Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of solvent mixture on nucleophilicity parameters: the case of pyrrolidine in methanol–acetonitrile†
Salma Souissi ab,
Wahiba Gabsi‡
a,
Abderraouf Echaieba,
Julien Roger
ab,
Wahiba Gabsi‡
a,
Abderraouf Echaieba,
Julien Roger b,
Jean-Cyrille Hierso
b,
Jean-Cyrille Hierso b,
Paul Fleurat-Lessard
b,
Paul Fleurat-Lessard *b and
Taoufik Boubaker*a
*b and
Taoufik Boubaker*a
aUniversité de Monastir, Faculté des Sciences, Laboratoire de Chimie Hétérocyclique, Produits Naturels et Réactivité (LR11S39), Avenue de l’Environnement, 5019 Monastir, Tunisia. E-mail: boubaker_taoufik@yahoo.fr
bInstitut de Chimie Moléculaire de l’Université de Bourgogne (UMR-CNRS 6302), Université Bourgogne Franche-Comté (UBFC), 9 Avenue Alain Savary, 21078 Dijon, France. E-mail: Paul.Fleurat-Lessard@u-bourgogne.fr
First published on 3rd August 2020
Abstract
The course of organic chemical reactions is efficiently modelled through the concepts of “electrophiles” and “nucleophiles” (meaning electron-seeking and nucleus-seeking reactive species). On the one hand, an advanced approach of the correlation of the nucleophilicity parameters N and electrophilicity E has been delivered from the linear free energy relationship log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k (20 °C) = s(N + E). On the other hand, the general influence of the solvent mixtures, which are very often employed in preparative synthetic chemistry, has been poorly explored theoretically and experimentally, to date. Herein, we combined experimental and theoretical studies of the solvent influence on pyrrolidine nucleophilicity. We determined the nucleophilicity parameters N and s of pyrrolidine at 20 °C in CH3OH/CH3CN mixtures containing 0, 20, 40, 60, 80 and 100% CH3CN by kinetic investigations of their nucleophilic substitution reactions to a series of 2-methoxy-3-X-5-nitrothiophenes 1a–e (X = NO2, CN, COCH3, CO2CH3, CONH2). Depending on the resulting solvation medium, the N parameters range from 15.72 to 18.32 on the empirical nucleophilicity scale of Mayr. The nucleophilicity parameters N first evolve linearly with the content of acetonitrile up to 60% CH3CN by volume, but is non linear for higher amounts. We designed a general computation protocol to investigate the solvent effect at the atomistic scale. The nucleophilicity in solvent mixtures was evaluated by combining classical molecular dynamic (MD) simulations of solvated pyrrolidine and a few density functional theory (DFT) calculations of Parr nucleophilicity. The pyrrolidine theoretical nucleophilicity 1/ω obtained in various CH3OH/CH3CN mixtures are in excellent agreement with Mayr's nucleophilicity (N) parameters measured. Analyses of the molecular dynamic trajectories reveal that the decrease of the nucleophilicity in methanol rich mixtures arises predominantly from the solvation of the pyrrolidine by methanol molecules through strong hydrogen bonds. Last, we proposed a simple model to predict and accurately reproduce the experimentally obtained nucleophilicity values.
k (20 °C) = s(N + E). On the other hand, the general influence of the solvent mixtures, which are very often employed in preparative synthetic chemistry, has been poorly explored theoretically and experimentally, to date. Herein, we combined experimental and theoretical studies of the solvent influence on pyrrolidine nucleophilicity. We determined the nucleophilicity parameters N and s of pyrrolidine at 20 °C in CH3OH/CH3CN mixtures containing 0, 20, 40, 60, 80 and 100% CH3CN by kinetic investigations of their nucleophilic substitution reactions to a series of 2-methoxy-3-X-5-nitrothiophenes 1a–e (X = NO2, CN, COCH3, CO2CH3, CONH2). Depending on the resulting solvation medium, the N parameters range from 15.72 to 18.32 on the empirical nucleophilicity scale of Mayr. The nucleophilicity parameters N first evolve linearly with the content of acetonitrile up to 60% CH3CN by volume, but is non linear for higher amounts. We designed a general computation protocol to investigate the solvent effect at the atomistic scale. The nucleophilicity in solvent mixtures was evaluated by combining classical molecular dynamic (MD) simulations of solvated pyrrolidine and a few density functional theory (DFT) calculations of Parr nucleophilicity. The pyrrolidine theoretical nucleophilicity 1/ω obtained in various CH3OH/CH3CN mixtures are in excellent agreement with Mayr's nucleophilicity (N) parameters measured. Analyses of the molecular dynamic trajectories reveal that the decrease of the nucleophilicity in methanol rich mixtures arises predominantly from the solvation of the pyrrolidine by methanol molecules through strong hydrogen bonds. Last, we proposed a simple model to predict and accurately reproduce the experimentally obtained nucleophilicity values.
