Open Access Article
Open Access ArticleElectrochemical synthesis of tin plasmonic dendritic nanostructures with SEF capability through in situ replacement
Jun Dong *a,
Feifei Wua,
Qingyan Hana,
Jianxia Qia,
Wei Gaoa,
Yongkai Wanga,
Tuo Lia,
Yi Yanga and
Mengtao Sun
*a,
Feifei Wua,
Qingyan Hana,
Jianxia Qia,
Wei Gaoa,
Yongkai Wanga,
Tuo Lia,
Yi Yanga and
Mengtao Sun *b
*b
aSchool of Electronic Engineering, Xi'an University of Posts and Telecommunications, Xi'an, 710121, China. E-mail: dongjun@xupt.edu.cn
bSchool of Mathematics and Physics, Beijing Key Laboratory for Magneto-Photoelectrical Composite and Interface Science, Center for Green Innovation, University of Science and Technology Beijing, Beijing, 100083, China. E-mail: mengtaosun@ustb.edu.cn
First published on 1st October 2020
Abstract
Dendrite nanostructures with noble metals, such as Au and silver, act as plasmonic substrates with excellent potential in enhanced fluorescence technology. However, tin dendritic nanostructures are poorly investigated. In this study, we proposed a method of in situ electrochemical synthesis replacement to fabricate highly branched tin dendritic nanostructures on aluminum substrates. The surface enhanced fluorescence performance of the tin dendrites was tested for the detection of rhodamine 6G as probe molecules, and the result showed that the enhancement factors can reach to 36.5-fold that of an aluminum substrate. The fabricated tin dendrites have numerous nanogaps between the stratified and adjacent ones, thereby creating many plasmon-active “hotspots” dedicated to enhanced fluorescence. Electrical field simulation results for the tin dendritic nanostructures proved that its nanogaps can enhance the nearby local electromagnetic field. As a result, tin dendritic nanostructures exhibit outstanding surface enhanced fluorescence and promising application in biomolecule detection and sensor devices.
1. Introduction
When excited by an external electric field, plasmonic nanostructures can manipulate the light field on the nanoscale and thus have received great attention in catalysis, surface enhanced Raman scattering (SERS), and surface enhanced fluorescence (SEF).1–5 SEF is one of the most important spectroscopic detection technologies and widely used in various fields such as bioassays, medical research, and photoelectric sensing.6–10 A highly sensitive fluorescence spectroscopy platform provides good development for fluorescence spectroscopy.11 Noble metal nanostructures with various morphologies and excellent plasmonic characteristics are widely used in SEF and related areas. Zhang et al.12 reported that 3D flower-like silver nanostructures can be applied as surface enhanced fluorescence substrates that can achieve the largest enhancement factor (EF) of 181. In addition, silver gel film13 and silver dendritic14 nanostructures also show good enhanced fluorescence effect. Although, conventional SEF substrates are mostly Ag, Au and Cu,15–18 Prof. Geddes19 first reported the enhanced fluorescence effect of tin nano-films prepared through thermal vapor deposition. With the increasing demand for the detection of low-concentration target molecules in a complex environment, developing an enhanced substrate with excellent plasmonic characteristic is important in the investigation. Actually, metallic dendritic nanostructures with multiple fractal structures, hierarchical self-similarity and peculiar optical response characteristics are widely used in various fields, such as solar energy storage,20 biomedical application,21,22 and spectroscopy.23 Moreover, metallic dendrites have many multi-level branched nanostructures, which allow a larger specific surface area. The corresponding complex nanostructures may be more conducive to the absorption of probe molecules,24 which have significant development in the field of detection. Silver or silver/gold alloyed dendritic nanostructures are being investigated for their potential application in catalysis and SERS.25,26 When studying the surface-enhanced fluorescence of metal dendritic nanostructures, silver dendritic nanostructures are mainly used to adjust the fluorescence signal.27 Here, we used a simple and rapid method to prepare tin dendritic nanostructures and investigated the effect of tin dendrites on fluorescence regulation.In this work, conventional metal tin instead of gold or silver was used to study its enhanced effect. Tin dendritic fractal nanostructures on aluminum (Al) were successfully prepared via in situ chemical reaction between Sncl2 solution and Al. In addition, the SEF performances of the tin dendrites were investigated for the detection of rhodamine 6G (Rh6G) as probe molecules. Tin dendritic nanostructures with different sizes and morphologies were generated by changing the reaction time and exhibited various enhanced fluorescence phenomena. The morphology of tin dendritic nanostructures with good enhancement effect and strong stability was simulated and theoretically analyzed. The results show that the tin dendrites on SEF-active substrates with good reliability and reproducibility can contribute to the practical applications of fluorescence detection in biomolecule discovery.
