Open Access Article
Open Access ArticleThe performance of emerging materials derived from waste organism blood and saponified modified orange peel for immobilization of available Cd in soil†
Zhuoxi HuangFuab,
Zongxin Ranab,
Yinpeng Moab,
Zichen Xuab,
Wei Weiab,
Jiang Yu *ab,
Bo Laia and
Xingrun Wangc
*ab,
Bo Laia and
Xingrun Wangc
aDepartment of Environmental Science and Engineering, College of Architecture and Environment, Sichuan University, No. 24 South Section 1, Yihuan Road, Chengdu, 610065, P. R. China. E-mail: yuj@scu.edu.cn
bInstitute of New Energy and Low Carbon Technology, Sichuan University, Chengdu, 610065, P. R. China
cInstitute of Soil and Solid Waste Environment, Chinese Research Academy of Environmental Sciences, Beijing, 100012, P. R. China
First published on 9th October 2020
Abstract
Waste organism blood (WOB) and orange peel are emerging stabilization materials obtained as by-products from agricultural processes, which are quite suitable for heavy metal immobilization in soil. In this work, waste organism blood and chemically modified orange peel (SOP) were investigated as potential sorbents for immobilization of available Cd in soil. Application of 5% WOB and SOP effectively immobilized cadmium (Cd) with an associated regulation of soil pH, among which the pH of acidic soil increased most significantly. While the application of 3% SOP alone stabilized almost the same amount of available Cd compared to WOB, it caused the highest stabilization rate of 58.85% when applied at 5%. By contrast, SOP combined with WOB (the mass ratio of the material is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at a 5% addition rate stabilized the available Cd in soils remarkably, with a stabilization rate of 57.74%. This study revealed that the soil particles after stabilization have a more compact and flaky structure, and the SOP and WOB had a particular pore structure, which was helpful for the adsorption of available Cd in soil. This study put forward new insights into the potential effects of Cd immobilization in contaminated soil by newly emerging stabilization biomass materials (WOB and SOP).
1) at a 5% addition rate stabilized the available Cd in soils remarkably, with a stabilization rate of 57.74%. This study revealed that the soil particles after stabilization have a more compact and flaky structure, and the SOP and WOB had a particular pore structure, which was helpful for the adsorption of available Cd in soil. This study put forward new insights into the potential effects of Cd immobilization in contaminated soil by newly emerging stabilization biomass materials (WOB and SOP).
1. Introduction
Cadmium (Cd) is highly toxic to plants, animals, and human beings as a typical heavy metal element, making soil ecology extremely risky.1 In China, 7% of the soil has excessive levels of cadmium and 0.5% of soil is severely polluted with cadmium, which typically results from anthropogenic sources, such as the application of phosphate fertilizers and pesticides, sewage irrigation, and industrial and mining waste emissions.2,3 Unlike organic pollutants, Cd in contaminated soil does not undergo microorganic digestion or chemical decomposition; instead, it persists in contaminated soil for a long time.Unlike organic pollutants, cadmium cannot be digested by microorganisms or chemically degraded, result in it stably persisting in soil for a long time with strong bioaccumulation, persistent toxicity, carcinogenic effect and high mobility, posing a persistent threat to human health through the food chain. To date, several remediation strategies for Cd-contaminated soils have been investigated in both field and controlled environment experiments, including phytoremediation and physical, chemical and microbial remediation,4,5 aiming to address the challenge of effective control of cadmium in the soil ecosystem.
Among these remediation methods, in situ immobilization has been shown to effectively reduce Cd migration and bioavailability with low interference with soil and the suitability for large-scale remediation of contaminated soils,6 therefore, it is widely used in the remediation of Cd-contaminated soils.7 It is difficult to find efficient and economical adsorbent materials for Cd remediation, which is the main factor restricting the development of chemical immobilization methods. At present, the most commonly utilized stabilization materials to implement soil Cd immobilization include alkaline fixatives, such as lime and fly ash, phosphate fixatives, such as hydroxyapatite and calcium dihydrogen phosphate, and natural or synthetic mineral passivators, such as zeolite, bentonite and sepiolite,8–10 which are still deficient in surface adsorption and cationic exchange potential.