1 Introduction
The course of organic chemical reactions is efficiently modelled through the concepts of “electrophiles” and “nucleophiles”.1 These important concepts were quickly embraced by the chemical community, and empirical approaches were proposed by many authors both experimentally2–4 and theoretically5,6 to obtain quantitative scales.In particular, the nucleophilicity parameters N and electrophilicity E, as introduced by Mayr group, allows to quantitatively predict the rate constant between an electrophile and a nucleophile based on a linear free energy relationship eqn (1) (ref. 4–10) in which k corresponds to the second-order rate constant, N and s are nucleophile-specific parameters, and E is the electrophilicity parameter.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k (20 °C) = s(E + N) k (20 °C) = s(E + N)
| (1) |
While the electrophilicity of a molecule is almost independent of the solvent, the nucleophilicity can strongly influenced by it.11 For example, the nucleophilicity parameter N of 4-(dimethylamino)pyridine jumps from 13.19 in water to 15.80 in dichloromethane.12 Being quantitative, Mayr's approach (eqn (1)) is an attractive entrance to study the general influence of solvent mixtures which are classically employed in preparative synthetic chemistry and was recently used for the synthesis of nanolympiadane.13
Previous studies have shown that the dependence of the nucleophilicity parameter N on the solvent mixture can be greatly non linear14 so that it appears difficult to predict the rate of a nucleophile–electrophile reaction in a new solvent or a new solvent mixture. Therefore, a careful study of solvent influence appeared to us indispensable for providing further advances to the general effort of scaling and predicting nucleophilic and electrophilic parameters.
As a starting point to develop a general approach, we choose to study electrophile/nucleophile reactivity of thiophenes and pyrrolidine into two miscible solvents (acetonitrile, methanol), prepared in mixture at various ratio.
On one hand, pyrrolidine and imidazolidinone derivatives are the object of current interest as organocatalysts used in contemporary organic synthesis.15 Their intrinsic reactivity has been examined in relation with bioactive compounds of natural or synthetic origin, which incorporate such nucleophilic scaffolds.16–19 Recently, Mayr group investigated the kinetics of the reactions of several pyrrolidine derivatives and they integrated the resulting constitutive parameters of pyrrolidines into the so-called Mayr's nucleophilicity scale.16
On the other hand, thiophenes are heterocycles of industrial interest,20,21 and substituted thiophenes are scaffold found within pharmaceuticals, conductive polymers, photochromic molecular switches, liquid crystals, etc.20 Regarding the general studies on nucleophilic and electrophilic parameters of heterocycles, Boubaker group investigated the reactions of 2-methoxy-3-X-5-nitrothiophenes electrophiles 1a–e (where X = NO2, CN, COCH3, CO2CH3 and CONH2) with a variety of N-based nucleophiles, in different solvents at 20 °C.22,23 The derived second-order rate constants have been employed to determine the reactivity parameters of these series of thiophenes 1a–e, according to the Mayr's linear free energy relationship. The electrophilic parameter E values of 1a–e have been found ranging from −19.09 to −15.26, going respectively, from 1e (X = CONH2) the least reactive thiophene derivative, to 1a (X = NO2), the most reactive specimen.
This set of results and collected data has been of high interest for the understanding of both individual intrinsic organic reactivity and mutual interaction of pyrrolidine and thiophene derivatives.17,22,23
The present work aimed at determining the nucleophile specific parameters N and s of pyrrolidine, as a model, in CH3OH/CH3CN mixtures. The changes in nucleophilicity parameters N values as a function of acetonitrile content was used to investigate the solvent-mixture overall effect at the atomistic level by combining experimental and theoretical approaches. As methanol and acetonitrile dielectric constant are rather close, a continuum model is not sufficient to properly describe the solvation effect on the nucleophilicity. To appreciate the effects of solvent on the parameters of nucleophilicity of pyrrolidine, we performed classical molecular dynamic simulations of a pyrrolidine molecule solvated by a mixture of methanol and acetonitrile solvent molecules. Our studies confirmed the strong dependence of the nucleophilicity (and thus reaction rate) on the nature of the solvation medium. In the present case it comes from a gradual methanol desolvation to pyrrolidine involving four to zero molecules when the acetonitrile amount is gradually increased. Our study provides a relevant model for the more systematic inclusion of varied solvent into the reactivity studies of valuable electrophile/nucleophile organic reagents such the present heteroaromatics.
2 Experimental section
2.1 Materials
The thiophenes 1a–e were prepared as previously described.24–26 Pyrrolidine was received from commercial source and distilled before use. Acetonitrile and methanol HPLC grade >99.9% were used without further purification.2.2 Kinetic measurements
The kinetic study was performed using a spectrophotometer (UV-1650 Shimadzu) equipped with a Peltier temperature controller (TCC-240 A), which is able to keep constant temperature within 0.1 K. The reactions were carried out under pseudo-first order conditions in which the pyrrolidine concentration (6 × 10−4 to 8 × 10−1 mol L−1) was at least 20 times greater than the substrate concentration (about 3 × 10−5 mol L−1). The first-order rate constants measured, kobsd, values, together with detailed reaction conditions, are summarized in Tables S1–S6 in the ESI.† Reproducible kinetics constants were measured from several consistent experimental runs within ±3–5% standard deviation (Table S7 in the ESI†).2.3 Theoretical models and computational details
All quantum calculations were performed in the framework of density functional theory (DFT) by using the Gaussian 09 software package.27 Energies and forces were computed with the B3LYP functional28,29 empirically corrected for dispersion effects using the D3 scheme of Grimme with the Becke–Johnson damping.30 The bulk effect of the solvent was described using a polarizable continuum model as implemented in Gaussian09. Geometry optimizations without symmetry constraints and the corresponding frequency calculations were conducted with the 6-311+G(d,p) basis set for all atoms.31Methanol and acetonitrile dielectric constant are fairly close with εr = 32.613 for methanol and εr = 35.688 for acetonitrile, thus a continuum model is not sufficient to properly describing the solation effect on the nucleophilicity. To more precisely appreciate the effects of solvent on the parameters of nucleophilicity of pyrrolidine, we performed molecular dynamic simulations with the Amber simulation suite of a pyrrolidine molecule explicitly solvated by a mixture of methanol and acetonitrile solvent molecules.32 Pure methanol, pure acetonitrile and mixture containing 9%, 20%, 40%, 50%, 60%, 80% and 90% of acetonitrile in methanol were simulated. The force field parameters and the technical details of the simulation are given in ESI Fig. S2 and S3.† For each simulation, we computed the distribution methanol molecules in the first solvation sphere or pyrrolidine. All calculation results were grouped in Tables S11 and S12 in the ESI.† These simulations showed that the first layer of solvation contains between zero and four methanol molecules.