2. Experimental section
2.1 Chemical reagents and materials
All reagents used were of analytical grade. Stannous chloride dihydrate (SnCl2·2H2O) and ethanol (C2H6O) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Aluminum foil (99.9995%) was obtained from Beijing Institute of Nonferrous Metals and Rare Earths. Rhodamine 6G (Rh6G, laser grade) was acquired from Exciton (USA).2.2 Apparatus
The samples were characterized by scanning electron microscopy (SEM, Carl Zeiss-Sigma), energy dispersive X-ray spectroscopy (EDS), and EDS elemental mapping. Green light was excited by a 532 nm laser as an excitation source. Fluorescence spectrometer (ocean optics) was used to measure the enhanced fluorescence spectrum. COMSOL Multiphysics software is used to simulate the sample.2.3 Fabrication of tin dendrites on aluminum substrate
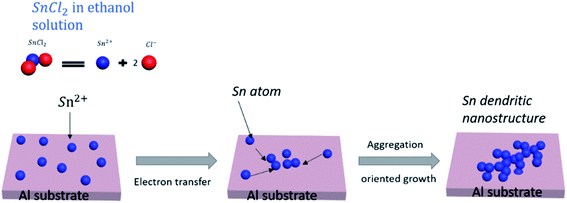
The tin dendritic nanostructure was prepared on aluminum substrate through in situ replacement. First, weigh 0.034 g of SnCl2·2H2O crystals and dissolve it in ethanol solution to obtain a concentration of 0.5 × 10−2 mol L−1 SnCl2–ethanol solution. Second, the aluminum substrate was polished with an 800 mesh sandpaper for 3 minutes, ultrasonically cleaned with ethanol, and dried using N2. Finally, the aluminum substrate with length of 1 cm and width of 0.5 cm was cut and placed in SnCl2 solution. At room temperature about 25 °C, the tin dendritic substrates with different morphologies were prepared by adjusting the reaction time. The reaction process was similar to a simple galvanic displacement. The metals transferred the electrons to reduce Sn2+ ions and form Sn atoms, which aggregated and grew into dendrites as shown in Fig. 1. Different morphologies of tin dendritic nanostructure were formed by changing the reaction time.2.4 Investigation of the surface enhanced fluorescence performance
Solutions of Rh6G with different concentrations (10−6, 10−7, 10−8, and 10−9 mol L−1) were prepared in ethanol. The prepared tin dendritic substrate was immersed in Rh6G solution for 25 min, and Rh6G molecules formed a uniform film on the sample surface. In the spectrum test, the confocal microscopy light path is used, the sample is under the objective lens, and the fiber optic spectrometer is used to receive the feedback fluorescence signal, and the fluorescence spectrum is displayed on the computer through the CCD. It should be noted that a 532 nm filter should be placed in front of the fiber spectrometer to eliminate the influence of the 532 nm laser on the feedback fluorescence signal. The same 50× microscope objective with an integration time of 0.1 s and a laser power of 5 mW was used for the fluorescence spectrum detection. Spectral testing needs to be carried out in a dark room to reduce the background light. The tested sample was washed with deionized water and alcohol several times to remove residual ions and molecules, and finally lightly dry it with N2 for repeated use.3. Results and discussion
3.1 Substrate characterization and spectroscopic measurements
The crystalline dendrite was fabricated by reducing Sn2+ in the SnCl2 solution on Al substrate. In this study, we displace Sn2+ in solution with aluminum to form tin dendritic micro-nano structures on the Al matrix. The formation of dendritic tin fractal nanostructures was investigated by adjusting the reaction time. Wenzhao Jia et al. fabricated the Tin nanodendrites by using galvanic replacement reaction between stannous chloride (SnCl2) and thermal evaporated zinc film composed of Zn nanodiscs.28 As the reaction time increases, the color of the aluminum surface changes from silvery white to dark gray, and finally to black with fine silver-white crystals. H2 will be generated during the entire reaction. The following is the chemical equation in the reaction:| Al + Sn2+ → Sn + Al3+ | (1) |
| Al + CH3CH2OH → (CH3CH2O)3Al + H2↑ | (2) |
| Sn2+ + 2e− = Sn, E0 = −0.140 V | (3) |
| Al3+ + 3e− = Al, E0 = −1.662 V | (4) |
It has been reported that in the preparation of silver dendritic structure using displacement reaction via Zn and AgNO3, under high silver ion concentration, strong anisotropic growth helps to form fine single crystal silver dendrites.29 Dendritic nanostructures are products formed under non-equilibrium conditions, and their morphology sets the properties of many metallic alloys, the dendrites grow into a supersaturated liquid and the growth is by solute diffusion the dendrite growth dynamics during the free-growth stage are diffusion limited.30 As the displacement reaction progress, the continuous growth of tin dendrites will deplete the monomer concentration in certain area. The Sn2+ ion concentration around previous grains drops to a certain level, small particles may have enough time to relax and grow oriented at the top part. In this paper, aluminum is more active as a reducing agent to reduce Sn2+ in the solution, hydroxyl hydrogen atoms in ethanol molecules will be replaced by aluminum, and generating hydrogen bubbles on the surface of aluminum. As the reaction progress, more and more tin atoms will accumulate on the Al surface, and hydrogen bubbles are constantly being produced. From the viewpoint of electrochemistry, there are two competitive reactions (Sn2+ reduction and H2 evolution). The Al substrate is artificially polished, and the roughness of the surface will also have a certain effect on the growth of dendrites. Fig. 2 shows the SEM morphology and the elemental composition of tin dendritic nanostructures prepared for 7 min. It can be observed that the Al substrate was covered with tin dendrites. The tin dendritic nanostructures are composed of multilevel branch structures, which generates large area stalactite-like morphology on Al surface.
