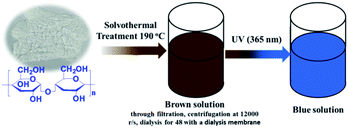 | ||
| Fig. 1 Schematic illustration of synthesis of N, P-CDs from maize starch via solvothermal treatment and its visible blue emission under UV of 365 nm. | ||
Detection Fe3+ by N, P-CDs
The selectivity measurements for metal ions were as following: 1 mL different metal ions stock solution (Fe3+, Cu2+, Ba2+, Ca2+, Zn2+, Al3+, Cd2+, Mn2+, K+, Hg2+) in Mili-Q ultra-pure water were added into 9 mL of N, P-CDs solutions and stirred for 10 min at room temperature for obtaining the mixture solutions with a uniform metal ions concentration of 50 μmol L−1, respectively. Then the fluorescence emission spectra of the above-formed mixture solutions were recorded and compared. Additionally, aiming at the sensitivity characterization, a series of Fe3+ stock solutions (1000 μmol L−1) with different volume were initially added into 10 mL N, P-CDs solution and then supplemented some Mili-Q ultra-pure water into the above-mentioned solutions for keeping a uniform concentration of N, P-CDs. The fluorescence emission spectra of the resulted solutions were recorded after reaction for 10 min at room temperature. It was noted that the final Fe3+ concentrations of the mixture solutions are varied from 0 μmol L−1 to 500 μmol L−1 and the optimal diluted N, P-CDs aqueous was utilized as the assay of sensitivity and selectivity measurements.Characterizations
The morphology of N, P-CDs was characterized by transmission electron microscopy (TEM JEOL-2100 F) with accelerating voltage of 200 kV. The Raman spectra were obtained via a Raman spectrometer (Renishaw, Britian) with a synapse CCD detector, and the spectrograph uses 600 g mm−1 gratings and a 532 nm He–Ne laser. The UV-vis absorption spectra were recorded on a Puxi TU-1900 UV-vis spectrophotometer. Fluorescence spectra measurement were performed on a Shimadzu RF-530PC spectrophotometer. The Fourier transform infrared (FT-IR) spectra were collected on a Thermo Nicolet 380 instrument with the scanning range from 4000 cm−1 to 500 cm−1 and the KBr tablet was used for holding the dried CQDs powder sample. The X-ray photoelectron spectroscopy (XPS) was performed with Thermo ESCALAB250Xi by Al Kα monochromatic X-ray radiation. Fluorescence emission and life decay spectra were collected in a FLS 920 luminescence spectrometer (Edinburgh, German). A well-known comparative method was adopted to determine the QY of the N, P-CDs. Specifically, quinine sulfate (QY of 54%) was utilized as a reference and the QY for the N, P-CDs was calculated by the following expression:3,14where Q denotes the QY, A is the absorbance intensity value recorded at the excitation wavelength of 340 nm, G is the measured integrated emission intensity in the wavelength range 380–700 nm and n represents the refractive index (1.33 for water). Because the quinine sulfate was dissolved in 0.1 M dilute H2SO4, the value of n/nst is approximately equal to 1.0. The subscript st represents the standard quinine sulfate. Additionally, the optical absorbance intensity values of N, P-CDs and quinine sulfate should be in the range of 0–0.1 at 340 nm for avoid any significant reabsorption deviation.