We computed the Parr nucleophilic indices ω−1 of pyrrolidine surrounded by n = 0, 1, 2, 3 and 4 explicit methanol molecules while the bulk solvation effects were described by a continuum. According to Parr,5 the nucleophilicity index is the inverse of the electrophilicity index ω that can be estimated using:
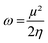 | (2) |
In which μ is the chemical potential and η the global hardness6 of pyrrolidine. Both can be evaluated in the context of DFT:
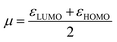 | (3) |
| η = εLUMO − εHOMO | (4) |
The pyrrolidine nucleophilicity are given in Table 1. These values are averaged using the distribution of methanol molecules in the first solvation layer of pyrrolidine obtained in the MD simulations (see ESI Table S10, Fig. S4 and S5†).
| Number of explicit methanol | 0 | 1 | 2 | 3 | 4 | 5 |
| Nucleophilic indices (ω−1) | 30.14 | 28.66 | 27.85 | 27.68 | 27.77 | 27.28 |
3 Results and discussion
3.1 Kinetics of the reactions of thiophenes 1a–e with pyrrolidine
The kinetics of the reactions of the series of thiophenes 1a–e employed as reference electrophiles with pyrrolidine (Scheme 1) are collected in Table 2.22,23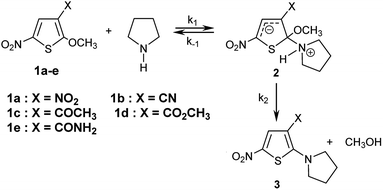 | ||
| Scheme 1 Reactions of 2-methoxy-3-X-5-nitrothiophenes 1a–e with pyrrolidine in CH3OH/CH3CN- mixtures at 20 °C. | ||
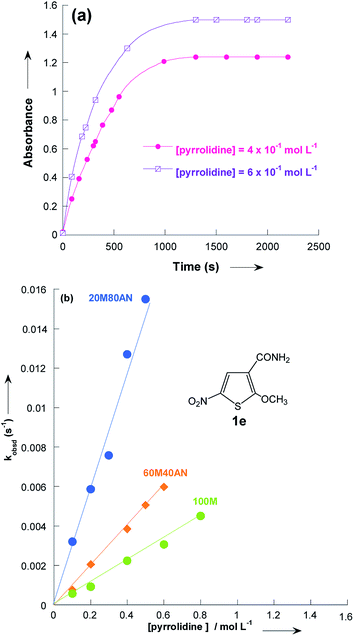
The reactions of 2-methoxy-3-X-5-nitrothiophenes 1a–e with pyrrolidine were followed spectrophotometrically by monitoring the formation of the products 3a–e at their absorption maxima (432–540 nm). An illustrative example is given in Fig. 1, which shows the set of UV-visible absorption spectra describing the progressive conversion of 1e to the product 3e (X = CONH2) resulting of the nucleophilic addition of pyrrolidine.
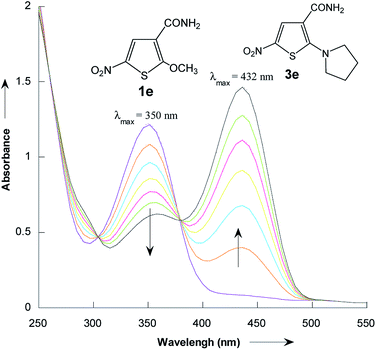 | ||
| Fig. 1 Time dependence of the electronic absorption spectrum of thiophene 1e (3 × 10−5 mol L−1) in the presence of pyrrolidine (10−1 mol L−1) in methanol at 20 °C. | ||
In all experiments, only one relaxation time was observed when the substitution products 3a–e were generated in the presence of a large excess of pyrrolidine. Typical results are given in Fig. 2a. The observed first-order rate constants kobsd were obtained from the correlation ln(A∞ − At) against time, where A∞ and At are the values of absorbance at the equilibrium and at time t, respectively. The plots of kobsd versus pyrrolidine concentrations are linear with R2 > 0.9987 passing through the origin. This indicates that the reactions are not base catalysed, and that the formation of the intermediate zwitterion 2 is rate determining (Scheme 1).22,23,33–35 The second-order rate constants k1 (mol−1 L s−1) for all reactions, which are listed in Table 3, can be readily derived from eqn (5).