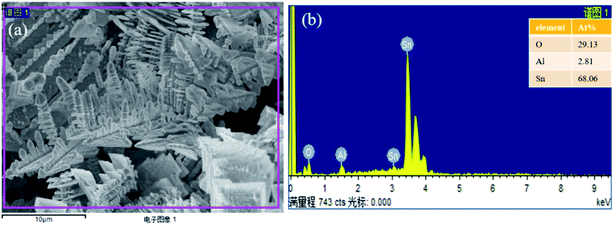 | ||
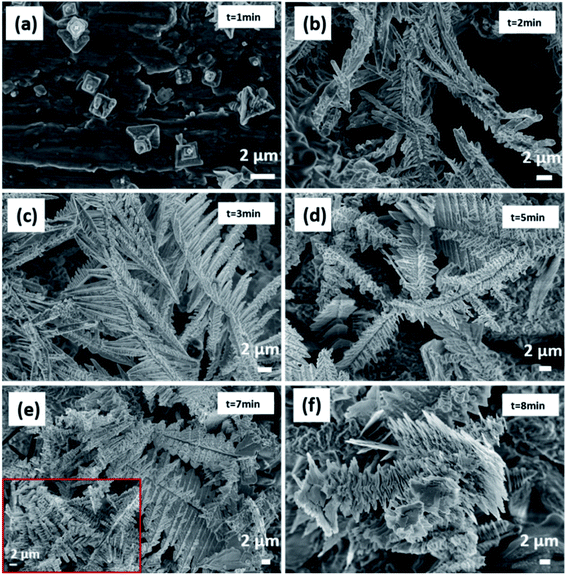
| Fig. 2 (a) SEM images of tin dendritic nanostructures prepared for 7 min, (b) the EDS analytical energy spectrum of tin dendritic with 7 min reaction time. | ||
The tin dendrites grew from square particles to highly branched dendritic nanostructures during morphological evolution, as shown in Fig. 3. When the reaction time is 1 min, Sn atoms are initially nucleated. It can be clearly to see the grinding marks on the aluminum substrate and the protruding square Sn particles growing on the aluminum surface, the nucleation grows on the surface of aluminum and is relatively dispersed in Fig. 3(a). The size of the Sn block structure is about 2 μm, similar to a tree stump structure. The bubbles greatly suppress the splitting and growth of additional branches by concealing growth sites for the branches, if the bubbles are small, it can promote the vertical growth of dendritic nanostructures and inhibit the formation of side branches.31,32 After 2 min reaction time, the reaction rate accelerated, and the initially grown tin nanoflowers with small particles propagated into thin rods as shown in Fig. 3(b). Long main stems and small branches can be observed. The size of the long main stem is about 5 microns, and the size of the small branches is 500 nm. When reaction time increased to 3 min, feather-like structure increases and formed hoarfrost-like large-area structures as shown in Fig. 3(c). Multiple flat main stems overlap each other, and multiple thin branches grow vertically on the main stem, and dendritic nanostructures are numerous and dense, forming a three-dimensional structure. When the reaction time increased to 5 min, a stepped folded structure is formed, the flat structure grows and expands laterally, and the square stacked structure perpendicular to its surface also grows slowly, as shown in Fig. 3(d). When reaction time increased to 7 min, high dendritic differentiation occurred, large complicated symmetrical tin dendritic were formed, and large-scale dendritic nano-structures grow into thin leaf-like flat structures, and there are a large number of square-shaped accumulations on the veins and surfaces of leaf branches (shown in insets), as shown in Fig. 3(e). In the reaction stage, the hydrogen bubble generation rate increases with the increase of the reaction time. After a certain period of time, the shape of the hydrogen bubble becomes larger, causing the dendritic structure to break and fall off on the aluminum substrate, when the reaction time was extended, the leaves became enlarged and collapsed as shown in Fig. 3(f).
