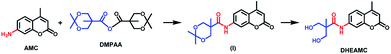Open Access Article
Open Access ArticleA novel strategy to polyurethanes with improved mechanical properties by photoactivation of amidocoumarin moieties†
Rodrigo Navarro *ab,
Rubén Seoane-Riveroc,
José María Cuevasc and
Ángel Marcos-Fernandezab
*ab,
Rubén Seoane-Riveroc,
José María Cuevasc and
Ángel Marcos-Fernandezab
aInstitute of Polymer Science and Technology (ICTP-CSIC), Juan de la Cierva 3, Madrid, 28006, Spain. E-mail: rnavarro@ictp.csic.es
bInterdisciplinary Platform for “Sustainable Plastics towards a Circular Economy” (SUSPLAST-CSIC), Madrid, Spain
cGAIKER Technology Centre, Basque Research and Technology Alliance (BRTA), Parque Tecnológico de Bizkaia, Ed. 202, Zamudio, E-487170, Spain
First published on 13th August 2020
Abstract
An attractive strategy involving photodimerization of a novel amidocoumarin moiety is presented to prepare polyurethane coatings with excellent mechanical properties. Two families of polyurethanes containing these chromophore units into soft or hard segments were easily synthesized by inserting a small fraction of amidocoumarin-diol (5 wt% or 10 wt%). A systematic study has been carried out comparing hard segment, chromophore content and the influence of this amidocoumarin unit within the hard or soft segment. For all synthetized polymers, mechanical properties of the coatings have been evaluated before and after an excitation of the coumarin units with UV light. The results show that the insertion of coumarin into the hard segment leads to a considerable improvement of the mechanical properties after irradiation. Additionally, the photochemical activity of amidocoumarin was studied by UV-Vis and Raman spectroscopies.
Introduction
Polyurethanes (PUs) are a type of versatile material that find a wide range of commercial applications in the field of coatings for anticorrosion protection, vehicles, railways, airplanes, freight ships, etc.1 Additionally, other important applications for the use of PUs are adhesives, sealants or elastomers, because these polymer systems combine excellent and attractive physicochemical properties at a competitive price.2,3 These systems are considered segmented block copolymers with phase-separated micro-structures: hard segments formed by isocyanates and chain extenders self-assemble via inter/intramolecular interactions (mainly hydrogen bonding) into hard domains, which embedded into a continuous matrix of flexible soft segments from long flexible chain diols (e.g. polyether, PDMS and polyester based diols). The hard segments mainly act as physical cross-links, giving rise to practicable mechanical properties.4 However, for some applications, such as protective coatings for vehicle bodies to resist weathering, excellent mechanical properties including toughness, tensile strength and even Young's modulus are urgently required. Therefore, improving the mechanical properties of polyurethane coatings is an important topic of research.To improve mechanical properties of polyurethanes, two different strategies have been proposed. One consists of incorporating nanofillers and the other of increasing chemical cross-linking density. Both methodologies can significantly improve the mechanical properties of polyurethanes. However, the introduction of nanofillers into the polymer matrix is complicated due to the tendency of these reinforcing fillers to agglomerate.5–7 To overcome this issue, several strategies have been proposed although their incorporation frequently leads to non-transparent coatings. These drawbacks strongly motivate us to exploit new ways to improve the mechanical properties (strength, toughness) of transparent coatings.
On the other hand, coumarins are attractive photoreactive chromophores that undergo a reversible dimerization process upon irradiation at specific wavelengths. This dimerization process consists on the creation of cyclobutane motif between two coumarin units through a [2π + 2π] cycloaddition reaction.8 It has been widely explored in the field of polymer science for the synthesis of polymer chains bearing coumarin motifs due to their high versatility, leading to tailor made polymeric materials.9–11 Indeed, the relevance of this chromophore in the polyurethane sector has recently been reviewed.12 Several works have focused on exploiting the photochemical characteristics of coumarin for different applications, for instance Velencoso et al.13 have synthesized a rigid polyurethane foam with a fluorescent soft segment from the insertion of a coumarin ring through a click reaction. In addition, these authors determined how the spatial conformation of the polyurethane seemed to be markedly affected by the coumarin moiety introduced into the polymer backbone. Later, Naeem et al.14 used coumarin 6 (C6) dye as fluorescent probe for monitoring drug entrapping and delivery efficiency of enzyme sensitive azo-polyurethane nanoparticles. This attractive dye is frequently used as fluorescent hydrophobic drug model to trace drug delivery systems via fluorescence spectroscopy.15 In parallel, the coumarin units have been previously introduced into the polyurethane chains to obtain polymers with better performance and advanced properties in the optical sector. As for example, the insertion of coumarin 102 dye as an effective UV light absorber for intraocular lenses was studied, where 0.1% was necessary to fully absorb irradiation at 450 nm for effective protection.16
In addition, the interesting photochemical character of coumarin has allowed improvements in the mechanical properties of polyurethanes. Its insertion into the polymer matrix has allowed the design of self-healing materials, taking advantage of the reversibility of the photo-dimerization reaction. Reversible photodimerization of the coumarin group on the fractured surface led to light-induced healing and partial recovery of the initial mechanical performance. However, the decay of the photochemical reversibility of coumarin through the cycles was previously devoted, which reduces the useful life of these polymers for certain applications.17–19 Meanwhile, some efforts focused on the design of new functional coumarin monomers capable of grafting into the soft or hard segment.20–22 For the incorporation of these monomers into the soft segment based on PCL, side reactions were detected that led to the exclusion of the coumarin ring from this segment.23 Despite the proposed alternatives, there is no systematic study comparing the influence exerted by coumarin when it is within the hard or soft segment.
In the present work, we innovatively introduce new coumarin units into hard and soft domains of segmented polyurethane coatings by partially replacing the classical components of PU coatings with functionalized amidocoumarin-diol, for the purpose of affording polyurethanes with improved stiffness and toughness through UV-irradiation. To this end, two polyurethane families functionalized with coumarin have been synthesized, in one family this chromophore has been inserted into the hard segment and in another family this photo-reactive product is embedded within the soft one. The central objective is to understand and evaluate the effect exerted by coumarin motif when it is present in a hard or soft segment on the improvements of the mechanical properties of polymers. In this way, it could be established where the coumarin ring should be inserted to achieve the best mechanical properties. In both segments, the formation of coumarin-dimer was confirmed by UV-Vis and Raman spectroscopy. Both strengthening and toughening were evaluated by mechanical tests.