| kobsd = k1 [pyrrolidine] | (5) |
| % CH3CN by volume | 0 | 20 | 40 | 60 | 80 | 100 |
|---|---|---|---|---|---|---|
| 1a: X = NO2 | 1.20 | 2.33 | 3.01 | 4.96 | 10.8 | — |
| 1b: X = CN | 1.66 × 10−1 | 2.85 × 10−1 | 4.74 × 10−1 | 7.41 × 10−1 | 1.48 | 10.9 |
| 1c: X = COCH3 | 3.89 × 10−2 | 5.72 × 10−2 | 1.04 × 10−1 | 1.51 × 10−1 | 2.88 × 10−1 | 1.91 |
| 1d: X = CO2CH3 | 1.15 × 10−2 | 2.08 × 10−2 | 3.74 × 10−2 | 5.15 × 10−2 | 9.53 × 10−2 | 8.14 × 10−1 |
| 1e: X = CONH2 | 5.60 × 10−3 | 7.00 × 10−3 | 1.03 × 10−2 | 1.72 × 10−2 | 3.09 × 10−2 | 3.32 × 10−1 |
3.2 Effect of the solvent mixture on the nucleophilic reactivity of the pyrrolidine
Fig. 3 and Table 3 show the contrasting changes in the reactivity of thiophenes 1a–e on transfer from CH3OH to CH3CN as a function of the amount of CH3CN in volume%. Similar curves are also obtained as a function of the CH3CN molar fractions, as shown in Fig. S1 and Table S9.† As can be seen, the second-order rate constants, k1, depend nonlinearly on the CH3CN volume%. The rate constant k1 significantly increases in the CH3CN-rich section and changes very little in the 0–60% CH3CN section. Surprisingly, k1 decreases while the polarity of the solvent mixture increase: the observed reactivity sequence being k1 [CH3OH] < k1 [80% CH3OH/20% CH3CN] < k1 [60% CH3OH/40% CH3CN] < k1 [40% CH3OH/60% CH3CN] < k1 [20% CH3OH/80% CH3CN] < k1 [CH3CN].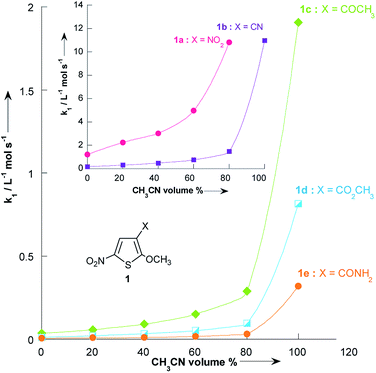 | ||
| Fig. 3 Plots of the second-order rate constants for the reactions of pyrrolidine with the 2-methoxy-3-X-5-nitrothiophenes 1a–e against volume % of CH3CN at 20 °C. Data from Table 3. | ||
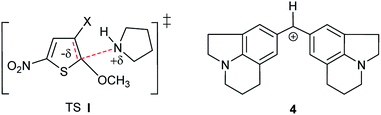
This behaviour might first seem in contradiction with the fact that this reaction is known to proceed via a dipolar transition state (TS I, Scheme 2), which should be more stabilized in methanolic solutions.36,37 However, as will be further shown later, it results from the progressive desolvation of pyrrolidine with decreasing CH3OH content upon addition of CH3CN.38,39 A comparable effect has been reported by Mayr and co-workers in the reactions of benzhydrylium cation (Scheme 2) with OH− in H2O–CH3CN mixtures.40
We further examined the solvation medium effect on the nucleophilic reactivity in terms of changes in solvent nucleophilicity parameters N1 using CH3OH/CH3CN mixtures.
We employed the linear dependence of the solvent nucleophilicity N1 on the amount of % CH3CN volume to interpolate the values of N1 for 40% and 60% of acetonitrile in methanol from the values reported in literature41 (see ESI†). N1 values are collected in Table 4.
In contrast to the nonlinear relationships observed between log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k1 and %CH3CN volume, excellent correlation coefficients (R2 > 0.9931) were found in all systems when the log
k1 and %CH3CN volume, excellent correlation coefficients (R2 > 0.9931) were found in all systems when the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k1 values were plotted versus the solvent nucleophilicity parameter N1,41 for solutions having N1 > 6.04 (i.e. less than 80% vol. CH3CN, Fig. 4). This result implies that the effect of solvent nucleophilicity is practically independent of the electronic nature of the substituent X. Most importantly, these linear correlations can be used to predict the unknown second-order rate constants, k1, values for reactions of thiophenes 1a–e with pyrrolidine in a given CH3OH/CH3CN mixture. This is particularly the case in CH3OH/CH3CN mixtures containing 9, 33, 50 and 67% CH3CN where the values of k1 for all thiophenes 1a–e have thus been obtained by extrapolation of the corresponding N1 data reported by Minegishi and co-worker41 (see Table S8 in the ESI†).
k1 values were plotted versus the solvent nucleophilicity parameter N1,41 for solutions having N1 > 6.04 (i.e. less than 80% vol. CH3CN, Fig. 4). This result implies that the effect of solvent nucleophilicity is practically independent of the electronic nature of the substituent X. Most importantly, these linear correlations can be used to predict the unknown second-order rate constants, k1, values for reactions of thiophenes 1a–e with pyrrolidine in a given CH3OH/CH3CN mixture. This is particularly the case in CH3OH/CH3CN mixtures containing 9, 33, 50 and 67% CH3CN where the values of k1 for all thiophenes 1a–e have thus been obtained by extrapolation of the corresponding N1 data reported by Minegishi and co-worker41 (see Table S8 in the ESI†).