It is found that the growth rate, morphology and structure of dendritic nanostructures are critical depended on the concentration of the reaction solution, reaction rate and reaction time.33 Here, by controlling the experimental condition, such as the reaction solution concentration, reaction time and so on, and the growth mechanism of tin dendritic nanostructures was investigated. It is found that with the increasing the reaction time, the size of the hydrogen bubbles increases, the tin dendritic nanostructures are easily broken. J. C. Millare. et al.34 reported that the addition of salt to the ethanol–water mixture solution will cause the anions to interact with the ethanol molecules, which will accumulate in the gas phase, resulting in an increase in bubble size. The solvent in the displacement reaction will significantly affect the reaction kinetics, thereby affecting the morphology of the product. It was found that the metal matrix, the ratio of ethanol and water and additives have a significant effect on the morphology of the obtained dendrites.36 S. P. Xie et al.35 reported the fast growth synthesis of silver dendrite based on a electroless galvanic displacement reaction between solid Cu and Ag(I) ion, and the influence of different anions (SO42−, NO3−) in the solute on the morphology and structure of silver branches is investigated. As a result, for the stability of the tin dendritic structure, additives can be added during the reaction to suppress or reduce the generation of hydrogen bubbles, and the solute and solvent can also be changed to obtain a structure with better performance.
3.2 SEF activities of tin dendritic nanostructures
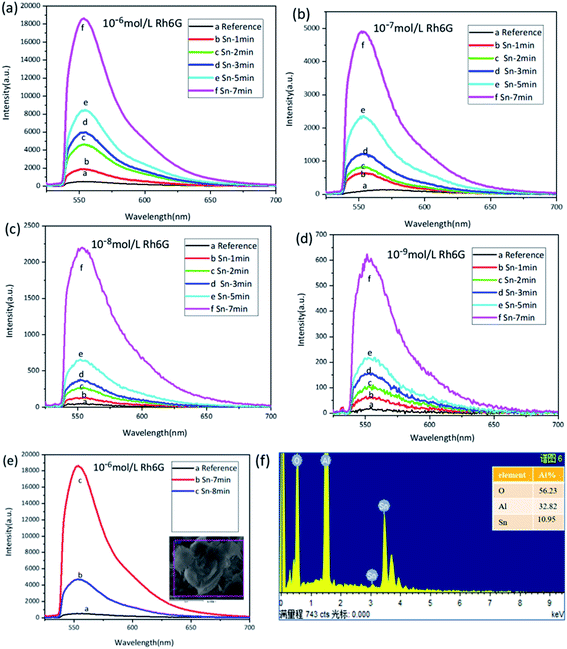
In order to investigate the regulation of tin dendritic nanostructures with different morphologies on the fluorescence spectrum we used rhodamine as a probe molecule and excited by a 532 nm laser, the aluminum substrate and the sample are treated in the same way during the test. The fluorescence spectrum of the Rh6G absorbed on the pure Al substrate was selected as a reference to inspect the SEF enhancement of tin branched dendritic nanostructure. As shown in Fig. 4, the fluorescence intensity of Rh6G deposited on the substrate gradually increased with the formation of the dendrites. However, when the reaction time was more than 8 min, the dendritic nanostructure collapsed, and the corresponding spectrum of Rh6G was weaker than that obtained from 7 min reaction time.Metal nanostructures can adjust the SPR band and local field enhancement frequency through the size, shape and environment of the particles, but the absorption or emission bands of the dye are relatively fixed.37 Due to the change of reaction time, the morphology of tin branches also changes significantly, the fluorescence spectra of Sn dendritic generated at different reaction times after the immersion of different Rh6G concentrations are shown in Fig. 4(a–d). The concentration of rhodamine molecules affects the number of fluorescent molecules deposited on the surface of the sample, resulting in different fluorescence intensities. The intensity of the fluorescence spectrum increases with time during the sample reaction time of 1 to 7 minutes. Fig. 4(e) shows the comparative spectra of tin dendrites at 7 min and 8 min. Compared with that at 8 min, the tin dendritic nanostructure prepared with 7 min reaction time has higher density and stable structure with excellent spectral intensity Fig. 4(f) is EDS analytical energy spectra of tin dendrites with 8 min reaction times.
