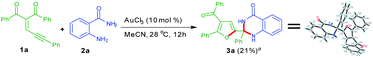DOI:
10.1039/D0RA06537D
(Paper)
RSC Adv., 2020,
10, 35681-35691
Gold(III) promoted formation of dihydroquinazolinones: double X–H activation by gold†
Received
28th July 2020
, Accepted 14th September 2020
First published on 28th September 2020
Abstract
An efficient 2-furyl gold–carbene promoted synthetic method was developed for the formation of dihydroquinazolinones from enynones by dual insertion of anthranilamides. In this organic transformation a new C–O and two C–N bond formations occurred and dihydroquinazolinones were obtained with a quaternary centre in moderate to very good yields in one-pot synthesis.
Introduction
Nitrogen-containing heterocyclic molecules1 such as quinazolinones have gained much attention due to their wide range of biological and pharmacological applications.2 Dihydroquinazolinone derivatives like fenquizone,3 and quinethazone4 are drugs for edema and hypertension. It was reported that bouchardatine exhibits antiobesity activitity,5 and penipanoid C exhibits tobacco mosaic virus inhibition6 (Fig. 1). Further, substituted dihydroquinazolinone derivatives displayed significant cytotoxic activity.7
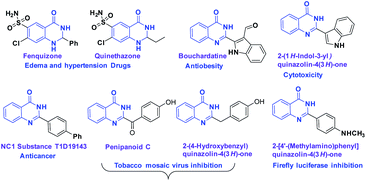 |
| | Fig. 1 Selected examples of important molecules containing dihydroquinazolinone core skeleton. | |
Hence, the development of new synthetic methods for the formation of dihydroquinazolinones is a limitless frontier. Cooperative catalysis8 has been established as a handy tool for the synthesis of several biologically valuable molecules and different procedures were reported for the synthesis of dihydroquinazolinone derivatives.9 Exploration of gold-catalyzed10 organic transformations has attracted much attention in recent years due to their broad functional group tolerance and selectivity for the formation of valuable heterocyclic molecules in one-pot reaction conditions.11 The recent literature indicating that exploitation of enynal/enynone has recognised as good donor or donor–donor carbene precursors for C–H/X–H insertion and cyclopropanation reactions.12 Several reports are available for synthesis of substituted furans from enynones in presence of metal catalysts via a 5-exo-dig cyclization.13
The reaction mechanism was proposed via (2-furyl) metal–carbene14 intermediate would react with one nucleophile15 to produce addition products (Scheme 1, eqn (1)). López and Vicente co-workers reported a method for synthesis of functionalized furans from enynones in the presence of zinc catalyst.16 Recently, Zhu et al. developed metal carbene promoted method for synthesis of vinyl-substituted dihydroindoles.17 Hashmi and co-workers studied the stabilization effects of gold carbene complexes.18
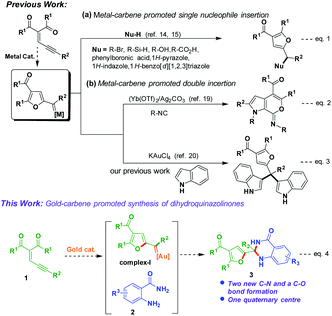 |
| | Scheme 1 Synthetic transformations of enynones. | |
Double insertion of isocyanides to enynones produced pyrrole-fused heterocyclic molecules via (2-furyl) metal–carbene intermediate was reported by Jia and Li co-workers (Scheme 1, eqn (2)).19 Very recently, we have reported formation of tetraarylmethane derivatives by reaction of enynones with indoles via (2-furyl) gold–carbene intermediate (Scheme 1, eqn (3)).20 Our current research21 efforts focused to explore the reactivity of enynones under gold catalysis. We envisioned that reaction of enynones (1) in the presence of gold-catalyst would produce gold–carbene complex-I, which would react with anthranilamide (2) may give corresponding dihydroquinazolinone derivative 3 (Scheme 1, eqn (4)).
Results and discussion
Accordingly, we have conducted an experiment by using substrates 1a and 2a in the presence of AuCl3 (Scheme 2). Very interestingly, 21% yield of the corresponding product 2-(4-benzoyl-5-phenylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 3a was observed. The product 3a was further confirmed by single crystal X-ray analysis.22 It is noteworthy that in this organic transformation two C–N bonds were formed by dual insertion of anthranilamide with a quaternary centre. This interesting observation encouraged us to optimize this reaction to get the better yields of the product 3a.