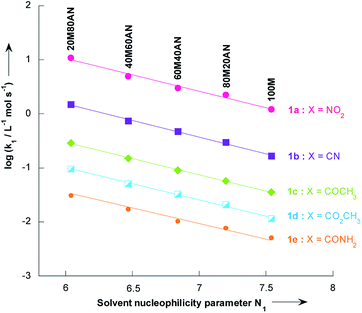 | ||
| Fig. 4 Plots of the second-order rate constants for the reactions of pyrrolidine with the 2-methoxy-3-X-5-nitrothiophenes 1a–e against solvent nucleophilicity parameter N1 at 20 °C. Mixtures of solvents given as (v/v). Abbreviations used for the solvents are AN (CH3CN) and M (CH3OH). Data from Tables 3 and 4 | ||
3.3 Mayr's nucleophilicity (N) parameters of pyrrolidine in methanolic acetonitrile solutions
The electrophilicity parameters E for the five thiophenes 1a–e,22,23 were employed to quantify the nucleophilicity parameters N and s of pyrrolidine in methanol/acetonitrile solutions.Using the data given in Tables 1 and 2, plots of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k1 versus electrophilicity parameters E of 1a–e have been constructed. As can be seen in Fig. 5, linear correlations were obtained, which yield the nucleophilicity parameters N and s, as defined by the linear free energy relationship (1). The nucleophilicity parameters N and s for pyrrolidine in various methanol/acetonitrile mixtures at 20 °C are reported in Table 5.
k1 versus electrophilicity parameters E of 1a–e have been constructed. As can be seen in Fig. 5, linear correlations were obtained, which yield the nucleophilicity parameters N and s, as defined by the linear free energy relationship (1). The nucleophilicity parameters N and s for pyrrolidine in various methanol/acetonitrile mixtures at 20 °C are reported in Table 5.
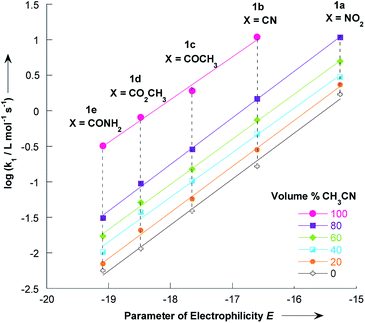 | ||
| Fig. 5 Correlations of the second-order rate constants (20 °C) for the reactions of pyrrolidine with the 2-methoxy-3-X-5-nitrothiophenes 1a–e in various CH3OH/CH3CN mixtures toward the electrophilicity parameters E. Data from Tables 1 and 2. | ||
The N value of 18.32 experimentally found in pure acetonitrile is consistent with the value of 18.64 reported by Kanzian and co-workers in the same solvent.42 It appears that adding acetonitrile to a methanolic solution of pyrrolidine resulted in only small changes in nucleophile specific parameter s.
The transfer from CH3CN to CH3OH corresponds to a relatively important decrease in the nucleophilicity of pyrrolidine (Table 5, ΔN = 2.60). This decrease of the nucleophilicity values in the CH3OH rich solvation medium could be attributed to a stronger solvation of pyrrolidine in this protic solvent, as confirmed below.
Literature data indicated a nucleophilicity parameter for piperidine of 17.35 in CH3CN,42 whereas the piperidine appears more nucleophilic in pure water (N = 18.13).43 For morpholine the N value is essentially the same in pure CH3CN and H2O: N(H2O) = 15.62,43 N(CH3CN) = 15.65.42 The changes in nucleophilicity parameter of pyrrolidine observed herein were also examined as a function of the variation in volume fraction of acetonitrile (%CH3CN). As seen from the Fig. 6, N linearly correlates with the vol% of CH3CN up to approximately 60% vol. CH3CN, following the eqn (6) with R2 = 0.9972:
| N = 15.732 + 0.0129% CH3CN | (6) |
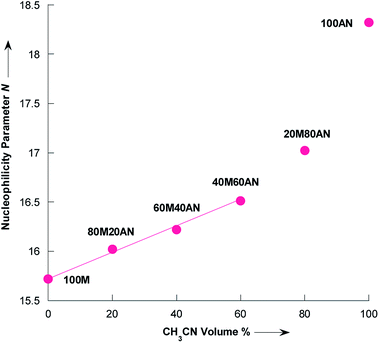 | ||
| Fig. 6 Dependence of the nucleophilicity parameter N of pyrrolidine on the composition of CH3OH/CH3CN mixtures at 20 °C. Dada from Table 4. Mixtures of solvents are given as (v/v), solvents: M = CH3OH and AN = CH3CN. | ||
Eqn (6) validity is supported by comparing with the experimental N value in the mixture of 91% CH3OH/9% CH3CN as reported by Phan et al.,44 to the interpolated value according eqn (6), as shown in Table 5. There is an excellent agreement between experimentally determined and calculated values with the average absolute error being only 0.12 N units.