Recently, the use of organic amendments for in situ stabilization of heavy metals has shown considerable economic potential in practical applications.11 Large amounts of organic waste are routinely produced by the livestock and poultry industries, which urgently need proper treatment to meet environmental regulations.11 Moreover, organic waste from the fruit industry demands rational utilization to prevent resource loss on the disposal of the discarded peel. Agricultural and forestry wastes such as orange peel and banana peel have been successfully used for the treatment of wastewater containing heavy metals and they have many advantages, including comprehensive sources and low cost.12 However, currently, remediation of heavy metal contaminated soil with peel has rarely been reported. As a major global orange producer, China's total citrus output reached 38.168 million tons in 2017, according to official data from the national bureau of statistics. Orange peel, which is considered a by-product of the orange industry, unlike commercially available inorganic chemical passivators, has abundant plant-derived biomolecules attached to the surface of the material. Moreover, the physical and chemical properties of soil, such as pH, organic matter and mineral composition, are important factors affecting the dissolution and adsorption of heavy metals, and are also different from those of wastewater13,14. Previous studies have shown that modifying orange peel by saponification using Ca(OH)2/Ca(OH)2 can increase the number of active functional groups and enhance the adsorption of Co2+, Ni2+, Zn2+, and Cd2+.15 It is acknowledged that there are comparable studies on the immobilization of Cd in soils with different physicochemical properties, especially under different pH conditions. Using modified orange peel to remediate Cd-contaminated soil not only conforms to the sustainable development concept but also provides an innovative idea that is beneficial to the remediation of heavy metals in contaminated soil. Furthermore, animal-derived blood meal, a natural/industrial by-products and a potential new sustainable soil amendment, is available in large volume and is typically inexpensive. At present, most slaughterhouses discard animal blood as waste, failing to use it to its full potential. Therefore, the use of animal blood not only fulfils its full potential, but also has the environmental benefits of waste recycling. Some researchers have investigated the use animal blood in studies on Cd-contaminated soil remediation.16,17 However, the mechanism of action of animal blood on cadmium was not discussed. We found that animal blood contains a large amount of organic matter and protein accounts for more than 80% of the composition after drying. The sulfhydryl and carboxyl functional groups in the protein have strong adsorption capacity for heavy metal ions.18,19 Especially for acidic soil, we speculated that the large amounts of biomolecules in WOB can activate iron-reducing bacteria, leading to the reduction of iron on the material surface and the consumption of H+, subsequently increasing the pH and thus improving the cadmium immobilization efficiency of the material.20 However, there are only a few special reports on the application of animal blood, such as treating wastewater containing cadmium.21 In this study, we prepared biochar-like compounds from animal blood using a high-temperature and long-term sterilization method that completely inactivates microorganisms without damaging the structure of the material. The treated material not only maintains the original excellent performance but also does not cause secondary pollution to the environment owing to the formation of a biochar-like structure. This agricultural waste can be used as an organic amendment for the remediation of soil contaminated with heavy metals.11
The fractions of Cd in the soil would influence the immobilization effect. Different types of immobilized materials also showed different specificity and selectivity for heavy metals.8,10,22 To date, research on immobilizing remediation for Cd-contaminated soil has mostly investigated the effect of single passivation. Few studies have focused on the synergistic remediation effects of multiple fixatives. In view of the extensive and complex non-point sources of Cd pollution in soils, this study proposes green and sustainable stabilization using orange peel and blood powder, which are easy to obtain, environmentally friendly and conform to the concept of “waste treatment” to mitigate the increased mobility/bio-accessibility of Cd and ameliorate the compromised soil quality. Extracted Cd concentrations and pH values were determined to explore the stabilization of Cd in polluted soils by waste orange peel/animal-derived blood meal. This study proposes a new way for the research and application of new immobilization materials in the remediation of Cd-contaminated soil.
2. Materials and methods
2.1. Stabilization materials
2.2. Soils sampling and analysis
The contaminated soil used for the stabilization experiment was obtained from the top layer (0–20 cm) in a rape field, located in Shuangliu, Chengdu City, Sichuan Province, southwest China (103.998E, 30.551N). The soil was sieved and 6 kg of the sieved fraction over 0.149 mm was evenly sprayed with 300 mL 0.2 g L−1 CdCl2, then the contaminated soil was put in a plastic pan and aged in a cool shelter for 2 weeks. Soil pH and electrical conductivity (EC) were determined in deionized water using soil![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ratios (v/v) of 1
water ratios (v/v) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 and 1
2.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, respectively. The soil was adequately turned over to maintain uniform ventilation during the period. 0.4 M K2Cr2O7–sulfuric acid solution was used to digest the soil samples and the ferrous sulfate titration method was used to determine the organic matter content. The total cadmium content was analyzed by microwave digestion with nitric acid, perchloric acid and hydrofluoric acid.15 The available Cd was determined as diethylenetriaminepentaacetic acid (DTPA) extractable Cd (EX-Cd) with a flame atomic absorption spectrophotometer.23 The Cd species in soil were analyzed by BCR method.24 Some properties of the soil and the concentrations of Cd in the soil samples as well as the national standard for Cd are listed in Table A (ESI†), and the results were as follows: pH: 5.53; EC: 155.8 μs kg−1; organic matter (OM): 34.579 g kg−1; total Cd: 0.175 mg kg−1; extracted-Cd (EX-Cd): 0.072 mg kg−1.
5, respectively. The soil was adequately turned over to maintain uniform ventilation during the period. 0.4 M K2Cr2O7–sulfuric acid solution was used to digest the soil samples and the ferrous sulfate titration method was used to determine the organic matter content. The total cadmium content was analyzed by microwave digestion with nitric acid, perchloric acid and hydrofluoric acid.15 The available Cd was determined as diethylenetriaminepentaacetic acid (DTPA) extractable Cd (EX-Cd) with a flame atomic absorption spectrophotometer.23 The Cd species in soil were analyzed by BCR method.24 Some properties of the soil and the concentrations of Cd in the soil samples as well as the national standard for Cd are listed in Table A (ESI†), and the results were as follows: pH: 5.53; EC: 155.8 μs kg−1; organic matter (OM): 34.579 g kg−1; total Cd: 0.175 mg kg−1; extracted-Cd (EX-Cd): 0.072 mg kg−1.