Experimental section
Materials
Anhydrous pyridine, 4-(N,N-dimethylamino)pyridine (DMPA), dichloromethane, sodium bisulphate, ε-caprolactone (ε-CL), stannous octoate SnOct2 and polycaprolactone diol of nominal molecular weight of 522 g mol−1 were supplied by Sigma Aldrich Química S.L. (Madrid, Spain) and used as received. 1,4-Butanediol (BD) was supplied by Sigma Aldrich Química S.L. and dried over magnesium sulphate, distilled and stored with nitrogen atmosphere before use. N,N-Dimethylacetamide (DMAc) was dried by distillation over commercial polymeric MDI.24 Amino-4-methylcoumarin (AMC) was provided by Carbosynth and used as received. Isophorone diisocyanate (IPDI) was a gift by Evonik Industries and used as received.Measurements
1H-NMR spectra were acquired on a Varian Unity Plus 400 instrument (Palo Alto, CA, USA) at room temperature, using deuterated chloroform and deuterated DMSO as solvents. All recorded spectra were referenced to the residual solvent signal 7.26 and 2.50 ppm for CDCl3 and DMSO-δ,6 respectively.The coumarin photoactivation processes were carried out in a cross-linking chamber supplied by Ultra-Violet Products (Upland, CA, USA) having two sets of lamps (5 × 8 W) with emission maxima at 365 nm (for crosslinking) and 254 nm (for scission).
Raman spectra were recorded on a Renishaw inVia laser micro-Raman spectrometer (Wotton-under-Edge, UK). A laser beam with wavelength of 785 nm as the excitation beam. The analysed region of each polymer film was about 1 μm2.
The mechanical properties were measured in a MTS Synergie 200 testing machine equipped with a 100 N load cell. All the test specimens analysed were cut with the dimensions established in ISO37 (type 4). The cross-head speed of 5 mm min−1 was used and the initial distance between cross-head was 10 mm. For all synthesized polymers, a minimum of 5 specimens were analysed.
Thermal transitions were obtained in a DSC on a Mettler Toledo DSC 822e calorimeter (Schwerzenbach, Switzerland). The conditions used to acquire the transitions consisted of several stages. Initially the samples were heated from 25 to 120 °C at a rate of 10 °C min−1, then cooled to −90 °C with the highest possible speed, then reheated from −90 °C to 120 °C with a rate of 10 °C min−1. All transitions were obtained in the last heating step. The glass transition temperature was determined at the midpoint of the observed transition.
Gel permeation chromatography (GPC) analyses were carried out with Styragel (300 × 7.8 mm, 5 mm nominal particle size) water columns. DMF with LiBr (0.1 w/w) was used as a solvent. Measurements were performed at 70 °C at a flow rate of 0.7 mL min−1 using a RI detector. Molecular weights of polymers were referenced to PS standards.
Synthesis of 2,2-bis(hydroxymethyl)propionamide of 7-amino-4-methyl-coumarin (DHEAMC)
The synthesis presents two steps. AMC (15.00 g, 85.50 mmol, 1.00 eq.) and DMAP (2.09 g, 17.13 mmol, 0.20 eq.) were dissolved in 150 mL anhydrous pyridine and diluted in 500 mL of dry dichloromethane. Afterwards, the previously synthesized isopropylidene-2.2-bis-(methoxy)propionic anhydride (DMPAA).22 (56.58 g, 171.3 mmol, 2.00 eq.) was added dropwise to the solution in 150 mL of dichloromethane. The reaction mixture was heated at 40 °C for 16 h. The reaction was monitored by TLC. The anhydride excess was removed by the addition of 60 mL pyridine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution. The organic phase was washed twice with sodium bisulphate (1 M) and then with brine. Finally, the organic layer was dried over anhydrous MgSO4 and afterwards, the solvent was removed under reduced pressure to obtain the product (I) (23.5 g, 83% yield). The yellowish solid was recrystallized in ethanol.
1) solution. The organic phase was washed twice with sodium bisulphate (1 M) and then with brine. Finally, the organic layer was dried over anhydrous MgSO4 and afterwards, the solvent was removed under reduced pressure to obtain the product (I) (23.5 g, 83% yield). The yellowish solid was recrystallized in ethanol.
Later the product (I) (23.5 g, 1.00 eq.) was dissolved in 300 mL of THF. Then, 150 mL of HCl (1.2 M, 4.00 eq.) were added dropwise onto coumarin solution. The mixture was stirring for 24 h. When the reaction was completed, the organic solvent was evaporated and the white solid DHEAMC was extensively washed with distilled water and finally dried (yield 19.6 g, 96%).
Synthesis of polycaprolactone-diols bearing coumarin unit (PCLcoum)
The preparation of this polyester was carried out following the standard procedure for ring opening of ε-caprolactone. In a 50 mL round bottom flask, 7.89 g of DHEAMC (23.31 mmol, 1.00 eq.), 23.36 g of ε-caprolactone (204.66 mmol, 8.78 eq.) and SnOct2 (0.1% wt respect to the monomer weight) were added. Subsequently, the reaction mixture was heated at 120 °C for 24 h. Keeping the reaction crude still warm, a vacuum pump was connected to remove residual monomer that had not reacted for 2 h. Finally, the product obtained (PCLcoum) is a white solid and the molecular weight was determined by 1H-NMR. The protocol for determining the molecular weight has been previously described elsewhere.22 The molecular weight determined by NMR was 1191 g mol−1 and the weight fraction of coumarin within this polymer was 24.4%.Synthesis of polyurethanes with functionalized coumarin polycaprolactone
All synthesized polyurethanes were obtained using anhydrous DMAc as the solvent, using 5 mol% excess of the diisocyanate with respect to the total mol content of diol. For this series of polyurethanes, functionalized PCLcoum and pristine PCL522 were used in an appropriate ratio to reach a final weight fraction of coumarin of 5 or 10%.As an example, the synthesis of polyurethane with 5% by weight coumarin and 40% in hard segment is described. In a 25 mL round bottom flask, 0.989 g of PCL522 (1.89 mmol, 1.00 eq.), 0.511 g of PCLcoum (0.429 mmol, 0.23 eq.), 0.140 g of BD (1.55 mmol, 0.82 eq.) and 0.860 g of IPDI (3.87 mmol, 2.05 eq.) were dissolved in 3 mL of anhydrous DMAc. Subsequently two drops of the tin catalyst were added and the entire reaction mixture was heated at 80 °C for 3 h, followed by 24 h stirring at room temperature. The final polymer solution was poured onto a level heating plate and the solvent was slowly evaporated by heating the plate to 60 °C. Once the solvent is removed, a polymer film with a homogeneous thickness is obtained.