3.4 Theoretical nucleophilicity (1/ω) parameters of pyrrolidine in methanolic mixtures with acetonitrile
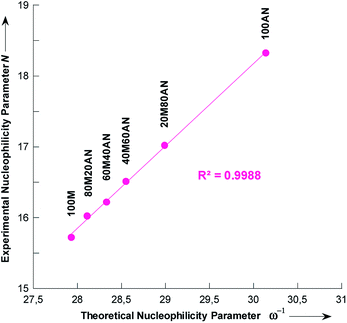
In the simulation process the number of methanol molecules n varies, and we found that on average one pyrrolidine molecule is surrounded by two molecules in pure methanol. This number decreases to zero when the content of the acetonitrile mixture is increased (Fig. S5†). In Table 6 is collected the theoretical nucleophilicity 1/ω of pyrrolidine in various methanolic CH3CN solutions, together with the Mayr's nucleophilicity (N) parameters measured in this study. A very good correlation was found, R2 = 0.9988, between theoretical 1/ω and experimental N values (Fig. 7),45–47 which is defined by eqn (7).| N = 1.1629 × ω−1 − 16.712 | (7) |
| % CH3CN | 0 | 9 | 20 | 40 | 60 | 80 | 91 | 100 |
|---|---|---|---|---|---|---|---|---|
| a From eqn (6).b Experimental nucleophilicity values were taken from ref. 44. | ||||||||
| Ω−1 | 27.93 | 28.01 | 28.11 | 28.33 | 28.55 | 28.99 | 29.37 | 30.14 |
| N from eqn (7) | 15.77 | 15.86 | 15.98 | 16.23 | 16.49 | 17.00 | 17.44 | 18.34 |
| N | 15.72 | 15.85a (15.97b) | 16.02 | 16.22 | 16.51 | 17.02 | 17.73b | 18.32 |
| Nmod | 15.88 | 15.94 | 16.03 | 16.24 | 16.57 | 17.13 | 17.65 | 18.32 |
Chamorro and co-workers have observed a linear correlations between the Mayr's nucleophilicity (N) parameters for a series of primary and secondary amines and the theoretical nucleophilicity index (ω−) obtained at the DFT level.45
The validity of eqn (7) was checked by estimating the nucleophilicity parameters N of pyrrolidine in CH3OH/CH3CN mixtures containing 9 and 91% CH3CN that had been experimentally measured.44 The detailed results are listed in Table 6. These results clearly show that the predicted values of N are in excellent agreement with the experimental data.
3.5 Modelling influence of the solvation
Our simulations reveal that the evolution of the pyrrolidine nucleophilicity on the composition of the methanol/acetonitrile mixture mainly comes from the fact that methanol forms a strong hydrogen bonds with the nitrogen lone pair and the NH bond of the pyrrolidine. Solvated molecules are much less nucleophilic: bare pyrrolidine nucleophilicity is 18.32 while it is only 15.67 for the bis-methanol pyrrolidine and 15.01 for the pyrrolidine solvated by five methanol. The proportion of pyrrolidine solvated by 0, 1, 2, 3 and 4 methanol molecules is shown in Fig. 8 as a function of the acetonitrile molar fraction.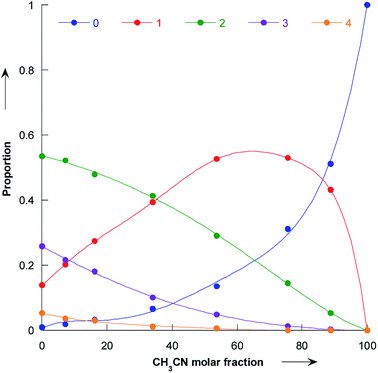 | ||
| Fig. 8 Plot of the proportion of bare pyrrolidine (dark blue), and pyrrolidine solvated by 1 (red), 2 (green), 3 (purple) and 4 (orange) molecules as a function of the molar fraction of acetonitrile. | ||
As the amount of methanol in the mixture is decreased, the amount of bare pyrrolidine molecule increases and so does the observed nucleophilicity. In particular, for molar fraction greater than 60%, the bare pyrrolidine is more abundant than the bis-methanol pyrrolidine. As a consequence, the average pyrrolidine nucleophilicity parameter N rises steeply, following the amount of unsolvated pyrrolidine.
It is interesting to note that solvated molecules have similar nucleophilicities, so that we could model the solvation process as a two states system:
| Pyr + M ⇌ (M–Pyr) | (8) |
The equilibrium constant of eqn (8) is denoted by 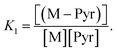 We decided to set the nucleophilicity of Pyr and (M–Pyr) to 18.32 and 15.01 respectively, and adjusted K1 on the experimental nucleophilicities: Ke1 = 0.11355.
We decided to set the nucleophilicity of Pyr and (M–Pyr) to 18.32 and 15.01 respectively, and adjusted K1 on the experimental nucleophilicities: Ke1 = 0.11355.
Using the definition of K1, the observed nucleophilicity is finally:
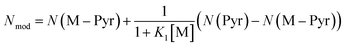 | (9) |
Results from Nmod are reported in Table 6. The very good agreement with the experimental values confirmed that the influence of the solvent on nucleophilicity can be modelled by our two states approach.
4 Conclusions
The reactions of 2-methoxy-3-X-5-nitrothiophenes 1a–e with pyrrolidine were studied kinetically by UV-visible spectroscopy in CH3OH, CH3CN and various CH3OH/CH3CN vol/vol. mixtures at 20 °C. The nucleophilicity parameters N and s for pyrrolidine in CH3OH/CH3CN mixtures of different compositions as defined by Mayr Equation log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k (20 °C) = s(E + N) have been determined and found to cover a range from 15.72 to 18.32. We have shown that the nucleophilicity parameters N for pyrrolidine are linearly related to the amount of acetonitrile (in % CH3CN volume) for ratio less than 60%. Finally, with the N and s values determined, it becomes possible to make predictions of second-order rate constants for reactions of pyrrolidine with others electrophiles of known E parameters. The nucleophilicity index 1/ω for pyrrolidine in solvent mixture of acetonitrile and methanol have been determined by combining DFT resampling of classical MD simulations. The theoretical values agree with the experimental ones, and the experimental dependence of the nucleophilicity parameters of pyrrolidine on mixture solvent methanol/acetonitrile was confirmed and supported by the simulation. The net dependence of the nucleophilicity parameter of pyrrolidine on solvent is mainly explained by a gradual methanol desolvation when the amount of acetonitrile is increased. The correlation between the experimental and theoretical values obtained herein indicates that this theoretical approach could be further processed to predict the nucleophilicity parameters of other nucleophiles in solvent mixtures and ultimately an automated tool for tuning electrophilic–nucleophilic reactivity as a function of a precise mixture of binary solvent. Further extension might concern ternary mixture of solvents.