Results and discussion
Commonly, CDs originated from the bio-mass carbon source exhibit relatively poor fluorescence properties due to its lower heteroatom (such as N) content in bio-mass molecular structure.24–26 Thus, with the aiming at obtaining highly fluorescent CDs by introducing much more heteroatom, we synthesized N, P-CDs with a facile solvothermal route by utilizing maize as the bio-mass carbon source and urea together with phosphate as the N and P source, respectively. After purifying via a process of successive filtration, centrifugation and dialysis for the solvothermal treated production, the N, P-CDs with brightly blue photoluminescence emission under UV (365 nm) and well mono-dispersion was successfully obtained. After measurement and calculation, the formed N, P-CDs has a QY value as high as 76.5%, which is relatively higher than that of the literatures' reports. The morphology of the above-obtained N, P-CDs was investigated by TEM measurement and the result was displayed in Fig. 2. As shown in Fig. 2A, the N, P-CDs clearly displays a morphology of quasi-spherical shape, and these N, P-CQDs are well dispersed in the solvent, which is nearly in agreement with the other CQDs demonstrated in previous literature.27–29 The statistical histogram based on the TEM image analysis shown in Fig. 2B revealed that the N, P-CDs exhibits a narrow particle size distribution range from 1 nm to 5 nm with an average diameter of ca. 2.5 nm.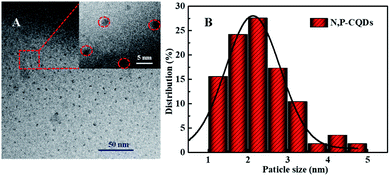 | ||
| Fig. 2 (A) The TEM image and (B) the statistical histogram of the particle size distribution for the synthesized N, P-CDs, inset of Fig. 1A is the magnification of TEM image. | ||
The surface functional groups of the synthesized N, P-CDs were investigated by FT-IR spectroscopy. As illustrated in Fig. 3A, the band at 3025–3674 cm−1 is broad and strong, which might be ascribed to the overlap of –OH/–NH stretching bands. Otherwise, a sharp band at 2924 cm−1 accompanied with a shoulder band at 2850 cm−1 was observed, which should be assigned to the C–H stretching of CC moiety present in the core of the N, P-CDs. The vibrational absorption peaks at 1623 and 1719 cm−1 corresponded to the (O, N)–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C
O/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration, confirming the presence of an unsaturated aromatic rings shaped with graphitic structure. Moreover, the absorption peaks around 607 cm−1, 1047 cm−1, 1119 cm−1 are devoted to the phosphate group (P
C stretching vibration, confirming the presence of an unsaturated aromatic rings shaped with graphitic structure. Moreover, the absorption peaks around 607 cm−1, 1047 cm−1, 1119 cm−1 are devoted to the phosphate group (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or P–O). The result confirmed the synthesized N, P-CDs were packaged with different functional groups, which could improve the aqueous solubility of N, P-CDs for potential applications in the bio-imaging and sensor fields.30,31 Moreover, as illustrated in Fig. 3B, two peaks with low intensity can be easily observed in the Raman spectrum of the prepared N, P-CDs. The peak at around 1580 cm−1 (G band) are attributed to the vibration of sp2-bonded ordered graphite carbon atom in a two-dimensional hexagonal lattice. Besides, the peak at about 1340 cm−1 (D band) is related to the vibrations of sp3-bonded (dangling bonds) carbon atoms in the peripheral plane of the disordered graphite or glassy carbon.32–34 Therefore, the Raman results suggest that the synthesized N, P-CDs have aromatic graphitic structure with minor surface defects.
O or P–O). The result confirmed the synthesized N, P-CDs were packaged with different functional groups, which could improve the aqueous solubility of N, P-CDs for potential applications in the bio-imaging and sensor fields.30,31 Moreover, as illustrated in Fig. 3B, two peaks with low intensity can be easily observed in the Raman spectrum of the prepared N, P-CDs. The peak at around 1580 cm−1 (G band) are attributed to the vibration of sp2-bonded ordered graphite carbon atom in a two-dimensional hexagonal lattice. Besides, the peak at about 1340 cm−1 (D band) is related to the vibrations of sp3-bonded (dangling bonds) carbon atoms in the peripheral plane of the disordered graphite or glassy carbon.32–34 Therefore, the Raman results suggest that the synthesized N, P-CDs have aromatic graphitic structure with minor surface defects.
