Open Access Article
Open Access ArticleMicrodroplet synthesis of azo compounds with simple microfluidics-based pH control
Daiki Tanaka *a,
Shunsuke Sawaib,
Shohei Hattorib,
Yoshito Nozakia,
Dong Hyun Yoon
*a,
Shunsuke Sawaib,
Shohei Hattorib,
Yoshito Nozakia,
Dong Hyun Yoon a,
Hiroyuki Fujitad,
Tetsushi Sekiguchia,
Takashiro Akitsuc and
Shuichi Shojib
a,
Hiroyuki Fujitad,
Tetsushi Sekiguchia,
Takashiro Akitsuc and
Shuichi Shojib
aResearch Organization for Nano & Life Innovation, Waseda University, Tokyo 162-0041, Japan. E-mail: d.tanaka@ruri.waseda.jp
bFaculty of Science and Engineering, Waseda University, Tokyo, 169-8555, Japan
cDepartment of Chemistry, Faculty of Science, Tokyo University of Science, Tokyo 162-8601, Japan
dCanon Medical Systems Corporation, Otawara, Tochigi 324-8550, Japan
First published on 23rd October 2020
Abstract
Conventional solution-phase synthesis of azo compounds is complicated by the need for precise pH and temperature control, high concentrations of pH control reagents, and by-product removal. In this work, we exploited the advantages of microdroplet chemistry to realize the simple and highly efficient synthesis of an azo compound using microfluidics-based pH control. Owing to the small size of microdroplets, heat exchange between a microdroplet and its environment is extremely fast. Furthermore, chemical reactions in microdroplets occur rapidly due to the short diffusion distance and vortex flow. Formation of the azo compound reached completion in less than 3 s at room temperature, compared with 1 h at 0 °C under conventional conditions. pH control was simple, and the pH control reagent concentration could be reduced to less than one-tenth of that used in the conventional method. No by-products were generated, and thus this procedure did not require a recrystallization step. The time course of the chemical reaction was elucidated by observing the growth of azo compound microcrystals in droplets at various locations along the channel corresponding to different mixing times.
1. Introduction
Azo compounds are versatile materials used in anticancer drugs, batteries, and numerous other applications. For example, Ganguly et al. reported that the copper complex of an azo compound exhibited anticancer activity by strongly binding to DNA,1 while Luo et al. used an azo compound to fabricate the organic electrode of a lithium-ion battery.2 Organic electrode materials are promising as materials for batteries but have the problem that they dissolve in the liquid electrolyte, suppressing battery capacity. The use of an azo compound electrode mitigates this problem. Although azo compounds have an array of applications in various fields, their synthesis remains challenging and is still an active area of research.Azo compounds are conventionally synthesized via a complicated solution-based procedure. For instance, the temperature should be maintained at 0 °C to remove the heat generated by the reaction. Furthermore, the diazotization step is typically conducted in highly concentrated aqueous hydrochloric acid solution to promote the chemical reaction. However, high concentrations of acidic or alkaline reagents lead to product degradation, so the pH should be returned to neutral. Zhao et al. reported the successful one-pot solution-phase preparation of various azo compounds by varying the synthesis time and temperature.3 They showed that prolonged stirring and strict temperature control were necessary for the synthesis of azo compounds. Einaga et al. systematically investigated organic/inorganic hybrid materials composed of metal Schiff base complexes and azo compounds in poly(methyl methacrylate) films as non-crystalline solids4 that could be made to regularly align upon light irradiation.5 They succeeded in photoisomerization and structural modification of azo compounds by attaching an azo group to a palladium complex. However, these conventional solution-phase synthetic methods involved a complicated procedure using a metal catalyst and strict control of the atmosphere and temperature.