Synthesis of polyurethanes with DHEAMC
These polyurethanes were also synthesized using DMAc as an anhydrous solvent and using 5 mol% excess of diisocyanate. For this series of polyurethanes, the 1,4-butanediol (BD) fraction was partially replaced by DHEAMC to reach a content of 5 or 10% by weight of coumarin in the final polyurethane. In addition, pristine PCL522-diol was used as a soft segment. The same experimental conditions described in the previous section were used to synthesize these polyurethanes.As an example, the synthesis of polyurethane bearing 5% by weight coumarin and 59% in hard segment is detailed. In a 25 mL round bottom flask, 1.025 g of PCL522 (1.96 mmol, 1.00 eq.), 0.250 g of DHEAMC (0.85 mmol, 0.43 eq.), 0.172 g of BD (1.91 mmol, 0.97 eq.) and 1.105 g of IPDI (4.97 mmol, 2.52 eq.) were dissolved in 3 mL of anhydrous DMAc. Subsequently two drops of the SnOct2 were added and the entire reaction mixture was heated at 80 °C for 3 h, followed by 24 h stirring at room temperature. The final polymer solution was poured onto a level heating plate and the solvent was slowly evaporated by heating the plate to 60 °C. Once the solvent is removed, a polymer film with a homogeneous thickness is obtained.
Results and discussion
Synthesis of amidocoumarin-diol (DHEAMC)
The synthetic route designed for the preparation of coumarin derivative (DHEAMC) is depicted in Scheme 1. This synthesis follows the same steps described in our previous work, except the last step of deprotection process.23 In the first stage, the coupling between 7-amino-4-methylcoumarin (AMC) and the anhydride DMPAA occurs leading to the intermediate amide (I), which was used directly for the next stage without isolation process. A complete NMR characterization of this intermediate compound is shown in Fig. S1.† Subsequently, the elimination reaction of acetonide protecting group was carried out using a diluted solution of HCl (1.2 M). In our previous work, it was necessary to use Dowex® exchange resin, due to the lability of the ester group of the product. It is well-known that the amide groups are more stable than their ester counterparts, therefore, the use of inorganic acid (such as HCl) was very effective for the elimination of the protective group. In short, hydrolysis of the amide group was not detected under the conditions tested. The overall yield of the synthetic route was 88% and the purity of the DHEAMC was high enough to be used directly in the following reaction steps, without the need for additional purification techniques. The 1H-NMR and 13C-NMR data of DHEAMC is shown in Fig. S2.†Synthesis and characterization of polycaprolactone-diol bearing amidocoumarin moiety (PCLcoum)
Polycaprolactone diol functionalized with DHEAMC was prepared by ring opening polymerization of ε-caprolactone in bulk with stannous octoate as catalytic agent and using DHEAMC diol as initiator. The monomer ε-caprolactone was fed in the appropriate ratio to obtain coumarin PCL-diol of approximately 1000 g mol−1. Most commercial linear thermoplastic polyurethanes are based on macrodiols with a molecular weight range between 1000 to 2000 g mol−1 which leads to good mechanical properties.25 Additionally, coumarin-containing PCL diols with a Mn around 1000 g mol−1 could avoid the crystallization of soft segment into the polyurethane matrix, leading to a transparent polymeric film which is desirable in coating technology.As shown in Fig. 1, using DHEAMC as initiator, a complex set of peaks was obtained by 1H-NMR spectroscopy. It is well-known that ring opening polymerization (ROP) of ε-caprolactone initiated by diols leads to a mixture of disubstituted species, monosubstituted species and unreacted initiator.22–24,26,27 The chemical structure of each of these species and their content (in mol) is depicted in Fig. S3.† To determine the percent (in mol) of each species, a derivatization of PCL was carried out using trifluoroacetic anhydride as modifying agent. This fluorinated agent reacts with the free chain ends in the medium and displaces the 1H-NMR signals, allowing the identification of all species. A comparison of the 1H-NMR spectra of the polyester PCLcoum before and after derivatization have been collected in Fig. S4.† In this figure, in addition, the assignment to each species and how the signals are shifted due to the reaction with the anhydride are detailed. Based on that assignment, in Fig. 1, the proton signals corresponding to each species have been identified with letters. The pristine initiator maintains its methylol signals (–CH2OH) in the same position as the starting product, at 3.75 ppm (labelled with a). For the monosubstituted species (identified as b and b*), its methylol groups are split, at 4.51 ppm and 4.12 ppm, corresponding to the reacted methylol (–CH2O–ε-CL) and unreacted methylol (–CH2OH), respectively. Indeed, as depicted in Fig. S4,† the signal at 4.12 ppm undergoes a shift in the derivatized spectrum, demonstrating that this signal is associated with free methylol (–CH2OH) group. Finally, the disubstituted species (labelled with c*) presents its signal at 4.33 ppm, and its position remains constant after the derivatization procedure. In fact, the positions of methylene groups are consistent with previous work.24,26,27
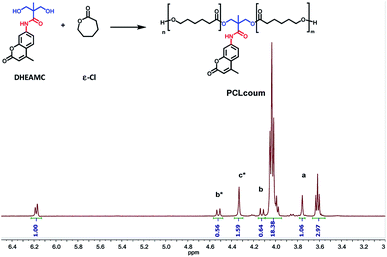 | ||
| Fig. 1 1H-NMR spectrum of polycaprolactone bearing coumarin moieties. (a) Pristine initiator, (b) monosubstituted specie and (c) disubstituted specie. | ||
From the ratio between the three areas described above, the percentage of each of the species presented in the reaction medium was easily calculated. The results were 27%, 31% and 42% for free initiator, monosubstituted and disubstituted PCL, respectively. It should be noted that there is a high molar percentage of unreacted initiator, this could be due to the fact that the initiator showed a low solubility in the polymerization medium, even at high temperatures and because the target molecular weight of PCLcoum was low. The higher the molecular weight of PCL, the lower content of free initiator.
On the other hand, taking into account the ratio of areas between 3.61 ppm and 4.03 ppm (chain end of PCL and backbone of PCL), the molecular weight of the functionalized PCL (PCLcoum), was established at 1191 g mol−1. This molecular weight was very close to theoretical, calculated by 1H-NMR from the integrals of the signals related to DHEAMC diol and caprolactone units.