The functional groups and composed elements of the N, P-CDs were further revealed by the XPS measurements. As depicted in Fig. 4A, we can clearly observe the peaks of P2p, P2s, C1s, N1s and O1s at 133.1 eV, 189.4 eV, 284.6 eV, 400.3 eV and 531.0 eV, respectively, indicating that the N, P-CDs is primarily composed of four types of elements including C, P, O, N. Undoubtedly, the N and P mainly be resulted from the heteroatom doping with urea and phosphate reaction precursors. Additionally, from the high-resolution XPS spectra of N1s, P2p and C1s shown in Fig. 4B–D, we can see that each of them can be divided into multiple peaks, which are ascribed to multiple kinds of chemical bonds. For example, the C1s exists as the states of C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N/C–O, C
C, C–N/C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–N, as well both of the XPS spectra of N1s and P2p consist of three peaks, behaving three types of the existence states in the N, P-CDs.9,15,18
O–N, as well both of the XPS spectra of N1s and P2p consist of three peaks, behaving three types of the existence states in the N, P-CDs.9,15,18
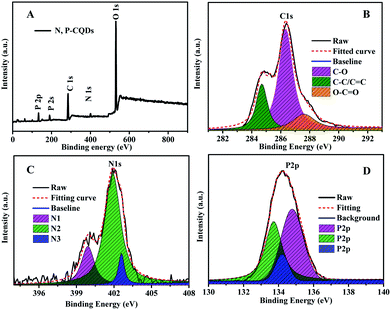 | ||
| Fig. 4 (A) The XPS survey spectrum (B) the C1s (C) N1s and (D) P2p XPS spectra of the synthesized N, P-CDs. | ||
The optical property of the N, P-CDs was initially investigated by performing the UV-vis absorption spectroscopy measurement. As displayed in Fig. 5, the absorption spectrum curve of N, P-CDs displays two peaks centered at 287 nm and 350 nm, respectively. As demonstrated in many previous works, the obvious absorption peak located at 287 nm likely be assigned to the π–π transition of sp2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in aromatic conjugate domains, which is the characteristic of synthesized CDs.35,36 Another peak at 350 nm may be associated with the n–π* transitions of C
C in aromatic conjugate domains, which is the characteristic of synthesized CDs.35,36 Another peak at 350 nm may be associated with the n–π* transitions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or others.27 Additionally, we can also observe from the inset of Fig. 5 that the N, P-CDs solution exhibits pale yellow color under sunlight and in contrary bright blue color irradiated with the UV light (365 nm), indicating that this N, P-CDs has significant photoluminescence property.
O or others.27 Additionally, we can also observe from the inset of Fig. 5 that the N, P-CDs solution exhibits pale yellow color under sunlight and in contrary bright blue color irradiated with the UV light (365 nm), indicating that this N, P-CDs has significant photoluminescence property.
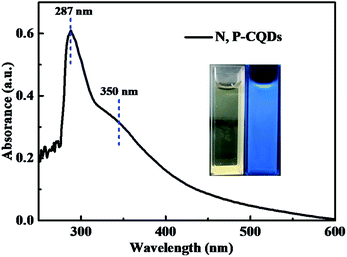 | ||
| Fig. 5 The absorption spectrum of the synthesized N, P-CDs, inset is the photographs of the N, P-CDs solution in ethanol irradiated under sunlight (yellow) and UV light (blue). | ||
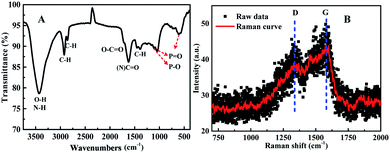
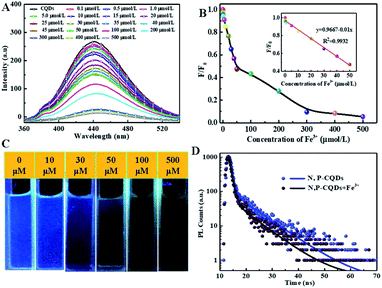
Sequentially, the fluorescence property of the N, P-CDs was studied by the photoluminescence (PL) spectroscopy. As shown in Fig. 6, no matter what excitation wavelength was selected range from 260 nm to 390 nm (Fig. 6A), the N, P-CDs wholly displays obvious strong PL emission in the region of around 360 nm to 550 nm (Fig. 6B). Furthermore, with the excitation wavelength increasing from 260 nm to 390 nm, the PL emission peak of the N, P-CDs shows a tiny shift from 445 to 460 nm, confirming that the fluorescence properties of the N, P-CDs are depend on the surface states of itself rather than the morphology and particle size, and the of the N, P-CDs should be rather uniform.37,38 Fig. 6C provided the CIE 1931 chromaticity diagram of the N, P-CDs. After calculating from the PL spectrum data under the excitation wavelength of 340 nm, we can get the CIE chromaticity coordinate value of (0.1590, 0.0257), further indicating a blue fluorescence emission property of the synthesized CDs. Fig. 6D shows the PL emission spectra of the N, P-CDs multiply diluted by H2O. We can see that the PL emission intensity of the N, P-CDs presents an obvious increase at a lower dilution multiple and then decrease with the continuous increase of the multiple dilution, indicating that the concentration of N, P-CDs is also an important decisive factor for the PL emission performance.