We previously established a simple method for azo compound synthesis using a Y-shaped microfluidic device under laminar flow.6 Although this method was superior to the conventional method in terms of reaction time and temperature, the resulting azo compound clogged the channel. Therefore, we herein describe a new method for the synthesis of azo compounds in which all of the complicated synthetic steps occur within microdroplets. The azo compound synthesized inside the microdroplets flows to the outlet without clogging the channel. The reagent introduced into the microdroplets diffuses instantaneously throughout, which enables efficient chemical reaction.7,8
Microdroplet-mediated chemical synthesis has been studied primarily in the field of organic chemistry.9–12 Song et al. compared the mixing of reagents during chemical reactions under laminar flow and inside microdroplets.13 Using a simplified diffusion model, Mortensen and Williams reported that mixing occurs in less than a millisecond, and the contribution of turbulence, estimated from times of coalescing ballistic microdroplets, suggests that complete mixing occurs within a few microseconds.14 Ursuegui et al. reported a microdroplet surface engineering strategy involving strain-promoted alkyne–azide cycloaddition, in which they designed and synthesized an azide PFPE–PEG based fluorosurfactant prone to react with strained alkyne derivatives via a copper-free click reaction.15 The use of microdroplets leads to more efficient mixing compared with laminar flow and is advantageous for chemical synthesis.16–19 Theberge et al. integrated two enabling technologies, namely, droplet-based microfluidics and fluorous biphasic catalysis, to create controlled catalytic interfaces for organic synthesis in flow.20 Although organic synthesis using microdroplets can provide superior results in terms of reaction time and temperature, no successful examples have yet been reported for reactions requiring strict pH control. In this study, we synthesized complex azo compounds using simple microfluidics-based pH control.
2. Experimental
2.1. Materials
Chemicals and reagents were of the highest grade commercially available (Tokyo Chemical Industry or Kanto Chemical) and were used as received without further purification.2.2. Synthesis concept and scheme
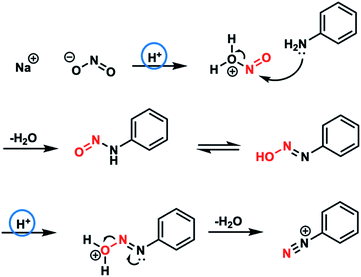
Fig. 1a shows the two-step reaction scheme used to synthesize an azo compound. The azo compound was synthesized from three reagents, namely, aniline, sodium nitrite, and o-vanillin; the conventional synthetic method using these reagents requires precise pH control. Each of the reagents was confined within microdroplets. Fig. 1b presents an overview of the microfluidic experiment. The individual microdroplets fused inside the microfluidic device, permitting the synthetic reaction to proceed. | ||
| Fig. 1 Synthesis of azo compounds: (a) synthetic scheme, (b) outline of the microfluidic experiment, and (c) design of microfluidic device. | ||
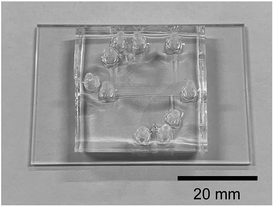
2.3. Device design and microfluidic experiments
Fig. 1c shows the design of the microdroplet-generating device used in this study. The device was constructed from polydimethylsiloxane (PDMS) with a channel width and depth of 200 and 100 μm, respectively.21 Solutions of the reagents were introduced via syringe using a syringe pump. Fig. 2 shows the microfluidic device used in this study. Microfluidic devices of the same design are placed one above the other to switch when there is a problem.The azo compound was synthesized using aniline (250 mmol L−1, reagent A), sodium nitrite (250 mmol L−1, reagent B), and o-vanillin (250 mmol L−1, reagent C). The aniline and o-vanillin were dissolved in aqueous HCl solution (500 mmol L−1) and aqueous NaOH solution (500 mmol L−1), respectively. The pH and reagent concentration were varied by altering the flow rate to examine their effect on the chemical reaction. The measured pH values were 3, 7, and 12.
The synthesized azo compound was isolated by filtration and analysed by infrared (IR) spectroscopy (FT/IR-6200, JASCO), fast atom bombardment mass spectrometry (FAB-MS; JMS-BU25, JEOL), and 1H and 13C nuclear magnetic resonance (NMR) spectroscopy (ECX500, JEOL).