2.3. Batch stabilization experiments
Stabilization test: the soil pH was adjusted to be acidic, neutral or alkaline by adding HCl or NaOH, depending on the initial pH. Soil culture experiments were carried out in contaminated soil by adding different ratios of stabilization materials. Two factors affecting the stabilization were controlled: soil pH and the addition rate of the stabilization material. Soil with no added stabilization material was used as the control group. Thirty treatments were set up for each material according to the influencing factors, as shown in Table 1. Each treatment was performed in triplicate. Soil samples were placed in beakers, sealed with cling film to maintain air permeability, cultured at room temperature of 25 ± 2 °C, and supplemented with deionized water regularly every other day to maintain a constant water content. After 14 days of cultivation, samples were taken to measure the pH and available Cd content in the soil.| Stabilization material | pH | Ratio (w/w) |
|---|---|---|
| SOP | 4 | 0%, 1%, 3%, 5% |
| 7 | 0%, 1%, 3%, 5% | |
| 10 | 0%, 1%, 3%, 5% | |
| WOB | 4 | 0%, 1%, 3%, 5% |
| 7 | 0%, 1%, 3%, 5% | |
| 10 | 0%, 1%, 3%, 5% | |
| SOP + WOB | 4 | 0%, 2.5% + 2.5% (5%) |
| 7 | 0%, 2.5% + 2.5% (5%) | |
| 10 | 0%, 2.5% + 2.5% (5%) |
3. Results and discussion
3.1. Characterization of the stabilization materials
The surface morphology of the SOP and WOB was characterized by scanning electron microscope (SEM, shown in Fig. 1). The SOP particles were observed to have a folded structure and a rough surface. It was speculated that in the process of alkali modification, apart from the neutralization of the excess components, such as pectin, the negative charge of the generated carboxylic acid group could destroy the fiber structure, thus turning the orange peel into a rough porous structure, which was more efficient for the adsorption of EX-Cd.25 SEM analysis of the WOB structure showed that the surface of WOB was less rough, with a more spherical and smooth structure than that seen on the SOP. However, the microscopic characterization of the surface fracture (Fig. 1(b2)) shows that the WOB particles contained a large number of dense pores, which could provide specific adsorption sites for the available Cd.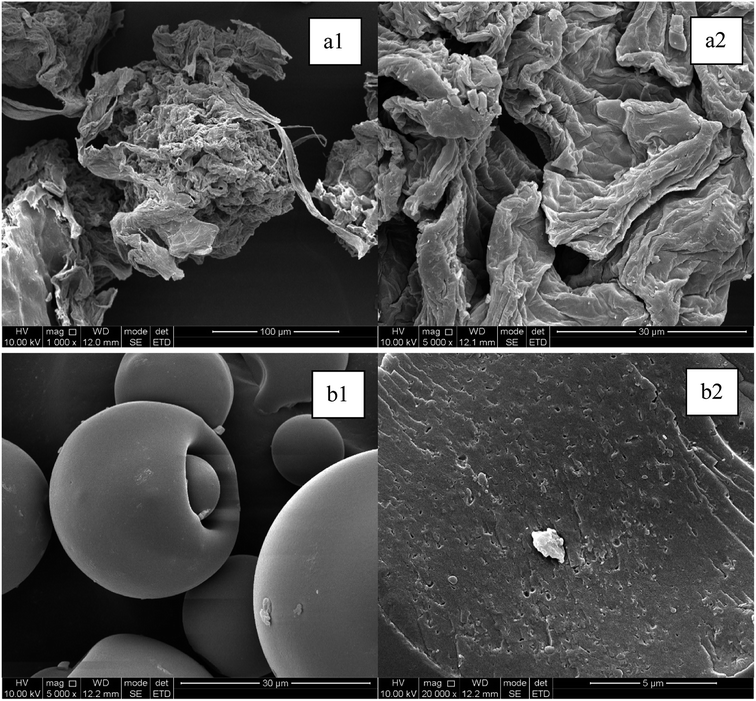 | ||
| Fig. 1 SEM images of two replicate areas of SOP ((a1) 1000 times, (a2) 5000 times) and WOB ((b1) 5000 times, (b2) 1000 times). | ||
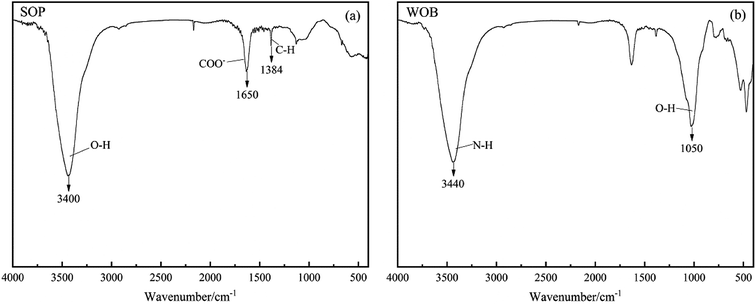
The functional groups on the SOP and WOB surfaces were determined by Fourier transform infrared spectroscopy (FTIR) characteristic peaks (Fig. 2). The band at 3640–3510 cm−1 was attributed to the stretching vibration of C–H.15 The band at 1740–1725 cm−1 was assigned to the COO and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations. The band at 1130–1000 cm−1 was attributed to the vibration of C–O–C, C–O–P and O–H of polysaccharides. The intense absorption peak observed at 3400 cm−1 exactly correlated with the O–H stretching from the cellulose, pectin, lignin, and hemicellulose of SOP (Fig. 2(a)), which was similar to the results of Feng.25 The peak observed at 1650 cm−1 could be attributed to the carboxylate ion (COO−) stretching band of pectin.26 The narrow peak at 1384 cm−1 could be due to the alkane (C–H) bending vibration. Deformation vibrations of methylene and methoxy groups in the plane could lead to the faint vibrations at 1400–1500 cm−1. Besides, the degradation of the cell walls, proteins and other organic components by alkaline pretreatment and further acid oxidation can form new functional groups for biomass, such as the peak at 1384 cm−1 in this study.15 Fig. 2(b) shows the FTIR spectrum for the WOB. The adsorption peak at 3440 cm−1 observed in the spectrum was attributed to the N–H (amide) stretching vibration. Compared to the SOP, the WOB showed a significant peak at 1050 cm−1, which was due to the O–H vibrations.27 Therefore, it could be speculated that there were plenty of protein hydrolysates in the WOB. Furthermore, the vibration frequencies of the external plane of C–H were between 650 and 1000 cm−1.