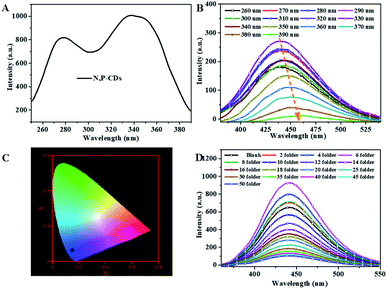
For investigating the selectivity and sensitivity of the as-prepared N, P-CDs to metal ion, the PL spectroscopy of the synthesized N, P-CDs in the presence of various kinds of metal ions with a uniform concentration of 50 μmol L−1 was performed. The corresponding PL spectra and relative fluorescence peak intensity (F0/F) are shown in Fig. 7 A and Fig. 7B, respectively. As shown in Fig. 7A and B, all listed metal ions could result in a fluorescence quenching effect to the N, P-CDs. However, Fe3+ can lead to a more significant effect to the N, P-CDs on fluorescence quenching. The result demonstrated that the N, P-CDs has a higher selectivity for detection of Fe3+ than that of others. Fig. 7C intuitionally illustrates the emission color of N, P-CDs solution containing different metal ions with concentration of 50 μmol L−1 under 365 nm excited wavelength. After the addition of 50 μM of Fe3+ to the N, P-CDs, the fluorescence color of the N, P-CDs almost disappeared versus that of other metal ions, which indicates the high selectivity of as prepared N, P-CDs towards the detection of Fe3+ owing to the stronger affinity of Fe3+ than the other metal ions. Furthermore, with the aiming at checking the impact of complicated environments on fluorescence quenching of the N, P-CDs as the sensor of Fe3+, one category of mixed ions solution including Zn2+, Hg2+ and K+ with concentration of 50 μM for each of the ion were utilized to investigate the fluorescence emission of N, P-CDs with or without the presence of Fe3+. As can be seen in Fig. S1,† the coexisting ions, almost similar with the single ions, triggered a slight fluorescence quenching of the N, P-CDs, however, the fluorescence emission of the N, P-CDs were almost completely quenched with an identical level by the Fe3+ regardless of the presence of other selected coexisting ions. All of this demonstrated the successful application of fluorescence sensor of the synthesized N, P-CDs for Fe3+ detection with a relatively high selectivity.