3. Results and discussion
The azo compound shown in Fig. 1a, which typically requires a complicated synthetic procedure, was synthesized inside microdroplets. The proposed method has several advantages over the conventional solution-phase method (Table 1). In particular, control of the reaction temperature was unnecessary and the reaction could be performed at room temperature (23 °C) rather than 0 °C, while the reaction time decreased from 1 h to 3 s or less.| Beaker | Microdroplet | |
|---|---|---|
| Synthesis temperature | 0–5 °C | Room temperature |
| Synthesis time | 3600 s | 3 s |
| Concentration of pH control reagents | 5.6 mol L−1 | 0.5 mol L−1 |
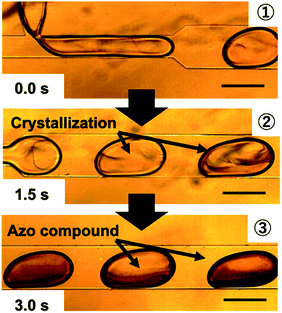
Fig. 3 shows microscopy images of the microfluidic experiment. Crystals of the azo compound formed immediately after the microdroplets fused. The chemical reaction inside the microdroplets reached completion in 1.5–3 s. The synthesis conditions were significantly different from those used in the conventional solution-phase method in a beaker. In the microdroplet method, the concentration of the pH control reagents (HCl and NaOH) could be decreased to 0.50 mol L−1, which is less than one-tenth of that used in the conventional method (5.62 mol L−1).
3.1 Product analysis
Fig. 4 shows the analytical results for the obtained azo compound. A peak corresponding to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (1580 cm−1) was observed in the IR spectrum (Fig. 4a). The FAB-MS spectrum contained a peak with a mass-to-charge ratio (m/z) of 257.10, which corresponds to the [M + H]+ ion of the expected product (Fig. 4b). Fig. 4c and d show the 1H and 13C-NMR spectra, respectively. The analytical results were consistent with those expected for the desired azo compound.
N (1580 cm−1) was observed in the IR spectrum (Fig. 4a). The FAB-MS spectrum contained a peak with a mass-to-charge ratio (m/z) of 257.10, which corresponds to the [M + H]+ ion of the expected product (Fig. 4b). Fig. 4c and d show the 1H and 13C-NMR spectra, respectively. The analytical results were consistent with those expected for the desired azo compound.
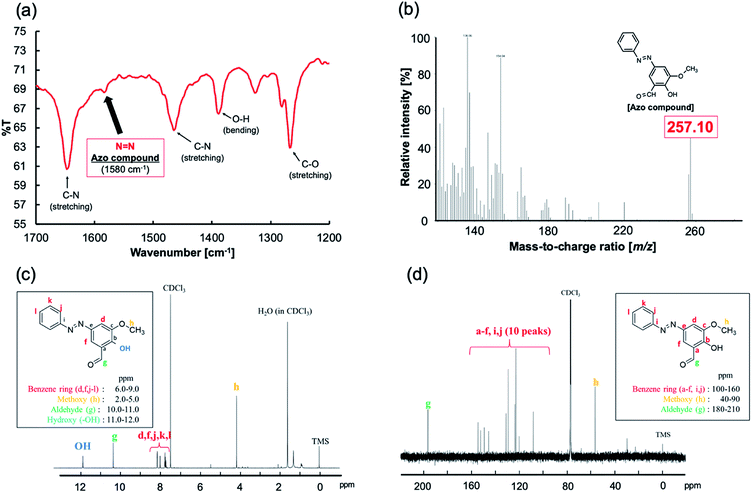 | ||
| Fig. 4 Characterization of the isolated azo compound: (a) IR spectrum, (b) FAB-MS spectrum, (c) 1H-NMR spectrum, and (d) 13C-NMR spectrum. | ||
3.2. Reaction rate in microdroplets
The synthesis of the azo compound inside the microdroplets reached completion in approximately 3 s. Furthermore, the concentration of the pH control reagents could be greatly reduced. It is considered that the diffusion distance of the chemical species inside the microdroplets was very short and these species diffused throughout the microdroplets instantaneously.The diffusion time of a chemical species is proportional to the square of the diffusion distance, as given by eqn (1):
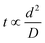 | (1) |
The diffusion time for the microdroplets employed in this study was calculated to be 3.125 s using eqn (2):
| d = (Dt)0.5 | (2) |
The mutual diffusion coefficient D of hydrochloric acid and water at 0.5 mol L−1 is approximately 3.2 × 10−9 m2 s−1, and the diffusion length d is 100 μm. In the microdroplet experiments, the reaction time for the synthesis of the azo compound was approximately 3 s, which is close to this theoretical value. However, other factors in addition to diffusion also play a role in actual experiments, such as the generation of eddy currents in the droplets.