O stretching vibrations. The band at 1130–1000 cm−1 was attributed to the vibration of C–O–C, C–O–P and O–H of polysaccharides. The intense absorption peak observed at 3400 cm−1 exactly correlated with the O–H stretching from the cellulose, pectin, lignin, and hemicellulose of SOP (Fig. 2(a)), which was similar to the results of Feng.25 The peak observed at 1650 cm−1 could be attributed to the carboxylate ion (COO−) stretching band of pectin.26 The narrow peak at 1384 cm−1 could be due to the alkane (C–H) bending vibration. Deformation vibrations of methylene and methoxy groups in the plane could lead to the faint vibrations at 1400–1500 cm−1. Besides, the degradation of the cell walls, proteins and other organic components by alkaline pretreatment and further acid oxidation can form new functional groups for biomass, such as the peak at 1384 cm−1 in this study.15 Fig. 2(b) shows the FTIR spectrum for the WOB. The adsorption peak at 3440 cm−1 observed in the spectrum was attributed to the N–H (amide) stretching vibration. Compared to the SOP, the WOB showed a significant peak at 1050 cm−1, which was due to the O–H vibrations.27 Therefore, it could be speculated that there were plenty of protein hydrolysates in the WOB. Furthermore, the vibration frequencies of the external plane of C–H were between 650 and 1000 cm−1.
3.2. Effects of amendments on soil pH
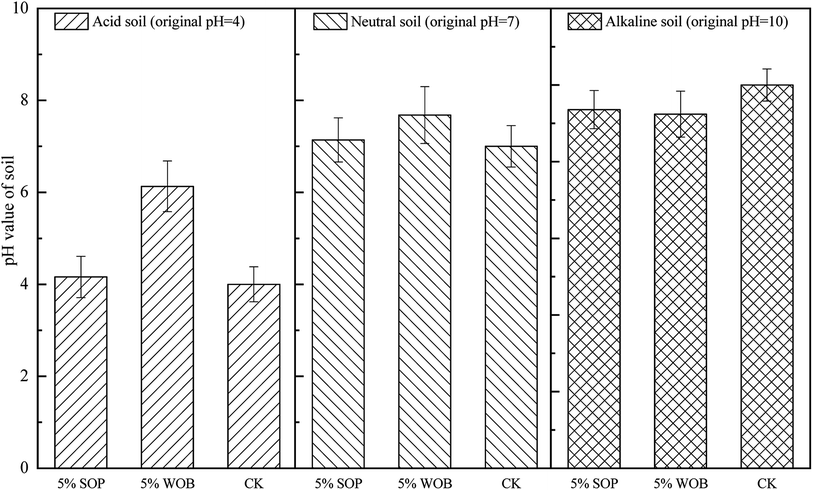
Soil pH is one of the most important factors affecting soil bioavailability.27 Fig. 3 depicts the effects of SOP/WOB addition on soil pH with different initial soil pH conditions, which showed an obvious influence on soil pH after 14 days of culture aging. Acid or alkali was added to all control treatments to regulate the initial soil pH (CK). The addition of 5% WOB led to a significant increase in the pH from 4 to approximately 6.13. The application of both SOP and WOB slightly increased the soil pH in neutral soil (initial soil pH = 7). The pH levels in neutral soil were not significantly affected by the two stabilization materials (5% SOP and 5% WOB), with a lower effect on soil pH (from 7 to approximately 7.14 and 7.75, respectively) as compared with that of the acid soil. The pH of alkaline soil (initial soil pH = 10) decreased by 0.64 and 0.61 units with the addition of 5% SOP or 5% WOB, respectively. For acid soil, it was speculated that the two immobilization materials increase the pH value, especially WOB, which activates iron-reducing bacteria by large amounts of biomolecules, leading to the reduction of iron on the material surface and the consumption of H+.20 Additionally, in general, the addition of SOP along with WOB to soil might be accompanied by organic matter, which might also influence soil pH; that is, the decarboxylation of organic anions consumed excess H+ in acidic soil, thus leading to a pH increase,28 which was also speculated as an appropriate reason for no significant change to the soil pH in neutral and alkaline soil.Previous research29 has studied the effects of combined stabilization materials on Cd stabilization in soil, showing a negative correlation between soil pH and available Cd content (r = −0.62). The availability of Cd decreased naturally with increasing soil pH. The dissolvability of Cd was increased, resulting in an increased risk of Cd entering the food chain in soils with high acidity. WOB, as a kind of organic material, contains a large amount of organic matter and organic functional groups, which could enhance the absorption of H+ and Al3+, thus decreasing the soil acidity. Besides, the mineralization of organic materials could lead to decarboxylation of organic anions and the release of alkaline substances, which also increases the pH value of acidic soils.28,30 Compared with WOB, a non-significant increase of soil pH by SOP at the 5% addition rate may be attributed to the orange peel being neutral after sodium hydroxide saponification and oxalic acid treatment. Moreover, it was speculated that the number of carboxyl functional groups and sodium alcoholate in the SOP increased, which balanced the adsorption of H+ and Al3+ by other macromolecular functional groups and lignocellulose to a certain extent.
