Open Access Article
Open Access ArticleA novel strategy to alleviate medium acidosis for simultaneously yielding more bacterial cellulose and electricity†
Qing Wang abc,
Dong Tian
abc,
Dong Tian ab,
Jinguang Huc,
Yongmei Zengab and
Fei Shen
ab,
Jinguang Huc,
Yongmei Zengab and
Fei Shen *ab
*ab
aInstitute of Ecological and Environmental Sciences, Sichuan Agricultural University, Chengdu, Sichuan 611130, P. R. China. E-mail: fishen@sicau.edu.cn
bRural Environment Protection Engineering & Technology Center of Sichuan Province, Sichuan Agricultural University, Chengdu, Sichuan 611130, P. R. China
cChemical and Petroleum Engineering, Schulich School of Engineering, The University of Calgary, Calgary, T2N 4H9, Canada
First published on 27th August 2020
Abstract
Bacterial cellulose (BC), a fascinating and renewable polymer, can be applied widely in various bio-based materials. However, its synthesis is generally limited by medium acidosis. Herein, we demonstrated a built-in galvanic cell within the BC fermentation medium to alleviate the acidosis, by which BC yield was promoted by 191%, and simultaneously the yield of electrical power of 0.68 W to 8.10 W during the incubation.
Bacterial cellulose (BC) is biologically synthesized by several bacteria, notably, the strain of Acetobacter xylinum.1 This fascinating and renewable polymer possesses remarkable chemical and physical attributes (e.g. high porosity, excellent mechanical strength, and large surface area, etc.) that can be applied in a wide range of bio-based materials and products.2–4 Its wide applications have motivated researchers to focus on the fermentation process, especially to promote the BC yield. However, the fermentation process is extensively related to many aspects, such as the employed strains, medium conditions (carbon source, nutrients, pH, and dissolved oxygen), and incubation conditions (the employed medium volume, duration, surface area).5–8
Among these factors, the medium acidosis caused by the released organic acids, such as glucuronic acid and acetic acid, in the medium will negatively affect the BC biosynthesis during bacterial metabolism, which could weaken the bacteria activities and reduce the BC yield as a consequence. Generally, the pH of the BC fermentation medium can be dramatically decreased from 5.0 to lower than 2.0 during the incubation if no pH adjustment strategy was employed.5,9,10 Current methods for BC incubation include static, agitated, and bioreactor cultures.11 For agitated and bioreactor cultures, the regulation of pH is relatively easy because of the culture system is under stirring. However, the static culture of BC is still an ideal way to obtain high-quality BC membranes for many novel applications.12–14 Unfortunately, it is quite difficult to adjust the pH of medium during the incubation because the formation of BC film in static culture will be seriously disturbed by the mechanical agitation. Therefore, to find an ingenious way to control the medium pH in a suitable range will be very critical to the BC production in practice.
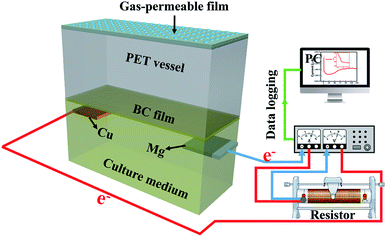
The essence of medium acidosis sources from the ionized hydrogen ions (H+) of the released organic acids in bacterial metabolic activity. If there is a proper approach to remove the H+ from culture medium in a controllable way, the BC production may significantly be promoted. Nowadays, the application of batteries has greatly facilitated people's life. This revolutionary device was originated from the prototype of “galvanic cell” (or “voltaic piles”), which was invented by Alessandro Volta in 1800.15 Even now, the galvanic cell still be employed to demonstrate the mechanism of battery for the educational aim, such as the fruit battery.16,17 Using lemons, oranges, grapefruits, potatoes, or apples which riches in citric acid, phosphoric acid, or malic acid as the electrolyte, and two different metallic electrodes were plugged into the fruit to form a battery. The fruit battery could provide continuous electricity to run various small devices, such as light-emitting diode (LED) and a digital clock. When the two metallic electrodes was connected with those devices by wire, the ionized H+ of organic acids inside of the fruit will be slowly consumed in anode and producing electric energy to drive those devices.18–20 Inspired by the principle of a galvanic cell, we assumed that the ionized H+ of accumulated organic acids during the BC fermentation can be consumed in the anode of the built-in galvanic cell to form H2, which will greatly weaken the acidified conditions of culture medium. Theoretically, a suitable pH condition for bacteria synthesis will promote the BC yield in a consequent. Meanwhile, the liberated metal ions from the cathode can serve as a suitable activator to the biochemical process of bacteria if the electrodes can be selected approximately, which also can potentially promote the BC yield.21 Based on this conception, the BC fermentation integrated a couple of electrodes to construct a galvanic cell system as its schematic diagram in Fig. 1 (the details of this built-in galvanic cell see ESI, page S2†). To check the possibility of this first attempt, the BC yield of this newly constructed system (GC-medium) was compared with that of the normal medium, and the performance of electricity release was investigated as well.