The microdroplet method allowed the concentration of the pH control reagents to be drastically reduced from 5.6 mol L−1 to 0.5 mol L−1. Theoretically, the diazotization reaction can proceed with 0.5 mol of HCl (H+) for 250 mmol of aniline (Fig. 5). However, 5.6 mol L−1 of HCl (H+) is typically used in conventional solution-phase synthesis, where the diffusion distance is long and the reaction does not proceed unless at least a tenfold excess of HCl is present.
On the other hand, in the microdroplet method, the diazotization reaction proceeded at a HCl concentration of only 0.5 mol L−1. These results indicate that the chemical species can rapidly diffuse inside the microdroplets and the chemical reaction can proceed in an optimal state.
When two liquids are mixed inside a microdroplet, a vortex is generated inside, thus promoting rapid mixing of the chemical species and increasing the efficiency of the chemical reaction beyond that possible via natural diffusion alone.
3.3. Efficiency of temperature control
Because the formation of an azo compound is an exothermic reaction, the conventional method requires the synthetic operations to be performed with cooling over ice. In contrast, with the microdroplet method, it was possible to perform the synthesis at room temperature (23 °C). Microdroplets have a very large surface area per unit volume, precise heat control can be easily achieved. In particular, for a synthetic scheme involving heat generation as in the current case, it is considered that the heat generated by the chemical reaction rapidly diffuses and dissipates to the surroundings. The microdroplet method thus enabled efficient removal of reaction heat, thereby suppressing side reactions.3.4. Microscopic reaction observation
In this study, the pH was controlled by the flow rates of the pH control reagents (HCl and NaOH), as summarized in Table 2. The concentration of the pH control reagents, and therefore the reaction conditions, could be easily controlled by varying the flow rates.| Reagent | Flow rate [μL min−1] | ||
|---|---|---|---|
| pH = 3 | PH = 7 | pH = 12 | |
| Carrier fluid | 2.0 | 2.0 | 2.0 |
| HCl | 1.2 (1.0 mol L−1) | 0.6 (0.5 mol L−1) | 0.2 (0.2 mol L−1) |
| NaOH | 0.6 (0.5 mol L−1) | 0.6 (0.5 mol L−1) | 0.6 (0.5 mol L−1) |
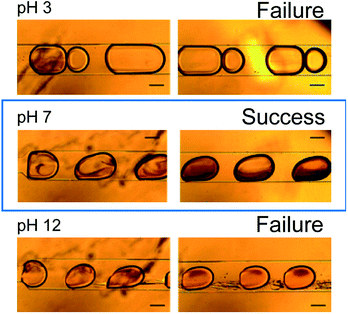
Fig. 6 shows microscopy images of the microdroplets synthesized at pH values of 3, 7, and 12. At pH 3, fine particles formed immediately after fusion of the droplets but disappeared after approximately 3 s. Immediately after the fusion of the droplets, it is considered that the pH changed to 7 in part of the droplet with formation of the azo compound, but after 3 s, the pH of the entire droplet became 3 and the reaction did not proceed further. At pH 7, formation of the azo compound proceeded steadily and the target compound was successfully synthesized. At pH 12, an oily product was observed. It is considered that replicative organisms and excess reactants were produced because the pH was too high.
4. Conclusion
The synthesis of azo compounds inside microdroplets has numerous advantages over conventional solution-phase synthesis. The microdroplet method permitted rapid synthesis of the desired azo compound at room temperature (23 °C), whereas conventional solution-phase synthesis in a beaker typically requires cooling to 0 °C over ice and a reaction time that is 1000 times longer. Moreover, pH control could be conveniently accomplished and the concentration of the pH control reagents could be reduced to one-tenth of that typically required. All of the complex synthetic operations such as pH adjustment occurred within the microdroplet. Furthermore, the microdroplet method efficiently afforded a high-purity product without the need for recrystallization. Thus, the described technique permits the convenient synthesis of azo compounds that typically require complex synthetic operations.Furthermore, the described microfluidic device allows the reagent concentration to be easily varied by controlling the flow rate, and synthetic experiments can be performed under various conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by Canon Medical Systems Corporation and a Grant-in-Aid for Basic Scientific Research (A) (No. 20H00336) from the Japanese Ministry of Education, Culture, Sports, Science and Technology. The authors would also like to thank the MEXT Nanotechnology Platform Support Project of Waseda University.References
- D. Ganguly, C. K. Jain, R. C. Santra, S. Roychoudhury, H. K. Majumder, T. K. Mondal and S. Das, ChemistrySelect, 2017, 2, 2044 CrossRef CAS.