It should be noted that no signals from the starting product AMC have been detected, demonstrating that the new initiator is stable and compatible with the ring-opening polymerization conditions. This finding prevented the side-reactions that led to the exclusion of the photo-reactive coumarin motif from the PCL backbone. We had previously detected this side-reaction in another coumarin-based system similar to DHEAMC and also based on the coumarin ring.23 In that other system, the lability of an ester group prevented a controlled grafting of coumarin heterocycle within the PCL chain.
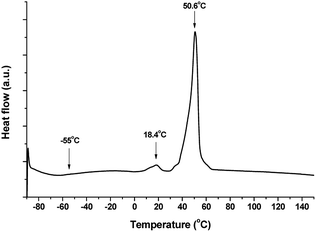
As shown in Fig. 2, the coumarin functionalized polyester was also studied using DSC. During the heating of the sample, a glass transition temperature around −55 °C was initially observed. Therefore, despite the incorporation of DHEAMC molecules into PCL, the mobility of polyester chains is not altered and the glass transition temperature is characteristic of PCL. At higher temperatures, there are two melting peaks with very different intensities, at 18.4 °C a small peak, while at 50.6 °C there is a much more intense peak. The small peak at low temperature could correspond to the fusion of small-size PCL crystals probably formed on cooling at low T, whilst the most intense peak would come from the crystalline fraction of PCL obtained at room temperature. This behaviour was expected, considering that the first run is depicted in Fig. 2, therefore the thermal history of the material has not been erased. After correcting the enthalpy (74.7 J g−1) with the percentage of caprolactone within the polyol and comparing it with the reference value (148.2 J g−1),28 the crystalline fraction of PCL within the polyol was 50.4%.
Finally, the characterization of this functional polyester was completed by determining the molecular weight by GPC. The GPC curve and the measured molecular weights are shown in Fig. S5.†
On the other hand, despite the presence of free initiator DHEAMC, the reaction conditions used have led to the controlled synthesis of PCLcoum, which is a key-element for the preparation of new photo-reactive coatings based on polyurethanes.
Synthesis and characterization of segmented polyurethanes
To assess the influence of coumarin on the features of segmented polyurethanes, two families of polyurethane were prepared following the synthetic routes described in Scheme 2.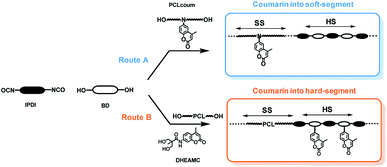 | ||
| Scheme 2 Synthetic routes for the preparation of coumarin-functionalized polyurethanes within the soft segment (route A) and hard segment (route B). | ||
In addition, the performance of these polyurethanes was assessed by paying attention to two factors, the hard-segment content (HS) and the fraction of photoreactive units. For that purpose, the coumarin content ranged between 5 and 10% by weight, whilst the hard-phase fraction varied between 40 and 60%. Following these considerations, 8 segmented polyurethanes were prepared in total. For all cases, polymers were prepared by the one-pot method in dry DMAc and SnOct2 as catalytic agent. High molecular weight polymers were obtained with good film forming properties. In Table 1, the GPC-results of the polymers are shown, polydispersity was high as expected for polycondensation polymers.
| Entry | Polyurethane | Mn (kDa) | Mw (kDa) | Ð |
|---|---|---|---|---|
| 1 | PU-5% PCLcoum-40HS | 66 | 150 | 2.3 |
| 2 | PU-5% PCLcoum-59HS | 178 | 306 | 1.7 |
| 3 | PU-10% PCLcoum-43HS | 180 | 280 | 1.5 |
| 4 | PU-10% PCLcoum-59HS | 60 | 246 | 3.9 |
| 5 | PU-5% DHEAMC-40HS | 48 | 92 | 1.9 |
| 6 | PU-5% DHEAMC-59HS | 52 | 90 | 1.8 |
| 7 | PU-10% DHEAMC-43HS | 45 | 170 | 3.7 |
| 8 | PU-10% DHEAMC-59HS | 60 | 140 | 2.4 |
Owing to the high versatility of the DHEAMC product, the insertion of photo-reactive coumarin ring into the hard and soft segment of polyurethane chains was carried out successfully. For the insertion of photo-active moiety into the soft segment (route A), the PCLcoum product was used (entries 1 to 4), whilst the use of DHEAMC as a chain extender leads to the functionalization of the hard segment (route B, entries 5 to 8). It is true that in the case of PCLcoum, a fraction of coumarin units are unreacted and therefore will be part of the hard segment thus the actual hard segment would be slightly higher than reflected in Table 1.
When PCLcoum was used in the formulation of the segmented polyurethane carrying coumarin into the soft-segment, the minimum coumarin content in the final polyurethane without adding pristine polyester PCL would be higher than 10% weight. Therefore, it was necessary to incorporate additionally into the formulation non-functionalized PCL-diol. To generate transparent coatings, a low molecular weight PCL diol (522 g mol−1) was selected because the soft segment crystallization within the polyurethane matrix was avoided. As for PCLcoum based polyurethanes, non-functionalized PCL diol has a fraction of unreacted initiator diol (diethylene glycol) similar to PCLcoum,24 and this initiator will be part of the hard segment thus the actual hard segment content for polyurethanes with DHEAMC as chain extender would be slightly higher than reflected in Table 1.
Other components used for the synthesis of segmented polyurethanes were the following: on the one hand as diisocyanate, an aliphatic and asymmetric diisocyanate isophorone diisocyanate (IPDI) was selected for these polyurethanes because of its high resistance to UV light and the lack of hard segment crystallization or phase segregation processes.29 Avoiding both processes, the polymer film would be transparent. This is a desired issue for polymeric coatings.20 On the other hand, the last component for these segmented polyurethanes was 1,4-butanediol (BD), which represents the most widely used chain-extender for polyurethane industry.2
For the preparation of polyurethanes bearing coumarin units within the hard segment (route B, entries 5 to 8) DHEAMC diol was used as chain extender, the rest of the components used were similar to those described above. Similarly, to ensure that the coumarin content was constant (at 5% or 10% wt), BD had also been used as chain extender. The soft segment for this polyurethane family was formed by a pristine PCL diol (PCL522) and IPDI as diisocyanate.
All the prepared polymers were completely transparent. In Fig. S6,† the photographs of the coatings are shown, comparing them with the reference systems lacking amidocoumarin moieties in their formulations.