By comparing the peak at 558 nm from the prepared metal substrates with that from the polished aluminum substrate in Fig. 4, the enhancement factor (EF) was proposed to express the enhanced fluorescent effects of tin branched dendritic nanostructure and was estimated through the following equation:38
| EF = (Isample − Ibackground)/(Ireference − Ibackground) | (5) |
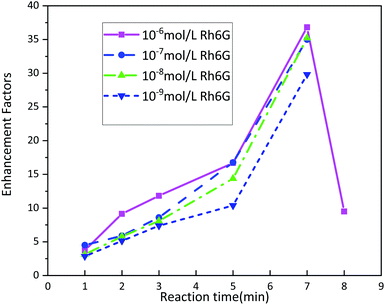 | ||
| Fig. 5 Fluorescence enhancement factors of Rh6G molecules deposited on tin dendritic substrate with different concentration. | ||
According to the fluorescence spectrum experimental data, the tin dendritic nanostructure exhibited fluorescence enhancement as shown in Fig. 5. Seven spectrum detection points on each sample were randomly selected to investigate the sample homogeneity under high magnification microscope. The EF critically depends on the reaction time of substrate. With the increase in reaction time, the EF value also increased. However, when the reaction time was longer than 7 min, the dendritic nanostructure had collapsed and reduced the fluorescence enhanced effect. Rh6G solution (10−6 mol L−1) was selected as the sample immersion solution. Under reaction times of 1, 2, 3, 5, 7, and 8 min, the EF was 3.75, 9.15, 11.82, 16.69, 36.81, and 9.50, respectively. With different Rh6G solution concentrations, the fluorescence EF reached an highest value at 7 min reaction time. When beyond this duration, EF value will be decreased. The metallic nanostructures and metallic colloidal nanoparticles interact with the proximal fluorophore and produce higher quantum yields.39 Experimental observations show that the fluorescence enhanced ability of the sample surface is strongly correlated with the sample morphology. The morphology of tin dendritic nanostructures formed under 7 min reaction time exhibits the best fluorescence enhancement effect. Careful examination of SEM revealed that the tin branched dendritic nanostructures had large-area stacked complex structures may be conducive to the absorption of Rh6G molecules. In particular, when metal dendrites were excited by an external light field, local field enhancement is generated at the end of branches, and young twigs can become needle tips that positively influence local EM field. In addition, strong EM coupling can occur in the space between two adjacent branches from the coupling of SPR and the nanogap formed in the nanostructures has a crucial role in enhancing the local EM field,40,41 the electromagnetic field intensity of these hotspots is much higher than other locations, resulting in strong fluorescence signals from nearby fluorophore molecules. As a result, the tin dendritic nanostructure exhibiting fluorescence enhanced efficiency.
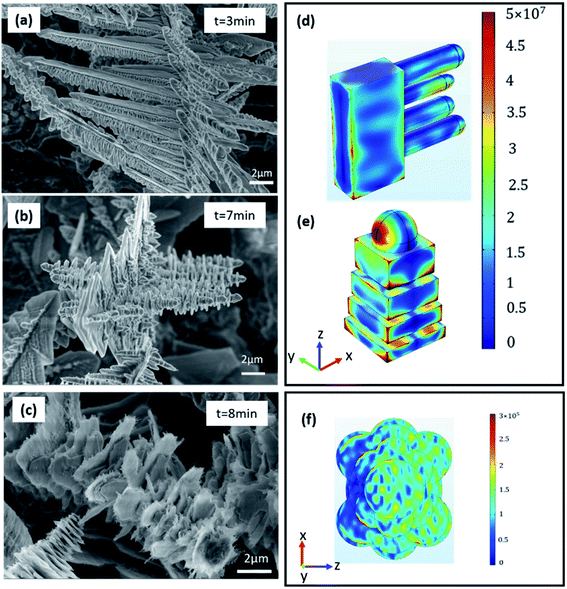
The EM field distributions of tin dendritic nanostructures on a planar surface were calculated using finite element method (FEM). The simulation model is based on the SEM picture of tin nanostructure as shown in Fig. 6(a–c). Select the characteristic parts of tin branches of different shapes and compare their electric field distributions. Fig. 6(d) and (e) show the spatial distribution of electric field for local EM fields were generated in the tin dendrites of gaps and adjacent dendrite structures excited via the 532 nm laser with Y polarization from Z axis. Strong EM “hot spots” were produced in the nanogap and tip. The strong electric field is distributed in the space between two adjacent branches as shown in Fig. 6(d). As seen in Fig. 6(e), the EM field was enhanced in the nanogap formed from the accumulation of small squares on tin dendrites. The highest enhancement of electric field was found at the top of the branch nanostructure. At the same electromagnetic scale, compared with branched and nano-gap structures, large blade-shaped structures have very weak electromagnetic field enhancement at the edge of the blade, changing the electromagnetic scale can better observe the part of electromagnetic enhancement as shown in Fig. 6(f). This simulation proves that the formed “hot spots” between the tip and nanogap are dedicated to generating surface plasmons and local EM field. In addition, the fluorescence signal was dramatically enhanced when the tin dendritic nanostructure was formed.