To detailedly study the sensitivity of N, P-CDs to Fe3+, the fluorescence emission spectra of N, P-CDs with different concentration of Fe3+ ranging from 0 to 500 μmol L−1 was measured. As exhibited in Fig. 8A, the fluorescence intensity of the N, P-CDs gradually decrease with the increase of Fe3+ concentration, indicating that the Fe3+ ions can quenched the fluorescence of the N, P-CDs due to interactive chelation between Fe3+ and N, P-CDs. Fig. 8B displays the correlation relationship between relative fluorescence intensity (F0/F) of the N, P-CDs and varied Fe3+ concentration, where F0 and F are denoted as the fluorescence emission peak intensity of N, P-CDs without and with addition of Fe3+, which resulted from the above-mentioned fluorescence emission spectra in Fig. 8A. It well noted that the F0/F decreased with the increase of the Fe3+ ions concentration. Furthermore, as exhibited in the inset of Fig. 8B, a good linear relationship can be evidenced between the F0/F of the N, P-CDs and Fe3+ concentration in the range of 0–50 μmol L−1 with a correlation coefficient (R2) of 0.9932. And the lowest detection concentration of Fe3+ is 0.1 μmol L−1, which is not only lower than other reported values for Fe3+ detection by bio-mass based CDs, but also much lower than the guideline limit of Fe3+ concentration of 5.36 μmol L−1 proposed by World Health Organization (WHO).39–41 In addition, the fluorescence quenching of the N, P-CDs due to Fe3+ addition with various concentration is visible with the naked eye as shown in Fig. 8C. These results suggest that the as-prepared N, P-CDs can sensitively detect Fe3+ ions in water even with a very low concentrations (mere 0.1 μmol L−1) of. Fig. 8D shows the photoluminescence lifetime decay spectra of the optimal diluted N, P-CDs aqueous in the absence and presence of Fe3+. We can observe that the excition lifetime of the N, P-CDs under 340 nm excitation shows a significant reduction from 6.3 ns to 5.1 ns, further confirming that fluorescence of N, P-CDs can be indeed quenched by Fe3+ through a dynamic process.42–44 At last, we have performed the analytical application of the synthesized N, P-CDs for the detection of Fe3+ ion in tap water via standard addition recovery method for giving a more comprehensive assessment. It needs to be noted that the tap water was initially treated by 0.22 μm membrane for removing the insoluble particles. The obtained results were summarized in Table 1. Obviously, the recovery rate shows a slight variation from 98.6% to 103.3, verifying the feasibility of Fe3+ detection by the N, P-CDs sensor.
| Water sample | Added Fe3+ (μmol L) | detected Fe3+ (μmol L) | Error (%) | Recovery (%) |
|---|---|---|---|---|
| Tap water | 0.152 | 0.157 | 3.29 | 103.29 |
| 0.233 | 0.240 | 3.00 | 103.00 | |
| 0.857 | 0.845 | −1.40 | 98.60 |
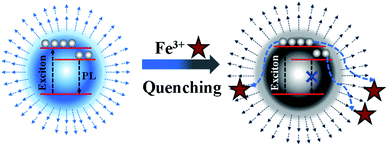
Based on the above systematic investigation, a possible mechanism was proposed to elucidate the fluorescence quenching mechanism of N, P-CDs by Fe3+ ion, which is shown in Fig. 9. It is well-known that the Fe3+ has half-filled 3d orbital which can act as the electron acceptor.33–35 On a contrary, the N, P-CDs has sorts of functional groups on the surface of N, P-CDs such as hydroxyl and amino, which can play as the electron donors. These will generate an effective coordination interaction between Fe3+ and N, P-CDs, which can make the excited electrons of the N, P-CDs easily transfer into the 3d orbital of Fe3+, finally leading to the fluorescence quenching to some extent and meanwhile the exciton lifetime reduction.
Conclusions
In summary, a kind of N, P-CDs with bright blue fluorescence were successfully synthesized utilizing natural maize starch as the carbon source and phosphate together with urea as the P and N source via a facile ethanol solvothermal route. The synthesized N, P-CDs have a morphology of quasi-spherical shape with an average diameter of ∼2.5 nm and exhibit the strongest fluorescence emission peak at 445 nm under the excitation wavelength of 340 nm. Furthermore, such a N, P-CDs can be desirably applied as the fluorescence sensor for selectively and sensitively detecting Fe3+ with a detection concentration range from 0.1 to 500 μmol L−1. The results indicate that N, P-CDs are an excellent metal fluorescent probe. Therefore, this prepared N, P-CDs are extremely promising for future practical application for the detections of hazardous and toxic ions.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21776144), the Natural Science Foundation of Heilongjiang Province of China (Grant No. LH2019B020), the Research Foundation of Education Bureau of Heilongjiang Province of China (Grant No. 135209211), the Special Project of Advantage Characteristic Discipline of Heilongjiang Province (Grant No. YSTSXK201841), the Postdoctoral Fund of China (Grant No. FJ3050A0670111) and Innovation Project of Graduate Education of Qiqihar University (Grant No. YJSCX2019034).Notes and references
- S. Y. Lim, W. Shen and Z. Gao, Chem. Soc. Rev., 2015, 44, 362–381 RSC.