Effect of photo-dimerization of coumarin moieties on the physical properties
Photo-dimerization of coumarin units were measured in the segmented polyurethanes bearing coumarin into the soft and hard segment. On the outer wall of a cuvette, a thin film of segmented polyurethane was deposited, using a solution in chloroform. The thickness of the deposited polymeric film was less than 2 microns, owing to thicker films led to saturated absorbance values in UV measurements. These thinner polymer films were irradiated with a set of 365 nm lamps to carry out the photo-dimerization of the coumarin moieties, while the set of 250 nm lamps allows photo-cleavage of the coumarin dimers. In Fig. 3, the UV-Vis absorption spectra for the dimerization and cleavage reaction of the polyurethanes (PU-5% PCLcoum-59HS and PU-5% DHEAMC-59HS) are depicted. Through irradiation at 365 nm, photo-dimerization took place and the peak with a maximum absorbance at 328 nm was gradually decreasing with increasing irradiation time (Fig. 3).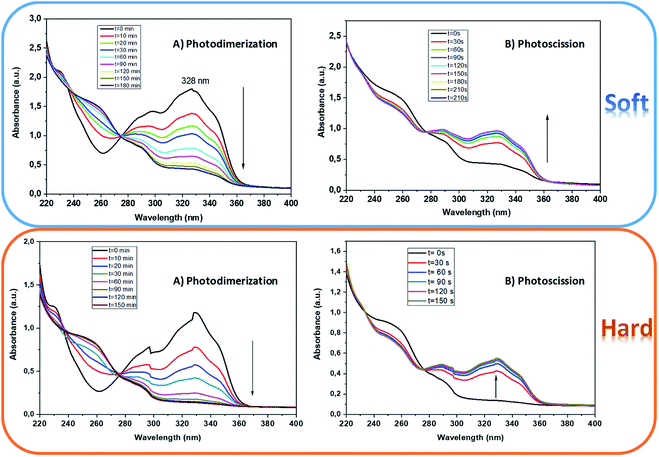 | ||
| Fig. 3 UV-Vis spectra of PU-5% PCLcoum-59HS (soft) and PU-5% DHEAMC-59HS (hard) (a) photodimerization and (b) photoscission. | ||
A decrease in the absorbance is expected for the cross-linked polymers because of the change from a coumarin moiety to a benzenoid chromophore after irradiation, that is the reduction is associated with the disappearance of the characteristic double bond of the coumarin ring and the corresponding cyclobutane ring formation ([2 + 2] cycloaddition reaction)30 On the contrary, when the 254 nm lamp set was used and illuminated on the polymer previously irradiated at 365 nm, the absorbance of the peak increases again, because the characteristic double bond of the coumarin ring is regenerated due to the scission of the photodimer (Scheme 3).
All segmented polyurethanes functionalized with coumarin presented a very similar photo-dimerization and photo-cleavage behaviour, in fact no significant differences were observed between these polymers even when the coumarin loading content varied from 5 to 10% wt.
In this study we attempt to analyse the reaction kinetic of the processes activated at 365 and 250 nm in the deposited films of polyurethanes bearing coumarins through the analysis of absorbance to provide kinetic information regarding the reaction occurring. The kinetics of the dimerization reaction showed an interesting dependence on the position of the coumarin ring within the polymer matrix. Thus, when the heterocycle is present within the soft segment, 90 minutes are required to achieve maximum photo-dimerization conversion, while photo-cleavage is optimized at 90 seconds of irradiation. However, the insertion of coumarin units into the hard segment leads to shorter irradiation times for photo-dimerization (60 minutes), and longer times for photo-cleavage (6 minutes). Despite the increase in the exposure time to 250 nm, the absorbance does not recover the initial values before irradiating at 365 nm, that is, some coumarin dimers remain completely unchanged. Our finding is consistent with differences in cleavage kinetics for photoreactive units when they are within a polymer matrix respect to when they are free.31,32
Additionally, a certain hysteresis has been detected in the photo-dimerization and photo-scission cycles, implying a decay of the photoreversibility. This fact has been previously addressed by other authors and was attributed to a balance between dimer and coumarin species and/or asymmetric cleavage of coumarin dimer which results in different by-products from pristine coumarins.22
In order to study the rest of the characterization techniques, thicker polymer coatings were used. Indeed, a polymer solution with 5% wt of solvent was deposited on a Teflon foil, and after evaporation of the solvent, the polymer thicknesses were around 180 microns. To achieve a homogeneous thickness along the polymer film, the Teflon sheet was supported on a level heating plate. Once the solvent was evaporated, the polymer films were dried thoroughly under vacuum to remove traces of solvent. For these thicker films, photo-dimerization and photo-cleavage reactions were monitored by Raman spectroscopy. Through this technique, the behaviour of dimerization kinetics of coumarin rings has been similar to that analysed by UV-spectroscopy. In Fig. 4 Raman spectra of a polyurethane with 5% by weight coumarin within the soft segment (PU-5% PCLcoum-40HS) and hard segment (PU-5% DHEAMC-40HS) respect to the irradiation time at 365 nm are shown. All Raman spectra were normalized with respect to the peak at 1442 cm−1, corresponding to the methylene groups, which remains constant throughout the photodimerization process. The band located at 1617 cm−1, coming from the double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of the heterocycle, gradually decreases its intensity with respect to the irradiation time. This is because coumarin dimers were forming. The intensity of this last band almost disappears when the irradiation times at 365 nm were 180 min. Moreover, the dimerization quantification was performed by establishing the ratio of the double bond peak at 1617 cm−1 and the reference peak 1442 cm−1.
C bond of the heterocycle, gradually decreases its intensity with respect to the irradiation time. This is because coumarin dimers were forming. The intensity of this last band almost disappears when the irradiation times at 365 nm were 180 min. Moreover, the dimerization quantification was performed by establishing the ratio of the double bond peak at 1617 cm−1 and the reference peak 1442 cm−1.