k (20 °C) = s(E + N) have been determined and found to cover a range from 15.72 to 18.32. We have shown that the nucleophilicity parameters N for pyrrolidine are linearly related to the amount of acetonitrile (in % CH3CN volume) for ratio less than 60%. Finally, with the N and s values determined, it becomes possible to make predictions of second-order rate constants for reactions of pyrrolidine with others electrophiles of known E parameters. The nucleophilicity index 1/ω for pyrrolidine in solvent mixture of acetonitrile and methanol have been determined by combining DFT resampling of classical MD simulations. The theoretical values agree with the experimental ones, and the experimental dependence of the nucleophilicity parameters of pyrrolidine on mixture solvent methanol/acetonitrile was confirmed and supported by the simulation. The net dependence of the nucleophilicity parameter of pyrrolidine on solvent is mainly explained by a gradual methanol desolvation when the amount of acetonitrile is increased. The correlation between the experimental and theoretical values obtained herein indicates that this theoretical approach could be further processed to predict the nucleophilicity parameters of other nucleophiles in solvent mixtures and ultimately an automated tool for tuning electrophilic–nucleophilic reactivity as a function of a precise mixture of binary solvent. Further extension might concern ternary mixture of solvents.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Education and Scientific Research of Tunisia. This work was also partly supported by the French CNRS, the University of Bourgogne, the Regional Council of Bourgogne through the Regional Action Plan for Innovation (PARI), and the European Union through the PO FEDER-FSE Bourgogne 2014/2020. The ANR-PRC 2016 program (ALCATRAS, ANR-16-CE07-0001-01) also contributed to financial support. Calculations were performed using HPC resources from DSI-CCUB (University of Bourgogne).Notes and references
- C. K. Ingold, Chem. Rev., 1934, 15, 225–274 CrossRef CAS.
- C. G. Swain and C. B. Scott, J. Am. Chem. Soc., 1953, 75, 141–147 CrossRef CAS.
- A. C. Legon and D. J. Millen, J. Am. Chem. Soc., 1987, 109, 356–358 CrossRef CAS.
- (a) H. Mayr and M. Patz, Angew. Chem., Int. Ed., 1994, 33, 938–957 CrossRef; (b) H. Mayr, B. Kempf and A. R. Ofial, Acc. Chem. Res., 2003, 36, 66–77 CrossRef CAS PubMed.
- R. G. Parr and W. Yang, Density Functional Theory of Atoms and Molecules, Oxford University Press, New York, 1989 Search PubMed.
- R. G. Parr and R. G. Pearson, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- S. Lakhdar, M. Westermaier, F. Terrier, R. Goumont, T. Boubaker, A. R. Ofial and H. Mayr, J. Org. Chem., 2006, 71, 9088–9095 CrossRef CAS PubMed.
- Q. Fuks, E. Zenz, I. Mayer, A. R. Ofial, H. Zipse, H. Mayr and H. Chen, J. Am. Chem. Soc., 2018, 140, 16758–16772 CrossRef PubMed.
- D. S. Timofeeva, A. R. Ofial and H. Mayr, Tetrahedron, 2019, 75, 459–463 CrossRef CAS.
- R. J. Mayer and A. R. Ofial, Org. Lett., 2018, 20, 2816–2820 CrossRef CAS PubMed.
- J. F. Bunnett, Annu. Rev. Phys. Chem., 1963, 14, 271–290 CrossRef CAS.
- F. Brotzel, B. Kempf, T. Singer, H. Zipse and H. Mayr, Chem.–Eur. J., 2007, 13, 336–345 CrossRef CAS PubMed.
- (a) S. Datta, Y. Kato, S. Higashiharaguchi, K. Aratsu, A. Isobe, T. Saito, D. D. Prabhu, Y. Kitamoto, M. J. Hollamby, A. J. Smith, R. Dagleish, N. Mahmoudi, L. Pesce, C. Perego, G. M. Pavan and S. Yagai, Nature, 2020, 583, 400–405 CrossRef CAS PubMed; (b) I. H. Um, H. J. Moon, Y. H. Shin and J. M. Dust, Can. J. Chem., 2018, 96, 922–945 CrossRef CAS; (c) I. H. Um, H. W. Yoon, J. S. Lee, H. J. Moon and D. S. Kwon, J. Org. Chem., 1997, 62, 5939–5944 CrossRef CAS.
- C. Nolte, J. Ammer and H. Mayr, J. Org. Chem., 2012, 77, 3325–3335 CrossRef CAS PubMed.
- A. Vega-Peñaloza, S. Paria, M. Bonchio, L. Dell'Amico and X. Companyó, ACS Catal., 2019, 9, 6058–6072 CrossRef.
- F. An, B. Maji, E. Min, A. R. Ofial and H. Mayr, J. Am. Chem. Soc., 2020, 142, 1526–1547 CrossRef CAS PubMed.