- Y. Wang and A. Hu, J. Mater. Chem. C, 2014, 2, 6921–6939 RSC.
- R. Das, R. Bandyopadhyay and P. Pramanik, Mater. Today Chem., 2018, 8, 96–109 CrossRef CAS.
- X. Li, M. Rui, J. Song, Z. Shen and H. Zeng, Adv. Funct. Mater., 2015, 25, 4929–4947 CrossRef CAS.
- Y. Xu, J. Liu, C. Gao and E. Wang, Electrochem. Commun., 2014, 48, 151–154 CrossRef CAS.
- J. Hou, J. Yan, Q. Zhao, Y. Li, H. Ding and L. Ding, Nanoscale, 2013, 5, 9558–9561 RSC.
- S. Thambiraj and R. Shankaran, Appl. Surf. Sci., 2016, 390, 435–443 CrossRef.
- F. Niu, Y.-L. Ying, X. Hua, Y. Niu, Y. Xu and Y.-T. Long, Carbon, 2018, 127, 340–348 CrossRef CAS.
- J. Xu, T. Lai, Z. Feng, X. Weng and C. Huang, Luminescence, 2015, 30, 420–424 CrossRef CAS.
- R. Wang, K.-Q. Lu, Z.-R. Tang and Y.-J. Xu, J. Mater. Chem. A, 2017, 5, 3717–3734 RSC.
- M. Sabet and K. Mahdavi, Appl. Surf. Sci., 2019, 463, 283–291 CrossRef CAS.
- D. Kumar, K. Singh, V. Verma and H. Bhatti, J. Bionanosci., 2014, 8, 274–279 CrossRef CAS.
- Y. Wang, X. Chang, N. Jing and Y. Zhang, Anal. Methods, 2018, 10, 2775–2784 RSC.
- H. Qi, M. Teng, M. Liu, S. Liu, J. Li, H. Yu, C. Teng, Z. Huang, H. Liu and Q. Shao, J. Colloid Interface Sci., 2019, 539, 332–341 CrossRef CAS.
- R. Zhang and W. Chen, Biosens. Bioelectron., 2014, 55, 83–90 CrossRef CAS.
- H. Wu, J. Jiang, X. Gu and C. Tong, Microchim. Acta, 2017, 184, 2291–2298 CrossRef CAS.
- V. Mahendran and J. Philip, Langmuir, 2013, 29, 4252–4258 CrossRef CAS.
- J. Shangguan, J. Huang, D. He, X. He, K. Wang, R. Ye, X. Yang, T. Qing and J. Tang, Anal. Chem., 2017, 89, 7477–7484 CrossRef CAS.
- N. Narayanaswamy and T. Govindaraju, Sens. Actuators, B, 2012, 161, 304–310 CrossRef CAS.
- S. Bothra, J. N. Solanki and S. K. Sahoo, Sens. Actuators, B, 2013, 188, 937–943 CrossRef CAS.
- F. Wu, M. Yang, H. Zhang, S. Zhu, X. Zhu and K. Wang, Opt. Mater., 2018, 77, 258–263 CrossRef CAS.
- Y. Zhang, Y. Xiao, Y. Zhang and Y. Wang, J. Nanosci. Nanotechnol., 2020, 20, 3340–3347 CrossRef CAS.
- Y. Dong, J. Ma, C. Liu and Y. Bao, Ceram. Int., 2020, 11115–11123 CrossRef CAS.