4. Conclusion
Tin dendritic nanostructure substrates with controllable morphology were prepared through in situ replacement without surfactant. The growth mechanism of tin dendritic nanostructures has been proposed. Under 532 nm laser excitation, the tin dendritic nanostructures can detect 10−9 mol L−1 Rh6G, and fluorescence enhancement can reach 29.81-fold that on pure Al substrate. Theoretical analysis proves that the large number of “hot spots” generated in tin dendritic nanostructures provide an advantage to excite surface plasmon resonance. Furthermore, local enhanced EM field excited among the dendritic nanostructures was observed based on FEM simulation. In this work, a simple, convenient, and low-cost method was used to fabricate tin dendritic nanostructure with fluorescence enhancement capability. This study provides reliable experimental support for fluorescence enhancement with great potential in SEF investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Shaanxi Province International Cooperation and Exchange Program (Grant No. 2019KW-027), Natural Science Foundation of Shaanxi Provincial (Grant No. 2018JQ6009), Shaanxi Provincial Research Plan for Young Scientific and Technological New Stars (Grant No. 2019KJXX-058), Xi'an University of Posts and Telecommunications Joint Postgraduate Cultivation Workstation (Grant No. CXJJLY2018057), and Xi'an University of Posts and Telecommunications Joint Postgraduate Cultivation Workstation (YJGJ201905).References
- Z. G. Dai, X. H. Xiao, W. Wu, Y. P. Zhang, L. Liao, S. S. Guo, J. J. Ying, C. X. Shan, M. T. Sun and C. Z. Jiang, Plasmon-driven reaction controlled by the number of graphene layers and localized surface plasmon distribution during optical excitation, Light Sci. Appl., 2015, 4, e342 CrossRef CAS.
- Y. Yin, P. Miao, Y. M. Zhang, J. C. Han, X. H. Zhang, Y. Gong, L. Gu, C. Y. Xu, T. Yao, P. Xu, Y. Wang, B. Song and S. Jin, Significantly Increased Raman Enhancement on MoX2 (X = S, Se) Monolayers upon Phase Transition, Adv. Funct. Mater., 2017, 27, 1606694 CrossRef.
- J. Xu, C. H. Li, H. P. Si, X. F. Zhao, L. Wang, S. Z. Jiang, D. M. Wei, J. Yu, X. W. Xiu and C. Zhang, 3D SERS substrate based on Au-Ag bi-metal nanoparticles/MoS2 hybrid with pyramid structure, Optics express, 2018, 26(17), 21546–21557 CrossRef CAS.
- H. Zong, X. Wang, X. Mu, J. Wang and M. Sun, Plasmon-Enhanced Fluorescence Resonance Energy Transfer, Chem. Rec., 2019, 19, 818–842 CrossRef CAS.
- X. Zhao, J. Dong, E. Cao, Q. Han, W. Gao, Y. Wang, J. Qi and M. T. Sun, Plasmon-exciton coupling by hybrids between graphene and gold nanorods vertical array for sensor, Appl. Mater. Today., 2019, 14, 166–174 CrossRef.
- S. E. Wang, Y. Huang, K. Hu, J. Tian and S. L. Zhao, A highly sensitive and selective aptasensor based on fluorescence polarization for the rapid determination of oncoprotein vascular endothelial growth factor (VEGF), Anal. Methods, 2014, 6(1), 62–66 RSC.
- C. Fan, X. Lv, F. Liu, L. Feng, M. Liu, Y. Cai, H. Liu, J. Wang, Y. Yang and H. Wang, Silver nanoclusters encapsulated into metal–organic frameworks with enhanced fluorescence and specific ion accumulation toward the microdot array-based fluorimetric analysis of copper in blood, ACS Sens., 2018, 3(2), 441–450 CrossRef CAS.
- J. Yang, A. Moraillon, A. Siriwardena, R. Boukherroub, F. Ozanam, A. Chantal Gouget-Laemmel and S. Szunerits, Carbohydrate microarray for the detection of glycan–protein interactions using metal-enhanced fluorescence, Anal. Chem., 2015, 87(7), 3721–3728 CrossRef CAS.
- N. Sui, L. Wang, F. Xie, F. Liu, H. Xiao, M. H. Liu and W. W. Yu, Ultrasensitive aptamer-based thrombin assay based on metal enhanced fluorescence resonance energy transfer, Microchim. Acta, 2016, 183(5), 1563–1570 CrossRef CAS.