- Z. Shao, J. Chen, Y. Tu, L. Lia and H. Zhang, Chem. Commun., 2003, 1918–1919 RSC.
- M. Z. Wrobel, A. Chodkowski, F. Herold, M. Marciniak, M. Dawidowski, A. Siwek, G. Starowicz, K. Stachowicz, B. Szewczyk, G. Nowak, M. Belka, T. Baczek, G. Satała, A. J. Bojarski and J. Turło, Eur. J. Med. Chem., 2019, 183, 111736–111753 CrossRef CAS PubMed.
- P. Han, Z. Y. Mao, C. M. Si, Z. Zhou, B. G. Wei and G. Lin, J. Org. Chem., 2019, 84, 914–923 CrossRef CAS PubMed.
- K. Smith and M. L. Barratt, J. Org. Chem., 2007, 72, 1031–1034 CrossRef CAS PubMed.
- (a) D. Gramec, M. L. Peterlin and M. S. Dolenc, Chem. Res. Toxicol., 2014, 27, 1344–1358 Search PubMed; (b) V. Ryabova and L. Ignatovich, Top. Heterocycl. Chem., 2015, 39, 43–108 Search PubMed; (c) A. Michalak, F. D. Proft, P. Geerlings and R. F. Nalewajski, J. Phys. Chem. A, 1999, 103, 762–771 CrossRef CAS.
- S. Souissi, W. Gabsi and T. Boubaker, Int. J. Chem. Kinet., 2018, 50, 582–590 CrossRef CAS.
- A. Echaieb, W. Gabsi and T. Boubaker, Int. J. Chem. Kinet., 2014, 46, 470–476 CrossRef CAS.
- G. Consiglio, C. Arnone, D. Spinelli, F. Sancassan, C. Dell'Erba, G. Leandri and F. Terrier, Gazz. Chim. Ital., 1987, 117, 267–274 CAS.
- D. Spinelli, G. Consiglio, R. Noto and A. Corrao, J. Chem. Soc., Perkin Trans. 2, 1975, 620–622 RSC.
- G. Baldini, G. Doddi, G. Illuminati and F. Stegel, J. Org. Chem., 1976, 41, 2153–2157 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, and D. J. Fox, Gaussian 09, Revision E.01, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 CrossRef CAS PubMed.
- K. Raghavachari, J. S. Binkley, R. Seeger and J. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef.
- P. A. Kollman, AMBER 2018, University of California, San Francisco, 2018 Search PubMed.
- F. Terrier, Modern Nucleophilic Aromatic Substitution, John Wiley & Sons, Weinheim, 2013 Search PubMed.
- J. F. Bunnett, S. Sekiguchi and L. Smith, J. Am. Chem. Soc., 1981, 103, 4865–4871 CrossRef CAS.
- H. Raissi, I. Jamaoui, R. Goumont and T. Boubaker, Int. J. Chem. Kinet., 2017, 49, 835–846 CrossRef CAS.
- F. Terrier, M. J. Pouet, J. C. Halle, S. Hunt, J. R. Jones and E. Buncel, J. Chem. Soc., Perkin Trans. 2, 1993, 1665–1672 RSC.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, Wiley, Weinheim, 4th edn, 2011 Search PubMed.
- C. F. Bernasconi and P. J. Wenzel, J. Am. Chem. Soc., 1996, 118, 11446–11453 CrossRef CAS.
- F. Terrier, E. Kizilian, R. Goumont, N. Faucher and C. Wakselman, J. Am. Chem. Soc., 1998, 120, 9496–9503 CrossRef CAS.
- S. Minegishi, S. Kobayashi and H. Mayr, J. Am. Chem. Soc., 2004, 126, 5174–5181 CrossRef CAS PubMed.
- S. Minegishi, S. Kobayashi and H. Mayr, J. Am. Chem. Soc., 2004, 126, 5174–5181 CrossRef CAS PubMed.
- T. Kanzian, T. A. Nigst, A. Maier, S. Pichl and H. Mayr, Eur. J. Org. Chem., 2009, 6379–6385 CrossRef CAS.
- F. Brotzel, Y. C. Cheung and H. Mayr, J. Org. Chem., 2007, 72, 3679–3688 CrossRef CAS PubMed.
- T. B. Phan, M. Breugst and H. Mayr, Angew. Chem., Int. Ed., 2006, 45, 3869–3874 CrossRef CAS PubMed.
- (a) E. Chamorro, M. Duque-Noreña and P. Pérez, J. Mol. Struct.: THEOCHEM, 2009, 896, 73–79 CrossRef CAS; (b) E. Chamorro, M. Duque-Noreña and P. Pérez, J. Mol. Struct.: THEOCHEM, 2009, 901, 145–152 CrossRef CAS.
- P. Jaramillo, P. Pérez, P. Fuentealba, S. Canuto and K. Coutinho, J. Phys. Chem. B, 2009, 113, 4314–4322 CrossRef CAS PubMed.
- O. Banjoko and I. A. Babatunde, Tetrahedron, 2004, 60, 4645–4654 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental kinetic data, computational details for molecular dynamics, methanol distribution around pyrrolidine, influence of PCM parameters. See DOI: 10.1039/d0ra06324j |
| ‡ Present address: Chemistry Department, College of Sciences and Arts, Jouf University, Algrayat, Saudi Arabia. |
| This journal is © The Royal Society of Chemistry 2020 |