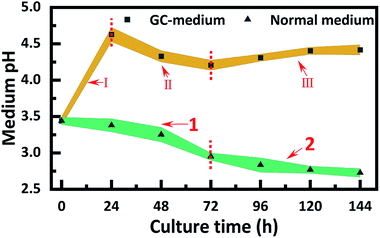
The medium pH was monitored at every 12 h during static incubation as shown in Fig. 2.22 It could be easily found that the pH of the normal medium displayed a continuous decrease as the fermentation time was prolonged to 144 h. By contrast, the pH in the GC-medium firstly increased to 4.6 after 24 h of incubation, then maintained in the range from 4.2 to 4.4. Obviously, the medium pH for BC fermentation can be stabilized via loading the galvanic cell to consume the extra acids from the medium process.
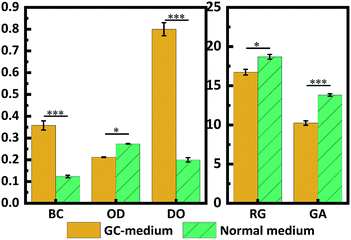
Correspondingly, the BC yield from the GC-medium was 0.358 g L−1, which was 2.9-fold higher than that of the normal medium (Fig. 3). Apparently, the pH adjustment by the galvanic cell can work well to yield more BC, verifying the possibility of the proposed conception. Besides, the residual glucose in the GC-medium was lower than that of the normal medium, as well as the residual glucuronic acid. These results again proved that more carbon source was converted into BC. When the BC film started to form at the air–liquid interface, the bacteria would be embedded into the film and fixed, which can result in a clearer medium. The relatively lower OD610 in the GC-medium indicated that the more bacteria were fixed result from the thicker BC film that produced in GC-medium.23 Moreover, the relatively higher DO (dissolved oxygen) was remained in the GC-medium after 6 days incubation also demonstrated that the conditions for cellulose biosynthesis of bacteria were better than that of the normal medium,24 which also suggested more sugar will be consumed for BC synthesis once the galvanic cell was built during the fermentation process.
The formed current with 2 Ω-load of the built-in galvanic cell can be detected by the digital multi-meter. As shown in Fig. 4a, the current decreased sharply from 2013 μA to 878 μA in the first 2 h, this result was related to the rapid consumption of the H+ that contained in the strain when the built-in galvanic cell was activated. When the bacteria started to self-replicate in the first 72 h, more organic acid was produced and released to the medium (refer to Fig. 2), which slowed down the rate of current reduction in the following 22 h. The prominent increase of current appeared in 24–72 h, proving that the medium was still rich in H+, meanwhile, the number of bacteria grew exponentially. These results also could be observed in Fig. 2, the changes of pH were well matched with the adjustment of the built-in galvanic cell. This correlativity between the medium pH and the formed current of GC has the potential to be designed as a fully automatic pH control system (ESI, Fig. S1†).
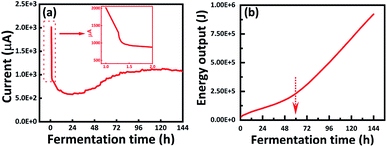 | ||
| Fig. 4 Electricity generation performance during the incubation. (a) Current monitoring with 2 Ω resistor; (b) cumulative energy output in the whole BC fermentative production. | ||
Afterward, the current was maintained around 1163 μA during the following 3 days, which can demonstrate the amount of Acetobacter xylinum in GC-medium reach a stable period. Meanwhile, the pH of GC-medium was still within a suitable range of 4.2–4.4 while the pH of the normal medium has been reduced to around 2.7–2.8, which was already negative to the bacterial metabolic activities (Fig. 2). Generally, the death period of bacteria was mainly attributed to the medium acidosis.10 Hence, the mechanism of improving the BC yield by this built-in galvanic cell was to prevent the medium acidosis and maintained the activity of bacteria, which can stabilize the BC biosynthesis successfully.