3.3. Effects of individual stabilization materials on available Cd content in soil
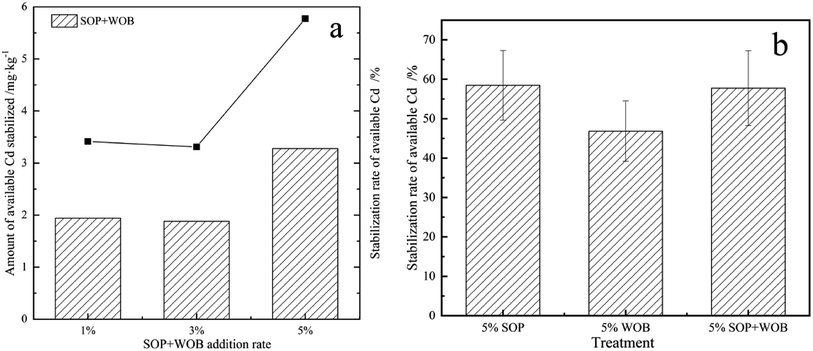
The exchangeable and carbonate bound fractions of Cd from all five combined forms could be considered as the bioavailable state in soil as a result of being efficiently absorbed by plants.31 In order to evaluate the influence of SOP and WOB on Cd bioavailability, the initial concentration of DTPA-extractable Cd was determined (5.68 mg kg−1) for stabilization by the addition of SOP and WOB for the contact time of 14 d. The EX-Cd was examined and the influence of the initial additional amount of stabilization material under different soil pH conditions was compared. The overall concentrations of available Cd were finally decreased after the incubation times, as shown in Fig. 4(a). With the increase of the level of SOP addition from 1% to 5% in acidic soil (initial soil pH = 4), the stabilization of available Cd increased from 2 mg kg−1 to 3.32 mg kg−1, showing that the stabilization rate of available Cd increased from 35.21% to 58.45%, with a continuing upward trend. A similar conclusion was obtained for neutral soil (initial soil pH = 7) and alkaline soil. Adding 1%, 3%, and 5% SOP to neutral soil, the stabilization of available Cd was 1.8–3.04 mg kg−1, respectively, and the corresponding available Cd stabilization rate was 32.73%, 48.73%, and 55.27%, respectively. Dosing 1%, 3%, and 5% SOP to alkaline soil, the available Cd stabilization rate was 50.62%, 54.73%, and 58.85%, respectively with a 2.46–2.86 mg kg−1 range of stabilized content. The reduction rate of available Cd reached a maximum of 58.85% at the 5% SOP addition rate. These results indicate that SOP and WOB could effectively absorb the available Cd in contaminated soil, resulting in inhibition of Cd mobilization.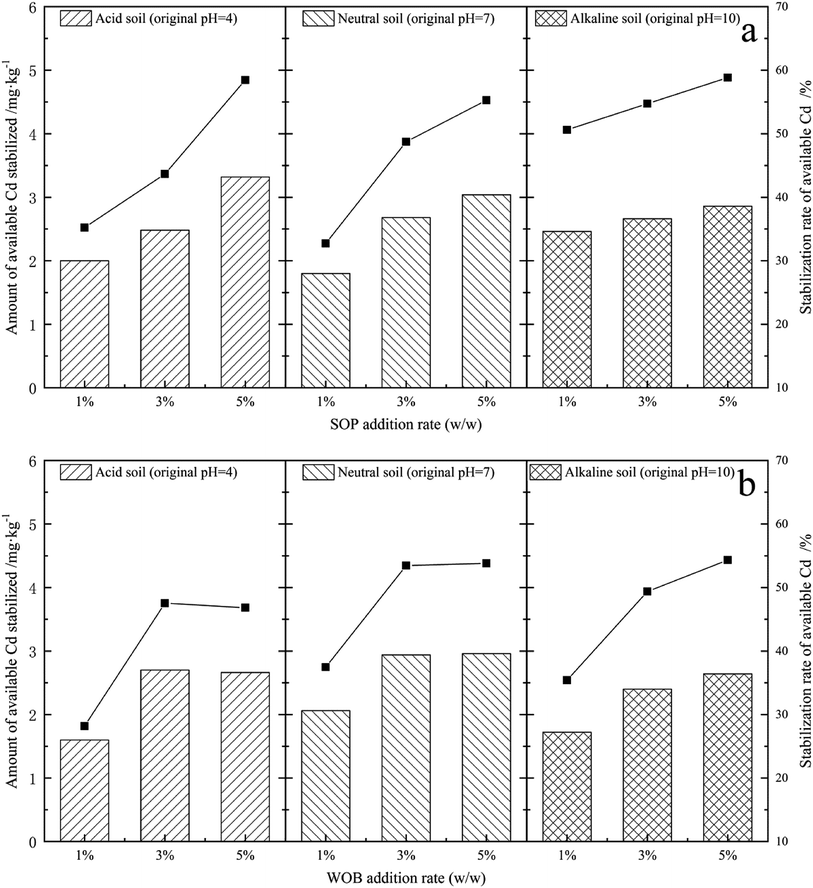 | ||
| Fig. 4 Stabilization rate and amount of stabilized available Cd after the application of SOP (a) and WOB (b) (addition rate: 1%, 3%, 5%) in soils with different pH values. | ||
Comparing the Cd stabilization effect of SOP in soil with different pH, SOP exhibited stronger binding of available Cd in alkaline soil. The decreasing rate of available Cd in acidic soil increased significantly as the addition rate increased from 1% to 5%. Meanwhile, the rate of increase was higher than that of neutral and alkaline soils. The available Cd stabilization in neutral soils approached saturation when the addition of stabilization material reached 5%. The Cd adsorption behavior of SOP was mainly based on the material's large specific surface area, porosity, and high levels of carboxyl and sodium alcoholate groups. Carboxyl groups (–COOH) were suggested to correspond with the binding of metal ions in some way.25 The cellulose, pectin, hemicellulose, and other main components in orange peel contain methyl ester, which is not significantly combined with heavy metals.25 However, these methyl esters could be modified to carboxylate ligands by NaOH and further increase the number of carboxyl functional groups on the surface of the adsorbent by saponifying the carboxyl groups of methyl ester on pectin molecules. The hydrolysis of methyl esters proceeds as follows:
In this study, 20% isopropyl alcohol with 0.1 M NaOH was utilized to remove pigment and hemicellulose from orange peel, and Na+ was introduced, which could enhance the adsorption of available Cd by the material.15 Finally, 0.6 M acid was applied to modify the material by saponification cross-linking, during which process a carboxyl group was introduced to the cellulose,15 thus increasing the binding with positively charged metal ions.32 A previous study proposed that when the pH value of the system increased from 2.5 to 4.5, the rate of adsorption of Cd2+ by SOP increased sharply.33 However, in this study, the adsorption rate remained almost unchanged with the continually increasing pH value. It was presumed that the negative charge on the surface of the orange peel increased with the increase of pH, which was conducive to Cd2+ approaching and adsorbing on the active site. However, metal ions would precipitate at pH values higher than 5.5 so the adsorption of metal ions by SOP could not be performed