- C. Luo, X. Ji, J. Chen, K. J. Gaskell, X. He, Y. Liang, J. Jiang and C. Wang, Angew. Chem., Int. Ed., 2018, 130, 8703 CrossRef.
- R. Zhao, C. Tan, Y. Xie, C. Gao, H. Liu and Y. Jiang, Tetrahedron Lett., 2011, 52, 3805 CrossRef CAS.
- Y. Einaga, R. Mikami, T. Akitsu and G. Li, Thin Solid Films, 2005, 493, 230 CrossRef CAS.
- Y. Aritake, T. Takanashi, A. Yamazaki and T. Akitsu, Polyhedron, 2011, 30, 886 CrossRef CAS.
- D. Tanaka, S. Sawai, D. H. Yoon, T. Sekiguchi, T. Akitsub and S. Shoji, RSC Adv., 2017, 7, 39576 RSC.
- H. Song, J. D. Tice and R. F. Ismagilov, Angew. Chem., Int. Ed., 2003, 42, 767 Search PubMed.
- M. Wojnicki, M. Luty-Błocho, V. Hessel, E. Csapó, D. Ungor and K. Fitzner, Micromachines, 2018, 9, 248 CrossRef.
- Z. T. Cygan, J. T. Cabral, K. L. Beers and E. J. Amis, Langmuir, 2005, 21, 3629 CrossRef CAS.
- T. Fukuda, N. Funaki, T. Kurabayashi, M. Suzuki, D. H. Yoon, A. Nakahara, T. Sekiguchi and S. Shoji, Sens. Actuators, B, 2014, 203, 536 CrossRef CAS.
- Y. Zheng, Z. Yu, R. M. Parker, Y. Wu, C. Abell and O. A. Scherman, Nat. Commun., 2014, 5, 5772 CrossRef CAS.
- E. K. Lumley, C. E. Dyer, N. Pamme and R. W. Boyle, Org. Lett., 2012, 14, 5724 CrossRef CAS.
- H. Song, J. D. Tice and R. F. Ismagilov, Angew. Chem., Int. Ed., 2003, 42, 767 Search PubMed.
- D. N. Mortensen and E. R. Williams, Anal. Chem., 2014, 86, 9315 CrossRef CAS.
- S. Ursuegui, M. Mosser and A. Wagner, RSC Adv., 2016, 6, 94942 RSC.
- M. Faustini, J. Kim, G. Y. Jeong, J. Y. Kim, H. R. Moon, W. S. Ahn and D. P. Kim, J. Am. Chem. Soc., 2013, 135, 14619 CrossRef CAS.
- R. O. Grigoriev, M. F. Schatza and V. Sharmab, Lab Chip, 2006, 6, 1369 RSC.
- F. Sarrazin, L. Prat, N. Di Miceli, G. Cristobal, D. R. Linkc and D. A. Weitz, Chem. Eng. Sci., 2007, 62, 1042 CrossRef CAS.
- C. Chen, Y. Zhao, J. Wang, P. Zhu, Y. Tian, M. Xu, L. Wang and X. Huang, Micromachines, 2018, 9, 160 CrossRef.
- A. B. Theberge, G. Whyte, M. Frenzel, L. M. Fidalgo, R. C. R. Wootton and W. T. S. Huck, Chem. Commun., 2009, 6225 RSC.
- D. Tanaka, W. Kawakubo, E. Tsuda, Y. Mitsumoto, D. H. Yoon, T. Sekiguchi, T. Akitsu and S. Shoji, RSC Adv., 2016, 6, 81862 RSC.
| This journal is © The Royal Society of Chemistry 2020 |