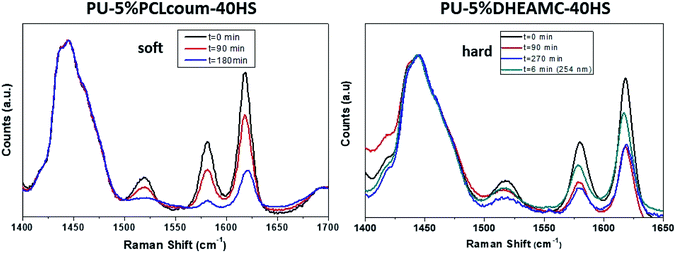 | ||
| Fig. 4 Raman spectra of polyurethanes bearing coumarin motif into the soft (a) and hard (b) segment. | ||
Table 2 collects the yields achieved for all the polymers after being irradiated for 180 min with a light of 365 nm, in addition the thicknesses of each coating are also indicated. Dimerization of coumarin within the hard segment (entries 5 to 8) is more hampered than in the soft segment (entries 1 to 4), in fact practically half of the coumarins in the hard segment remain unaltered after photoexcitation at 365 nm.
| Entry | Polyurethane | Thickness (μm) | Dimerization yield (%) |
|---|---|---|---|
| 1 | PU-5% PCLcoum-40HS | 148 ± 10 | 72.2 |
| 2 | PU-5% PCLcoum-59HS | 220 ± 13 | 76.2 |
| 3 | PU-10% PCLcoum-43HS | 209 ± 17 | 74.4 |
| 4 | PU-10% PCLcoum-59HS | 204 ± 15 | 75.2 |
| 5 | PU-5% DHEAMC-40HS | 189 ± 24 | 49.6 |
| 6 | PU-5% DHEAMC-59HS | 204 ± 11 | 51.4 |
| 7 | PU-10% DHEAMC-43HS | 156 ± 13 | 52.7 |
| 8 | PU-10% DHEAMC-59HS | 202 ± 16 | 50.1 |
Similarly, in this case of PU-5% DHEAMC-40HS (Fig. 4b), the intensity of the characteristic band of the double bond of coumarin (at 1617 cm−1) also decreased respect to the irradiation time after 90 minutes of exposure. However, after 90 min of irradiation at 365 nm, intensity variations of this band were not appreciated even when the irradiation times increased (up to 270 minutes).
Therefore, some coumarin moieties still remain un-reacted and the highest cross-linking achieved by this series of polyurethanes is lower than that of other series (functionalized polyurethanes in the soft segment). However, in principle, this should not imply an improvement in the mechanical properties of irradiated polyurethanes. This unexpected behaviour could be due to reduced mobility of coumarin within the hard segment. This mobility reduction could be mainly due to two factors. On the one hand, the fraction of intermolecular interactions (hydrogen bonds) within the hard segment is higher than in the soft segment, so the mobility of these chain fragments is more hindered. Furthermore, on the other hand, as dimerization reactions occur within the hard segment, the mobility of the chains within hard segment is more hampered, which could be an additional impediment to subsequent dimerization reactions. Therefore, in the hard segment, coumarins have a higher difficulty in diffusing and being able to react with each other correctly, so that the dimerization reaction takes place.
It should be noted that the reversibility of photochemical crosslinking of the coumarin unit is also compromised. In Fig. 4b, the Raman spectrum of the irradiated sample at 254 nm for 6 minutes has been represented in green. Under this UV-light, the coumarin dimer is split into two coumarin units, which have regenerated the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond (Scheme 3). Therefore, as the double bonds regenerate, the bands associated with these bonds should increase again. However, the intensity of this band (at 1617 cm−1) does not become similar to the initial intensity, that is, there are coumarin dimers that are not cleaved despite forcing the photo-breakage of the dimer by increasing the exposure times to 254 nm for several minutes. This fact has been previously devoted and could be related to the stereochemistry of the coumarin dimer.19 Indeed, in the solid state, the photochemical reactions are greatly affected by topological effects and surrounding crystalline environment, which acquires a special relevance in the segmented structure of polyurethane chains.33–36 The rest of polyurethanes, bearing higher content of coumarin (10% wt) and higher content of hard segment, presented behaviour similar to that described above, only the intensity relationship between the reference band and those associated with the coumarin double bond varied.
C double bond (Scheme 3). Therefore, as the double bonds regenerate, the bands associated with these bonds should increase again. However, the intensity of this band (at 1617 cm−1) does not become similar to the initial intensity, that is, there are coumarin dimers that are not cleaved despite forcing the photo-breakage of the dimer by increasing the exposure times to 254 nm for several minutes. This fact has been previously devoted and could be related to the stereochemistry of the coumarin dimer.19 Indeed, in the solid state, the photochemical reactions are greatly affected by topological effects and surrounding crystalline environment, which acquires a special relevance in the segmented structure of polyurethane chains.33–36 The rest of polyurethanes, bearing higher content of coumarin (10% wt) and higher content of hard segment, presented behaviour similar to that described above, only the intensity relationship between the reference band and those associated with the coumarin double bond varied.
Effect of photo-dimerization of coumarin moieties on mechanical properties of segmented polyurethanes
Mechanical properties of the segmented polyurethanes before and after irradiation were evaluated by static uniaxial tensile testing. For all synthetized polyurethanes, typical stress–strain curves were obtained, although they exhibited marked differences in toughness and stress before and after irradiation. As shown in Table 3, before irradiation with UV-light, polyurethanes functionalized with coumarin within the soft segment (entries 1 to 4) (except for PU-5% PCLcoum-40HS) showed good mechanical properties (max. stress), whilst polyurethanes with coumarin as chain extender (entries 5 to 8) had poor mechanical properties and were soft. This could be due to the difference in reactivity of the monomers employed during the polymerization reaction.27 When the coumarin unit is inside the polyester PCL (PCLcoum), the chain ends of this polyester would easily react with the isocyanate groups, which would lead to high molecular weights. However, when coumarin is used as a chain extender, its reactivity could be hindered (intramolecular hydrogen bonds between OH groups), thereby decreasing the molecular weight of the final polyurethane. In fact, GPC values could confirm this difference in reactivity. To justify in more detail this issue, it would be necessary to conduct more systematic studies of the reactivity of coumarin-diol under different conditions, however all this work is outside the framework of the present work.| Entry | Polyurethane | Thickness (μm) | Stress (MPa) | |
|---|---|---|---|---|
| Non-irrad. | Irrad. 365 nm | |||
| 1 | PU-5% PCLcoum-40HS | 148 ± 10 | 1.3 ± 0.2 | 5.0 ± 1.5 |
| 2 | PU-5% PCLcoum-59HS | 220 ± 13 | 16.7 ± 4.2 | 48.0 ± 7.4 |
| 3 | PU-10% PCLcoum-43HS | 209 ± 17 | 31.8 ± 3.7 | 37.5 ± 9.3 |
| 4 | PU-10% PCLcoum-59HS | 204 ± 15 | 40.2 ± 2.9 | 42.8 ± 8.8 |
| 5 | PU-5% DHEAMC-40HS | 189 ± 24 | 0.4 ± 0.3 | 77.2 ± 5.6 |
| 6 | PU-5% DHEAMC-59HS | 204 ± 11 | 1.6 ± 0.5 | 56.8 ± 3.5 |
| 7 | PU-10% DHEAMC-43HS | 156 ± 13 | 0.6 ± 0.2 | 42.4 ± 4.7 |
| 8 | PU-10% DHEAMC-59HS | 202 ± 16 | 2.9 ± 0.5 | 74.3 ± 6.4 |
Surprisingly, when polymers were irradiated at 365 nm, mechanical properties underwent significant variations. The variation on the mechanical properties cannot be due to the increase in the Tg, which was very limited. The variation of glass temperature will be addressed in detail in the next section. After the dimerization reaction activated by UV-light, the mechanical properties of the polyurethanes with DHEAMC were triggered (entries 5 to 8). As hard domains act as “reinforcing fillers” in polyurethanes, the stronger intermolecular forces among hard segments brought by the dimerization of coumarin moieties make the fillers more rigid. Then the remarkable increases in stress are fully understandable from the aspect of rigid filler reinforcement. This interesting improvement of mechanical properties is achieved even whilst maintaining practically half of unreacted coumarin units, since according to data obtained by Raman, dimerization of coumarin is more impeded into the hard segment (Fig. 4). It is also important to highlight how increasing the coumarin content does not induce an improvement in mechanical properties. Therefore, a small variation of a commercial coating formulation would be enough to achieve new coatings with advanced performance and without compromising their initial properties of the material, for example around its transparency.