The cumulative energy output was calculated and showed in Fig. 4b, although the initial pH of GC-medium was relatively lower than that after 48 h, the energy output had a remarkable promotion. This result proved the major factors for the energy output was not only affected by the apparent medium pH but also related to the ability to provide H+ within bacteria metabolism. This ability was mainly contributed by the number of bacteria and the bacterial activity, which would be in a better condition if there was a built-in galvanic cell. Previous studies have suggested that the H+ concentration in the electrolyte, metal type of electrode, and the number of galvanic cells to be assembled in series are the key factors that influenced the output voltage of the galvanic cell.17 In this study, the magnesium (Mg) anode, copper (Cu) cathode, and the H+-rich culture medium formed an integrated galvanic cell system. The voltage was determined as 1.55–1.65 V for this built-in galvanic cell during the BC production, which can light the LED bulb (ESI, Fig. S2†). Of course, it also can provide enough electricity to drive digital clocks, sensors, and other low-power devices in the whole incubation time. These results substantially proved that the conception of this work to produce electricity and promote BC production can be achieved via loading the built-in galvanic cell.
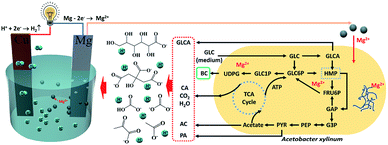
The schematic mechanism diagram of acidification, pH adjustment, and electricity generation of the galvanic cell were illustrated with Fig. 5, the metabolic by-products, such as citric acid (CA), acetic acid (AC), gluconic acid (GLCA), and pyruvic acid (PA), are generally derived from the tricarboxylic acid (TCA) cycle, pentose phosphate pathway (HMP) and glycolytic pathway (EMP).25 At the beginning of fermentation, glucose (GLC) in the medium was high enough to support the cell self-replication through the aerobic respiration of bacteria in the TCA cycle, which results in an amount of GLCA released to the medium. In this process, the by-products, such as CO2, CA, and H2O, also could be generated correspondingly. In addition, a considerable amount of AC and some PA can be released from the HMP and EMP, respectively.24 These by-products, including GLCA, AC, CA, and PA or CO2, were mainly responsible for medium acidosis, which inhibited bacteria activity and reduced the BC production as a consequence. Besides, the produced GLCA would inhibit the Acetobacter xylinum biosynthesis for BC.26 The dissolved CO2 in the medium also limited the aerobic respiration of bacteria, which will make the bacteria produce more CA and PA as a feedback regulation. This could create a vicious circle to deteriorate the medium pH and cease in BC production eventually. However, when the galvanic cell was built inside of the culture medium, those organic acids could provide the ionized H+ to maintain the galvanic reaction. The H+ would move to the Cu-electrode and be reduced as hydrogen gas by the acceptance of the electrons. The Mg-electrode would lose electrons and be oxidized to Mg2+, which can involve several cell life activities and potentially promote the BC yield (the effect of Mg2+ on BC production shown in ESI, Fig. S3†). During this process, the directional transfer of electrons in the external circuit created the current, and the electric energy will be continuously output throughout the fermentation. It can image that this built-in galvanic cell can yield considerable power once a large-scale BC production was considered. Moreover, the conception of built-in galvanic cell in bioconversion processes that need pH control, such as anaerobic digestion of easy-acidification substrates, and ethanol fermentation by yeast can also be considered to apply this conception to maintain the stable and suitable pH.
Conclusions
A facile built-in galvanic cell was designed in the BC fermentation system, by which the medium pH can be well controlled from serious acidification by consuming the extra organic acids derived from metabolism. Correspondingly, the stabilized pH conditions of medium promoted BC yield significantly. Meanwhile, a decent amount of electricity can be lastly generated to successfully drive some low-powered devices. This conception makes the dynamic medium pH regulation to be possible during the biochemical process, and it offers an implication to extend this conception in other scenarios that need the on-line pH monitoring/regulation, such as the processes of biodegradation and biosynthesis, to promote the bioconversion efficiency, as well as electricity output.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of this work by the National Natural Science Foundation of China (21978183) and the Department of Science and Technology of Sichuan Province (2017HH0047).Notes and references
- M. Iguchi, S. Yamanaka and A. Budhiono, J. Mater. Sci., 2000, 35, 261–270 CrossRef CAS.
- L. Fu, J. Zhang and G. Yang, Carbohydr. Polym., 2013, 92, 1432–1442 CrossRef CAS PubMed.