- J. Peng, B. Shang, Y. Xu, Z. Feng and V. Calatayud, Environ. Pollut., 2020, 256, 113466 CrossRef CAS.
- W. S. Koe, W. C. Chong, Y. L. Pang, C. H. Koo, M. Ebrahim and A. W. Mohammad, Journal of Water Process Engineering, 2020, 33, 101068 CrossRef.
- M. Javed, A. N. S. Saqib, B. Ali, M. Faizan, D. A. Anang, Z. Iqbal and S. M. Abbas, Electrochim. Acta, 2019, 297, 250–257 CrossRef CAS.
- K. Wang, Q. Ji, J. Xu, H. Li, D. Zhang, X. Liu, Y. Wu and H. Fan, J. Fluoresc., 2018, 28, 759–765 CrossRef CAS.
- C.-W. Lei, M.-L. Hsieh and W.-R. Liu, Dyes Pigm., 2019, 169, 73–80 CrossRef CAS.
- F. Wu, H. Su, X. Zhu, K. Wang, Z. Zhang and W.-K. Wong, J. Mater. Chem. B, 2016, 4, 6366–6372 RSC.
- A. F. Shaikh, M. S. Tamboli, R. H. Patil, A. Bhan, J. D. Ambekar and B. B. Kale, J. Nanosci. Nanotechnol., 2019, 19, 2339–2345 CrossRef CAS.
- X. Sun, H. Liu, L. Yang, X. Wang, W. Yang, M. Wei, X. Liu, J. Cao, J. Yang and S. G. Xing, Nanomaterials, 2018, 8, 635 CrossRef.
- Q. Yang, J. Duan, W. Yang, X. Li, J. Mo, P. Yang and Q. Tang, Appl. Surf. Sci., 2018, 434, 1079–1085 CrossRef CAS.
- H. Xu, S. Zhou, L. Xiao, H. Wang, S. Li and Q. Yuan, J. Mater. Chem. C, 2015, 3, 291–297 RSC.
- J. Yu, C. Xu, Z. Tian, Y. Lin and Z. Shi, New J. Chem., 2016, 40, 2083–2088 RSC.
- J. Shen, S. Shang, X. Chen, D. Wang and Y. Cai, Mater. Sci. Eng., C, 2017, 76, 856–864 CrossRef CAS.
- Z. Li, H. Yu, T. Bian, Y. Zhao, C. Zhou, L. Shang, Y. Liu, L. Z. Wu, C. H. Tung and T. Zhang, J. Mater. Chem. C, 2015, 3, 1922–1928 RSC.
- H. Zhang, H. Wang, Y. Wang and B. Xin, Appl. Surf. Sci., 2020, 145751 CrossRef CAS.
- Z. Song, X. Chen, X. Gong, X. Gao, Q. Dai, T. T. Nguyen and M. Guo, Opt. Mater., 2020, 100, 109642 CrossRef CAS.
- V. Raveendran, A. R. S. Babu and N. K. J. R. a. Renuka, RSC Adv., 2019, 9, 12070–12077 RSC.
- A. Sachdev and P. J. A. Gopinath, Analyst, 2015, 140, 4260–4269 RSC.
- R. Vikneswaran, S. Ramesh and R. J. M. L. Yahya, Mater. Lett., 2014, 136, 179–182 CrossRef CAS.
- C. Wang, H. Shi, M. Yang, Y. Yan, E. Liu, Z. Ji and J. J. M. R. B. Fan, Mater. Res. Bull., 2020, 124, 110730 CrossRef CAS.
- Y. Liu, X. Tang, T. Zhu, M. Deng, I. P. Ikechukwu, W. Huang, G. Yin, Y. Bai, D. Qu and X. J. J. o. M. C. C. Huang, Chem. Commun., 2018, 6, 4793–4799 CAS.
- X. Sheng, Y. Liu, Y. Wang, Y. Li, X. Wang, X. Wang, Z. Dai, J. Bao and X. J. A. M. Xu, Adv. Mater., 2017, 29, 1700150 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06209j |
| This journal is © The Royal Society of Chemistry 2020 |