- K. Sugawa, T. Tamura, H. Tahara, D. Yamaguchi, T. Akiyama, J. Otsuki, Y. Kusaka, N. Fukuda and H. Ushijima, Metal-enhanced fluorescence platforms based on plasmonic ordered copper arrays: wavelength dependence of quenching and enhancement effects, ACS Nano, 2013, 7(11), 9997–10010 CrossRef CAS.
- J. Dong, Z. L. Zhang, H. R. Zheng and M. T. Sun, Recent progress on plasmon-enhanced fluorescence, Nanophotonics, 2015, 4(1), 472–490 CAS.
- Y. Zhang, C. L. Yang, X. J. Xiang, P. G. Zhang, Z. H. Peng, Z. L. Cao and L. Xuan, Highly effective surface-enhanced fluorescence substrates with roughened 3D flowerlike silver nanostructures fabricated in liquid crystalline phase, Appl. Surf. Sci., 2017, 401, 297–305 CrossRef CAS.
- J. J. Wang and Z. H. Jia, Metal nanoparticles/porous silicon microcavity enhanced surface plasmon resonance fluorescence for the detection of DNA, Sensors, 2018, 18(2), 661 CrossRef.
- A. Lotfi, M. Nikkhah and A. Moshaii, Development of metal-enhanced fluorescence-based aptasensor for thrombin detection using silver dendritic nanostructures, Plasmonics, 2019, 14(3), 561–568 CrossRef CAS.
- C. R. Simovski, M. S. M. Mollaei and P. M. Voroshilov, Fluorescence quenching by plasmonic nanoantennas, Phys. Rev. B, 2020, 101, 245421 CrossRef CAS.
- A. M. Gabudean, M. Focsan and S. Astilean, Gold nanorods performing as dual-modal nanoprobes via metal-enhanced fluorescence (MEF) and surface-enhanced Raman scattering (SERS), J. Phys. Chem. C, 2012, 116(22), 12240–12249 CrossRef CAS.
- Z. Zhang, J. Malicka, I. Gryczynski and J. R. Lakowicz, Surface-enhanced fluorescence of fluorescein-labeled oligonucleotides capped on silver nanoparticles, J. Phys. Chem. B, 2005, 109(16), 7643–7648 CrossRef.
- J. Zhu, K. Zhu and L. Huang, Using gold colloid nanoparticles to modulate the surface enhanced fluorescence of Rhodamine B, Phys. Lett. A, 2008, 372(18), 3283–3288 CrossRef CAS.
- Y. Zhang, A. Dragan and C. D. Geddes, Metal-enhanced fluorescence from Tin nanostructured surfaces, J. Appl. Phys., 2010, 107(2), 024302 CrossRef.
- O. Amiri, M. Salavati-Niasari, N. Mir, F. Beshkar, M. Saadat and F. Ansari, Plasmonic enhancement of dye-sensitized solar cells by using Au-decorated Ag dendrites as a morphology-engineered, Renewable energy, 2018, 125, 590–598 CrossRef CAS.
- L. Liu, K. Zhuo, Z. Yuan, Q. Zhang, W. Zhang and S. Sang, Nano-branched Silver Dendrites Modified Glassy Carbon Electrode for Detection of Heavy Metal Ions, Int. J. Electrochem. Sci., 2019, 14, 5317–5330 CrossRef CAS.
- X. X. Wang, D. D. Yang, L. Z. Chen, B. Liu, Z. G. Teng, N. Y. He and Z. F. Wang, 2D Dendritic Gold Nanostructures Formed on Silica Nanosheets: Transferability, Clean Surface, and Their Biomedical Application, Part. Part. Syst. Char., 2018, 35(10), 1800268 CrossRef.
- G. Xing, K. Wang, P. Li, W. Q. Wang and T. Chen, 3D hierarchical Ag nanostructures formed on poly (acrylic acid) brushes grafted graphene oxide as promising SERS substrates, Nanotechnology, 2018, 29(11), 115503 CrossRef.
- L. H. Lu, A. Kobayashi, Y. Kikkawa, K. Tawa and Y. Ozaki, Oriented attachment-based assembly of dendritic silver nanostructures at room temperature, J. Phys. Chem. B, 2006, 110(46), 23234–23241 CrossRef CAS.
- W. Q. Zhang, J. Liu, W. X. Niu, H. Yan, X. M. Lu and B. Liu, Tip-Selective Growth of Silver on Gold Nanostars for Surface-Enhanced Raman Scattering, ACS Appl. Mater. Interfaces, 2018, 10(17), 14850–14856 CrossRef CAS.