well, which is due to the high concentration of hydroxide ions in the solution.34 Therefore, with the increase of environmental pH, the adsorption capacity of SOP for metal ions tended to be stable, which verified that there was no significant difference in the stabilization capacity of available Cd in soils of different pH when adding the same dose of SOP (neutral and alkaline conditions). F. Ningchuan et al.33 further analyzed the adsorption mechanism of SOP by infrared spectroscopy. The results showed that the hydroxyl polymerization degree of SOP decreased after adsorption of heavy metals, indicating that hydroxyl groups participated in the adsorption of Cd. This was speculated to be one of the reasons for the effective stabilization of available soil Cd.
By analyzing the residual available Cd content in soil with different pH, the stabilization amount and rate of available Cd by the single application of WOB under different treatments are summarized in Fig. 4(b). The available Cd stabilization amount in soil reached 1.6 mg kg−1, 2.06 mg kg−1 and 1.72 mg kg−1 with stabilization rates of 28.17%, 37.45%, and 35.39% by adding 1% WOB in acidic soil (original pH = 4), neutral soil (original pH = 7) and alkaline soil (original pH = 10), respectively. Moreover, the stabilization rate of available Cd increased significantly, reaching 47.53%, 53.45%, and 49.38%, respectively, as the dose of stabilization material increased from 1% to 3%. On further increasing the addition rate of WOB to 5%, the stabilization rate of available Cd in acidic and neutral soils remained stable, while that in alkaline soils increased slightly. Thus showed that stabilization material addition at a rate of 3% achieved saturated stabilization of available Cd in soil. WOB is mainly composed of red blood cells and other related components, such as serum protein, sodium, and potassium particles, and contains more than 80% protein after drying. We speculated that WOB has an abundance of biomolecules that might release organic matter into the soil, in which decarboxylation of organic anions consumes H+, further improving soil pH.28 Moreover, the DOC in WOB-treated soil was expected to increase since WOB contains large amounts of biomolecules that originate from the animal blood, which are capable of reducing the migration and transformation of cadmium in the soil. Since organic carbon with abundant ligands has the ability to capture Cd,35 the stabilization capacity of WOB significantly improved as the dose increased. Clay minerals in soil colloids, such as montmorillonite and kaolin, are negatively charged and show significant adsorption capacity for amphoteric colloidal proteins. The carboxyl groups in the WOB protein have strong particle binding ability, especially for low concentrations of Cd.21 In this study, relative to the WOB treatments, under acid soil conditions, the concentration of available Cd decreased significantly by 28.17% to 47.53%. In contrast, in neutral soil treatments, the available Cd was ultimately greatly stabilized and exhibited a significantly higher immobilizing rate than that of acid soil treatments. In comparison, no significant difference was found between neutral and alkaline soils (Fig. 4(a)).
The presence of OH− leads to the precipitation of Cd.9,36 In general, a high pH will cause the solubility of most heavy metals in soils to decrease, leading to the reduction of the biological availability of heavy metals,37 but conversely leads to poor adsorption by SOP.34 Therefore, the adsorption performance under alkaline conditions was limited to some extent in this study.
In conclusion, the available Cd decreased markedly with SOP or WOB treatment under different soil pH conditions, with each material showing different stabilization capacities on soil available Cd owing to their different stabilization mechanisms. Generally, except for applying WOB in neutral soil, a single application of SOP had the best effect on decreasing the content of available Cd in soils with different pH. Among the two stabilization materials, SOP showed a higher stabilization rate of available Cd at the 5% addition rate. The stabilization rate of available Cd in soil tended to be stable with a 3% addition rate of WOB.