On the contrary, dimerization of coumarin within the soft segment (entries 1 to 4) achieves slightly improve the mechanical properties of photo-crosslinked polyurethanes. For this series, a small fraction of unreacted coumarin in PCLcoum forms part of the hard segment and the influence of coumarin within soft and hard segment cannot be separated. However, the majority of the coumarin that is within the soft segment is more reactive to dimerization than the coumarin within the hard segment and therefore, the effect on the mechanical properties will come from the coumarin in the soft segment. For this family of polyurethanes, the maximum tension at break has been established around 48 MPa, in contrast, their hard counterpart polyurethanes reached maximum tension values of 77.2 MPa. This supposes a new limit for polyurethanes functionalized with coumarins, since previously it had been described that the maximum tension would be 60 MPa.22,23 Additionally, in Table S1,† the strain values of these polyurethanes are collected before and after irradiation. As expected, a photochemical crosslinking reduced strain for all systems. Thus, the coumarin presented in this work (DHEAMC) has an added value with respect to the coumarins described in previous articles, not only with respect to its synthetic route but also with respect to the improvements in the mechanical properties that it introduces into the coating. The variation of mechanical properties with the irradiation of two representative polymers are depicted in Fig. 5.
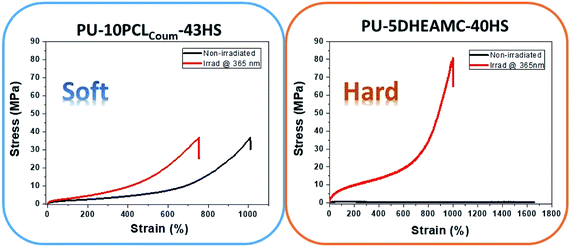 | ||
| Fig. 5 Mechanical properties of two functionalized polymers PU-10% PCLcoum-43HS (soft) and PU-5% DHEAMC-40HS (hard) before and after photo-crosslinking at 365 nm during 180 min. | ||
Effect of coumarin-dimer on soft segment dynamics
It is known that the mobility of soft segments could influence the mechanical properties of polyurethanes and reflect the intermolecular interactions of hard segments. Therefore, the soft segment dynamics was analysed by the glass transition temperature, through this measure the influence of the hard segment on the molecular dynamics of the soft segment can be evaluated. The Tg values of each polyurethane before and after irradiation have been collected in Table 4.| Entry | Polyurethane | Tg (°C) non-irrad. | Tg (°C) irrad. |
|---|---|---|---|
| 1 | PU-5% PCLcoum-40HS | 1.1 | 4.9 |
| 2 | PU-5% PCLcoum-59HS | 14.6 | 20.1 |
| 3 | PU-10% PCLcoum-43HS | 8.1 | 14.5 |
| 4 | PU-10% PCLcoum-59HS | 25.0 | 27.5 |
| 5 | PU-5% DHEAMC-40HS | −0.3 | 3.2 |
| 6 | PU-5% DHEAMC-59HS | 15.6 | 20.7 |
| 7 | PU-10% DHEAMC-43HS | −0.9 | 3.8 |
| 8 | PU-10% DHEAMC-59HS | 23.0 | 26.4 |
For all synthesized polymers, a single glass transition temperature was detected, indicating that the polymers did not show the characteristic phase separation of these materials. The absence of structural regularity of the isocyanate used (IPDI) and the presence of coumarin translate into a greater difficulty for the hard segments to segregate and crystallize. This finding is in line with the transparency shown by all polymer coatings (Fig. S6†).
After carrying out the chemical cross-linking by ultraviolet light, for all synthesized polyurethanes the Tg values increased slightly. From the values in Table 4, it can be deduced that polyurethanes with a low hard segment content (odd entries) will remain soft materials because their Tg is lower than room temperature before and after irradiation. In contrast, polyurethanes with a high hard segment content (even entries) will be relatively rigid before irradiation, but will increase their stiffness when they are cross-linked by UV light. Therefore, although hard and soft segments are directly linked by covalent bonds, dimerization of coumarin units does not exert a significant effect on the molecular dynamics of the soft segment.
Conclusions
The preparation of polyurethanes by inserting a small fraction of coumarin units is a well-defined strategy to promote the general mechanical properties of polyurethanes by dimerizing these photo-reactive units. A new amidocoumarin diol capable of being easily introduced into the hard or soft segment of the polyurethanes has been synthesized. A small fraction of this compound (5% by weight) is enough to significantly trigger the mechanical properties of these materials, after chemical crosslinking by UV-light. Especially, when this chromophore group is present in the hard segment, the improvements induced by its dimerization are much more significant and relevant over the mechanical properties of the coating, although the dimerization process is more hindered. The formation of the coumarin dimer was the predominant molecular mechanism behind the simultaneous effects of hardening, strengthening and toughness. Since the hard domains act as reinforcement fillers in polyurethanes, an increase in the stiffness of these segments produces better reinforcement and strengthening effects. The present work can provide an effective approach to comprehensively improve the mechanical properties of polyurethanes, varying slightly their initial composition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support by the Ministerio de Ciencia e Innovación through the research projects RTC-2016-4887-4, MAT2017-87204-R and RTI2018-096636-J-100 and by the Basque Government within the framework ELKARTEK through the research projects KK-2018/00108 (FRONTIERS IV) and KK-2019/00077 (FRONTIERS V). The authors also thanks to Evonik Industries for the diisocyanate employed.References
- H.-W. Engels, H.-G. Pirkl, R. Albers, R. W. Albach, J. Krause, A. Hoffmann, H. Casselmann and J. Dormish, Angew. Chem., Int. Ed., 2013, 52, 9422–9441 CrossRef CAS PubMed.