- S. Wang, T. Li, C. J. Chen, W. Q. Kong, S. Z. Zhu, J. Q. Dai, A. J. Diaz, E. Hitz, S. D. Solares, T. Li and L. B. Hu, Adv. Funct. Mater., 2018, 28, 1707491 CrossRef.
- M. Gao, J. Li, Z. Bao, M. Hu, R. Nian, D. Feng, D. An, X. Li, M. Xian and H. Zhang, Nat. Commun., 2019, 10, 437 CrossRef CAS PubMed.
- A. Krystynowicz, W. Czaja, A. Wiktorowska-Jezierska, M. Goncalves-Miskiewicz, M. Turkiewicz and S. Bielecki, J. Ind. Microbiol. Biotechnol., 2002, 29, 189–195 CrossRef CAS PubMed.
- A. Kurosumi, C. Sasaki, Y. Yamashita and Y. Nakamura, Carbohydr. Polym., 2009, 76, 333–335 CrossRef CAS.
- D. R. Ruka, G. P. Simon and K. M. Dean, Carbohydr. Polym., 2012, 89, 613–622 CrossRef CAS PubMed.
- A. Basu, S. V. Vadanan and S. Lim, Carbohydr. Polym., 2019, 207, 684–693 CrossRef CAS PubMed.
- M. E. Embuscado, J. S. Marks and J. N. Bemiller, Food Hydrocolloids, 1994, 8, 407–418 CrossRef CAS.
- K. A. Zahan, N. Pa'e and I. I. Muhamad, Arabian J. Sci. Eng., 2015, 40, 1881–1885 CrossRef CAS.
- J. Wang, J. Tavakoli and Y. Tang, Carbohydr. Polym., 2019, 219, 63–76 CrossRef CAS PubMed.
- N. Tang, S. Zhang, Y. Si, J. Yu and B. Ding, Nanoscale, 2019, 11, 17851–17859 RSC.
- C. Vilela, A. C. Silva, E. M. Domingues, G. Gonçalves, M. A. Martins, F. M. Figueiredo, S. A. Santos and C. S. Freire, Carbohydr. Polym., 2020, 230, 115604 CrossRef PubMed.
- Q. Fang, X. Zhou, W. Deng, Z. Zheng and Z. Liu, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- M. Piccolino, Trends Neurosci., 2000, 23, 147–151 CrossRef CAS PubMed.
- A. M. Khan and M. Obaid, J. Energy South Afr., 2015, 26, 90–99 CrossRef.
- P. Y. Furlan, T. Krupa, H. Naqiv and K. Anderson, J. Chem. Educ., 2013, 90, 1341–1345 CrossRef CAS.
- J. D. Worley and J. Fournier, J. Chem. Educ., 1988, 65, 158 CrossRef.
- https://knowledge.electrochem.org/encycl/art-v01-volta.htm, accessed August 2020.
- D. J. Swartling and C. Morgan, J. Chem. Educ., 1998, 75, 181–182 CrossRef CAS.
- Y. Nishi, M. Uryu, S. Yamanaka, K. Watanabe, N. Kitamura, M. Iguchi and S. Mitsuhashi, J. Mater. Sci., 1990, 25, 2997–3001 CrossRef CAS.
- The initial pH of the medium was adjusted to approximately 5.0, then inoculated the strain according to a volume ratio of 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (strain/fermentation medium). Afterwards, the final medium pH was 3.5 due to the produced organic acids in the incubation of strain.
10 (strain/fermentation medium). Afterwards, the final medium pH was 3.5 due to the produced organic acids in the incubation of strain. - M. Liu, C. Zhong, X. Y. Wu, Y. Q. Wei, T. Bo, P. P. Han and S. R. Jia, Biochem. Eng. J., 2015, 101, 85–98 CrossRef CAS.
- M. U. Islam, M. W. Ullah, S. Khan, N. Shah and J. K. Park, Int. J. Biol. Macromol., 2017, 102, 1166–1173 CrossRef PubMed.
- C. Zhong, G. C. Zhang, M. Liu, X. T. Zheng, P. P. Han and S. R. Jia, Appl. Microbiol. Biotechnol., 2013, 97, 6189–6199 CrossRef CAS PubMed.
- R. E. Cannon and S. M. Anderson, Crit. Rev. Microbiol., 1991, 17, 435–447 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06245f |
| This journal is © The Royal Society of Chemistry 2020 |