3.4. Effects of combined stabilization materials on available Cd content in soil
Based on the above results, the combined application of the two materials for stabilization of available Cd was explored by the addition of the stabilization materials to the soil at different rates without adjusting the pH (pH = 5.76), and the stabilization effect was compared after 14 days of soil culture, as shown in Fig. 5(a). The results showed that the combined application of SOP and WOB had a significant effect on the removal of available soil Cd. With the increase of the stabilization material dose from 1% to 5%, the reduction rate of available soil Cd increased from 34.15% to 57.74%. The maximum reduction rate was achieved with the stabilization material addition rate of 5%. It can be seen from Fig. 5(a) that as the material dosage increases from 1% to 5%, there is no significant difference between the immobilization effect of 1% and 3%, and the immobilization effect is the best when the material dosage is 5%. When the material dosage was 5%, the reduction rate of available cadmium by the combined stabilization materials was slightly lower than that for SOP alone, but it was significantly higher than that for WOB alone (Fig. 5(b)). Particularly for acidic soil, the combination of materials gives a better immobilization effect.The production cost for SOP was significantly higher than that for WOB. Thus, the combination of WOB and SOP was suggested to maintain the treatment effect and reduce the cost.
3.5. SEM analysis of the Cd-contaminated with soil-stabilization material
Through SEM morphology characterization of contaminated soil before applying 5% SOP and WOB (Fig. 6(a)), it can be observed that the original contaminated soil had a fine granular micro-structure, and the soil characteristics are sparse, irregular, less pores, and a small number of polygonal shallow pores. The overall distribution of the fine granular fraction was uniform, causing high particle fluidity, which posed a risk to the environment.38 After the stabilization with 5% SOP and WOB, the surface of the soil granules had a laminar structure, showing regular clusters and sheet-like granules, and the structure was compact (Fig. 6(b)). The observed structure and morphology are the surface characteristics of the contaminated soil particles agglomerated with the organic matter in the SOP and WOB. Carboxyl and sodium alcoholate have strong particle binding ability so they cross-link with Cd in the soil, making it difficult to leach. | ||
Fig. 6 SEM images of the contaminated soil before ((a) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times) and after ((b) 10 000 times) and after ((b) 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times) adding 5% SOP + WOB. 000 times) adding 5% SOP + WOB. | ||
4. Conclusions
SOP and WOB were effectively applied for the immobilization of Cd in three soils with different pH values. The soil pH became more neutral with the application of the stabilization material. In particular, the application of 5% WOB alone exhibited the most substantial effect on acid soil pH, while the application of SOP alone showed little impact. However, both stabilization materials had a non-significant effect on the pH of alkaline soil. The observed changes in soil pH were speculated to be attributed to the influence of biomolecules on Fe reduction on the surface of the stabilization materials. In addition to the soil pH variation being affected by the incorporation of organic matter, the carboxyl functional groups and sodium alcoholate are also affected in SOP, especially. In the comparison to SOP and WOB for the immobilization of available Cd, we concluded that the addition of SOP alone exhibited different effects on available Cd content in different pH soils, and the stabilization effect increased significantly with the increase of the stabilization material dose. Generally, the available Cd stabilization capacity of the two green and sustainable stabilization materials is SOP > WOB, which to a large extent is because of the addition of carboxyl functions of SOP but the effect of SOP on soil DOC was basically consistent with that of WOB. The combination of WOB and SOP was suggested to maintain the treatment effect and reduce the cost, which has a similar optimal available Cd immobilization capacity to SOP alone. Besides, SOP + WOB were agglomerated with the soil particles, making soil particles more compact. Thus, this study strongly suggests that SOP and WOB singly and in combination as new green and sustainable low-cost stabilization materials with ideal stabilization ability could be applied for the effective immobilization of Cd in soils with various pH levels.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Key Research and Development Program (2018YFC1802605) and the Nature Science Foundation of Sichuan Province (2017GZ0383, 2017SZ0181).References
- H. Zhang, X. Zhang, T. Li and F. Huang, Environ. Earth Sci., 2014, 71, 277–286 CrossRef CAS.
- X. Tan, Z. Wang, G. Lu, W. He, G. Wei, F. Huang, X. Xu and W. Shen, J. Hazard. Mater., 2017, 329, 299–309 CrossRef CAS.
- H. Yin, N. Tan, C. Liu, J. Wang, X. Liang, M. Qu, X. Feng, G. Qiu, W. Tan and F. Liu, Chemosphere, 2016, 161, 181–189 CrossRef CAS.
- R. Bian, S. Joseph, L. Cui, G. Pan, L. Li, X. Liu, A. Zhang, H. Rutlidge, S. Wong, C. Chia, C. Marjo, B. Gong, P. Munroe and S. Donne, J. Hazard. Mater., 2014, 272, 121–128 CrossRef CAS.
- Z. Yao, J. Li, H. Xie and C. Yu, in Seventh International Conference on Waste Management and Technology, ed. L. Jinhui and H. Hualong, 2012, vol. 16, pp. 722–729 Search PubMed.
- N. S. Bolan, D. C. Adriano, P. Duraisamy, A. Mani and K. Arulmozhiselvan, Plant Soil, 2003, 250, 83–94 CrossRef CAS.
- N. T. Basta, R. Gradwohl, K. L. Snethen and J. L. Schroder, J. Environ. Qual., 2001, 30, 1222–1230 CrossRef CAS.