- M. Ghasemlou, F. Daver, E. P. Ivanova and B. Adhikari, Ind. Crops Prod., 2019, 142, 111841 CrossRef CAS.
- J. O. Akindoyo, M. Beg, S. Ghazali, M. R. Islam, N. Jeyaratnam and A. R. Yuvaraj, RSC Adv., 2016, 6, 114453–114482 RSC.
- M. F. Sonnenschein, Polyurethanes: science, technology, markets, and trends, John Wiley & Sons, 2014, vol. 11 Search PubMed.
- S. Pourhashem, E. Ghasemy, A. Rashidi and M. R. Vaezi, J. Coat. Technol. Res., 2019, 1–37 CAS.
- J. Pavličević, M. Špírková, A. Aroguz, M. Jovičić, D. Kojić, D. Govedarica and B. Ikonić, J. Therm. Anal. Calorim., 2019, 138, 2043–2055 Search PubMed.
- S. Roy, S. K. Srivastava, J. Pionteck and V. Mittal, Polym. Int., 2016, 65, 93–101 CrossRef CAS.
- H. Chang, M. Shi, Y. Sun and J. Jiang, Chin. J. Polym. Sci., 2015, 33, 1086–1095 CrossRef CAS.
- S. R. Trenor, A. R. Shultz, B. J. Love and T. E. Long, Chem. Rev., 2004, 104, 3059–3078 CrossRef CAS PubMed.
- G. L. Fiore, S. J. Rowan and C. Weder, Chem. Soc. Rev., 2013, 42, 7278–7288 RSC.
- I. L. Hia, V. Vahedi and P. Pasbakhsh, Polym. Rev., 2016, 56, 225–261 CrossRef CAS.
- J. M. Cuevas, R. Seoane-Rivero, R. Navarro and Á. Marcos-Fernández, Polymers, 2020, 12, 630 CrossRef CAS PubMed.
- M. M. Velencoso, A. S. B. Gonzalez, J. C. García-Martínez, M. J. Ramos, A. De Lucas and J. F. Rodriguez, Polym. Int., 2013, 62, 783–790 CrossRef CAS.
- M. Naeem, W. Kim, J. Cao, Y. Jung and J.-W. Yoo, Colloids Surf., B, 2014, 123, 271–278 CrossRef CAS PubMed.
- J. H. Finke, C. Richter, T. Gothsch, A. Kwade, S. Büttgenbach and C. C. Müller-Goymann, Eur. J. Lipid Sci. Technol., 2014, 116, 1234–1246 CrossRef CAS.
- P. Bruin, E. A. J. Meeuwsen, M. V. Van Andel, J. G. F. Worst and A. J. Pennings, Biomaterials, 1993, 14, 1089–1097 CrossRef CAS PubMed.
- J. Ling, M. Z. Rong and M. Q. Zhang, Polymer, 2012, 53, 2691–2698 CrossRef CAS.
- J. Ling, M. Z. Rong and M. Q. Zhang, J. Mater. Chem., 2011, 21, 18373–18380 RSC.
- N. Yonezawa, T. Yoshida and M. Hasegawa, J. Chem. Soc., Perkin Trans. 1, 1983, 1083–1086 RSC.
- C. Salgado, M. P. Arrieta, L. Peponi, M. Fernández-García and D. López, Macromol. Mater. Eng., 2017, 302, 1600515 CrossRef.
- R. Seoane Rivero, P. B. Solaguren, K. G. Zubieta, L. Peponi and A. Marcos-Fernández, eXPRESS Polym. Lett., 2016, 10, 84 CrossRef.
- R. Seoane Rivero, P. Bilbao Solaguren, K. G. Zubieta, A. Gonzalez-Jimenez, J. L. Valentin and A. Marcos-Fernandez, Eur. Polym. J., 2016, 76, 245–255 CrossRef CAS.
- R. Seoane Rivero, R. Navarro, P. B. Solaguren, K. G. Zubieta, J. M. Cuevas and A. Marcos-Fernández, Eur. Polym. J., 2017, 92, 263–274 CrossRef CAS.
- A. Marcos-Fernández, G. A. Abraham, J. L. Valentín and J. San Román, Polymer, 2006, 47, 785–798 CrossRef.
- D. Randall and S. Lee, The polyurethanes book, John Wiley & Sons New York, 2002 Search PubMed.
- G. A. Abraham, A. Marcos-Fernández and J. S. Román, J. Biomed. Mater. Res., Part A, 2006, 76, 729–736 CrossRef PubMed.
- J. E. Báez, Á. Marcos-Fernández, R. Lebrón-Aguilar and A. Martínez-Richa, Polymer, 2006, 47, 8420–8429 CrossRef.
- D. W. van Krevelen, Properties of polymers: their correlation with chemical structure, their numerical estimation and prediction from additive group contributions, Elsevier, 1990 Search PubMed.
- F. E. Golling, R. Pires, A. Hecking, J. Weikard, F. Richter, K. Danielmeier and D. Dijkstra, Polym. Int., 2019, 68, 848–855 CrossRef CAS.
- M. Jiang, N. Paul, N. Bieniek, T. Buckup, N. Hampp and M. Motzkus, Phys. Chem. Chem. Phys., 2017, 19, 4597–4606 RSC.
- M. V. S. N. Maddipatla, D. Wehrung, C. Tang, W. Fan, M. O. Oyewumi, T. Miyoshi and A. Joy, Macromolecules, 2013, 46, 5133–5140 CrossRef CAS.
- Z. S. Kean, G. R. Gossweiler, T. B. Kouznetsova, G. B. Hewage and S. L. Craig, Chem. Commun., 2015, 51, 9157–9160 RSC.
- G. M. J. Schmidt, J. Chem. Soc., 1964, 2014–2021 RSC.
- K. Gnanaguru, N. Ramasubbu, K. Venkatesan and V. Ramamurthy, J. Org. Chem., 1985, 50, 2337–2346 CrossRef CAS.
- M. D. Cohen, G. M. J. Schmidt and F. I. Sonntag, J. Chem. Soc., 1964, 2000–2013 RSC.
- M. D. Cohen, Angew. Chem., Int. Ed. Engl., 1975, 14, 386–393 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06372j |
| This journal is © The Royal Society of Chemistry 2020 |