- J. Dong, S. X. Qu, H. R. Zheng, Z. L. Zhang, J. N. Li, Y. P. Huo and G. A. Li, Simultaneous SEF and SERRS from silver fractal-like nanostructure, Sensor. Actuator. B Chem., 2014, 191, 595–599 CrossRef CAS.
- A. Parfenov, I. Gryczynski, J. Malicka, C. D. Geddes and J. R. Lakowicz, Enhanced fluorescence from fluorophores on fractal silver surfaces, J. Phys. Chem. B, 2003, 107(34), 8829–8833 CrossRef CAS.
- W. Z. Jia, L. Su, X. P. Li, L. C. Zhang, Z. Y. Gu, P. X. Gao and Y. Lei, Synthesis of tin nanodendrites via galvanic replacement reaction and their thermal conversion to nanodendritic tin oxide for ultrasensitive electrochemical sensing, RSC Adv., 2011, 1(8), 1500–1505 RSC.
- J. X. Fang, H. J. You, P. Kong, Y. Yi, X. P. Song and B. J. Ding, Dendritic silver nanostructure growth and evolution in replacement reaction, Cryst. Growth Des., 2007, 7(5), 864–867 CrossRef CAS.
- J. W. Gibbs, K. A. Mohan, E. B. Gulsoy, A. J. Shahani, X. Xiao, C. A. Bouman, M. De Graef and P. W. Voorhees, The three-dimensional morphology of growing dendrites, Sci. Rep., 2015, 5(1), 1–9 Search PubMed.
- C. L. Wang, C. S. Lin, Y. Hwu, C. H. Chen, L. Chang, H. Je and G. Margaritondo, Hydrogen bubbles and the growth morphology of ramified zinc by electrodeposition, J. Electrochem. Soc., 2008, 155(5), D400 CrossRef.
- T. Kim, K. Hong, D. Sohn, M. Kim, D. Nam, E. Cho and H. Kwon, One-step synthesis of multilayered 2D Sn nanodendrites as a high-performance anode material for Na-ion batteries, J. Mater. Chem. A, 2017, 5(38), 20304–20315 RSC.
- J. S. Yang and Z. D. Jiang, Facile fabrication of dendritic silver structures and their surface enhanced Raman spectroscopic properties, J. Chem. Sci., 2015, 127(1), 173–176 CrossRef CAS.
- J. C. Millare and B. A. Basilia, Nanobubbles from Ethanol-Water Mixtures: Generation and Solute Effects via Solvent Replacement Method, ChemistrySelect, 2018, 3(32), 9268–9275 CrossRef CAS.
- S. P. Xie, X. C. Zhang, D. Xiao, M. Chin Paau, J. Huang and M. M. F. Choi, Fast growth synthesis of silver dendrite crystals assisted by sulfate ion and its application for surface-enhanced Raman scattering, J. Phys. Chem. C, 2011, 115(20), 9943–9951 CrossRef CAS.
- X. T. Bai, Y. A. Gao and L. Q. Zheng, Galvanic replacement mediated growth of dendritic gold nanostructures with a three-fold symmetry and their applications to SERS, CrystEngComm, 2011, 13(10), 3562–3568 RSC.
- J. Zhu, K. Zhu and L. Q. Huang, Using gold colloid nanoparticles to modulate the surface enhanced fluorescence of Rhodamine B, Phys. Lett. A, 2008, 372(18), 3283–3288 CrossRef CAS.
- J. Dong, H. Zheng, X. Yan, Y. Sun and Z. Zhang, Fabrication of flower-like silver nanostructure on the Al substrate for surface enhanced fluorescence, Appl. Phys. Lett., 2012, 100(5), 051112 CrossRef.
- Y. Jeong, Y. Kook, K. Lee and W. Koh, Metal enhanced fluorescence (MEF) for biosensors: general approaches and a review of recent developments, Biosens. Bioelectron., 2018, 111, 102–116 CrossRef CAS.
- Z. Q. Cheng, Z. W. Li, J. H. Xu, R. Yao, Z. L. Li, S. Liang, G. Cheng, Y. Zhou, X. Luo and J. Zhong, Morphology-controlled fabrication of large-scale dendritic silver nanostructures for catalysis and SERS applications, Nanoscale Res. Lett., 2019, 14(1), 1–7 CrossRef CAS.
- H. J. Yin, Z. Y. Chen, Y. M. Zhao, M. Y. Lv, C. N. Shi, Z. L. Wu, X. Zhang, L. Liu, M. L. Wang and H. J. Xu, Ag@Au core-shell dendrites: a stable, reusable and sensitive surface enhanced Raman scattering substrate, Sci. Rep., 2015, 5, 14502 CrossRef.
| This journal is © The Royal Society of Chemistry 2020 |