- G. Guo, Q. Zhou and L. Q. Ma, Environ. Monit. Assess., 2006, 116, 513–528 CrossRef CAS.
- S.-H. Lee, J.-S. Lee, Y. J. Choi and J.-G. Kim, Chemosphere, 2009, 77, 1069–1075 CrossRef CAS.
- Y.-F. Zhou and R. J. Haynes, Crit. Rev. Environ. Sci. Technol., 2010, 40, 909–977 CrossRef CAS.
- M. A. Khan, S. Khan, A. Khan and M. Alam, Sci. Total Environ., 2017, 601, 1591–1605 CrossRef.
- M. Thirumavalavan, Y.-L. Lai, L.-C. Lin and J.-F. Lee, J. Chem. Eng. Data, 2010, 55, 1186–1192 CrossRef CAS.
- J. J. Gonzalez Costa, M. J. Reigosa, J. M. Matias and E. F. Covelo, Sci. Total Environ., 2017, 593, 508–522 CrossRef.
- U. Kraus and J. Wiegand, Sci. Total Environ., 2006, 367, 855–871 CrossRef CAS.
- X. Li, Y. Tang, X. Cao, D. Lu, F. Luo and W. Shao, Colloids Surf., A, 2008, 317, 512–521 CrossRef CAS.
- A. A. D. Cintra, M. D. Revoredo, W. J. Melo and L. T. Braz, in Sustainability of Horticultural Systems in the 21st Century, ed. L. Bertschinger and J. D. Anderson, 2004, pp. 259–265, DOI:10.17660/ActaHortic.2004.638.34.
- P. Du, Y. Xie, S. Wang, H. Zhao, Z. Zhang, B. Wu and F. Li, Environ. Sci. Pollut. Res., 2015, 22, 3498–3507 CrossRef CAS.
- S. A. Lynch, A. M. Mullen, E. E. O'Neill and C. A. Garcia, Compr. Rev. Food Sci. Food Saf., 2017, 16, 330–344 CrossRef.
- J. A. Ofori and Y.-H. P. Hsieh, Crit. Rev. Food Sci. Nutr., 2014, 54, 687–697 CrossRef.
- M. Mar Gil-Diaz, A. Perez-Sanz, M. Angeles Vicente and M. C. Lobo, Clean: Soil, Air, Water, 2014, 42, 1776–1784 CAS.
- L. I. Sitong, S. Fangfang, W. Nan, C. Hongbing, L. I. Yadong and L. I. Shunyi, Chin. J. Environ. Eng., 2017, 11, 5001–5006 Search PubMed.
- C. W. Lee, H. B. Kwon, H. P. Jeon and B. Koopman, J. Cleaner Prod., 2009, 17, 683–687 CrossRef CAS.
- V. Antoniadis, J. S. Robinson and B. J. Alloway, Chemosphere, 2008, 71, 759–764 CrossRef CAS.
- J. Xu, Q. Cai, H. Wang, X. Liu, J. Lv, D. Yao, Y. Lu, W. Li and Y. Liu, Environ. Monit. Assess., 2017, 189, 1–11 CrossRef CAS.
- N.-c. Feng and X.-y. Guo, Trans. Nonferrous Met. Soc. China, 2012, 22, 1224–1231 CrossRef CAS.
- R. Gnanasambandam and A. Proctor, Food Chem., 2000, 68, 327–332 CrossRef CAS.
- J. Madejova and P. Komadel, Clays Clay Miner., 2001, 49, 410–432 CrossRef CAS.
- F. Yan, S. Schubert and K. Mengel, Soil Biol. Biochem., 1996, 28, 617–624 CrossRef CAS.
- C. X. Yu, L. L. Zhang, L. J. Yang, D. P. Li and Z. J. Wu, Chin. J. Ecol., 2017, 36, 1941–1948 Search PubMed.
- N. V. Hue, Soil Sci., 2011, 176, 543–549 CrossRef CAS.
- C. Wu, L. Shi, S. Xue, W. Li, X. Jiang, M. Rajendran and Z. Qian, Sci. Total Environ., 2019, 647, 1158–1168 CrossRef CAS.
- R. E. Wing, Ind. Crops Prod., 1996, 5, 301–305 CrossRef CAS.
- F. Ningchuan, G. Xueyi, L. Sha and T. Qinghua, Chin. J. Environ. Eng., 2011, 5, 11–15 Search PubMed.
- P. X. Sheng, Y. P. Ting, J. P. Chen and L. Hong, J. Colloid Interface Sci., 2004, 275, 131–141 CrossRef CAS.
- J. Lin, M. Sun, B. Su, G. Owens and Z. Chen, Sci. Total Environ., 2019, 659, 491–498 CrossRef CAS.
- M. Lee, I. S. Paik, I. Kim, H. Kang and S. Lee, J. Hazard. Mater., 2007, 144, 208–214 CrossRef CAS.
- Y.-S. Ok, H. Lee, J. Jung, H. Song, N. Chung, S. Lim and J.-G. Kim, J. Appl. Biol. Chem., 2004, 47, 143–146 CAS.
- W.-q. Xie, X.-m. Li, C. Chen, X.-f. Chen, Y. Zhong, Z.-y. Zhong, Y. Wan and Y. Wang, Huanjing Kexue, 2015, 36, 4609–4614 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06411d |
| This journal is © The Royal Society of Chemistry 2020 |