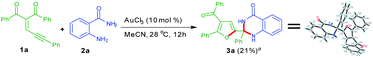 |
| | Scheme 2 Reaction of enynone (1a) with anthranilamides (2a) for formation of 3aa [CCDC 1863534].22 | |
Gold(I) catalysts were screened with the substrates 1a with 2a to produce moderate yields of 3a along with 3a′ (Table 1, entries 1 and 2). Whereas when experiments were conducted in the presence of AuBr3 and KAuCl4 moderate yields of product 3a was observed (Table 1, entries 3 and 4). Complex mixture was obtained while reaction was performed in the presence of IPrAuCl (Table 1, entry 5). In the presence of AuClPPh3, poor yields of desired product 3a was found (Table 1, entry 6). Then reactions were conducted by utilizing gold catalysts in combination of selectfluor (Table 1, entries 7–10), yields of desired product 3a was not improved. Reactions were performed by employing KAuCl4 in combination with K2S2O8, CF3COOH, Cu(OAc)2, K2CO3, pyridine N-oxide and PhI(OAc)2 (Table 1, entries 11–16), moderate yield of 3a was observed. Nevertheless, KAuCl4 and FeCl3 combination afforded very good yield of (81%) of product 3a (Table 1, entry 17). An experiment was conducted by utilizing only FeCl3, poor yields of product 3a was observed along with 3a′ (Table 1, entry 18). Reactions were screened by using series of solvents like toluene, MeOH, THF, DMF and DCE, none of them gave better yield than MeCN (Table 1, entries 19–23). When the gold-catalyst loading decreased from 10 mol% to 5 mol% and 7 mol%, the product yield also reduced to 52% and 58%, respectively (Table 1, entries 24 and 25). Two reactions were conducted without utilizing FeCl3 and these cases poor yields of product 3a observed (Table 1, entries 26 and 27).
Table 1 Optimization of the reaction conditionsa
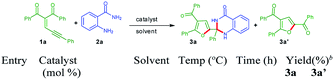
|
| Entry |
Catalyst (mol%) |
Solvent |
Temp (°C) |
Time (h) |
Yieldb (%) |
| 3a |
3a′ |
| Reaction conditions: all reactions were carried out under nitrogen atmosphere with 1a (0.15 mmol), and 2a (0.225 mmol) and solvent (2 mL) in oil bath. Yields are for isolated products; eq.: equivalent. cm: complex mixture. Entries 26 and 27 were conducted without FeCl3.23 |
| 1 |
[Au(JohnPhos)(MeCN)][SbF6](10) |
MeCN |
28 |
12 |
45 |
48 |
| 2 |
(C20H15AuF6NO4PS2)2C7H8 (10) |
MeCN |
28 |
12 |
46 |
43 |
| 3 |
AuBr3 (10) |
MeCN |
28 |
12 |
42 |
— |
| 4 |
KAuCl4 (10) |
MeCN |
28 |
12 |
35 |
— |
| 5 |
IPrAuCl (10) |
MeCN |
100 |
12 |
cmc |
|
| 6 |
AuClPPh3 (10) |
MeCN |
70 |
24 |
32 |
— |
| 7 |
AuClPPh3 (10), Selectfluor (20) |
MeCN |
70 |
24 |
30 |
— |
| 8 |
IPrAuCl (10), Selectfluor (20) |
MeCN |
70 |
24 |
cmc |
|
| 9 |
KAuCl4 (10), Selectfluor (20) |
MeCN |
70 |
24 |
49 |
— |
| 10 |
AuCl3 (10), Selectfluor (20) |
MeCN |
70 |
24 |
48 |
— |
| 11 |
KAuCl4 (10), K2S2O8 (20) |
MeCN |
28 |
14 |
49 |
— |
| 12 |
KAuCl4 (10), CF3COOH (1 eq.) |
MeCN |
28 |
14 |
cmc |
|
| 13 |
KAuCl4 (10), Cu(OAc)2 (20) |
MeCN |
28 |
14 |
40 |
— |
| 14 |
KAuCl4 (10), K2CO3 (1 eq.) |
MeCN |
28 |
14 |
45 |
— |
| 15 |
KAuCl4 (10), Py N-oxide (1.2 eq.) |
MeCN |
28 |
14 |
10 |
— |
| 16 |
KAuCl4 (10), PhI(OAc)2 (1.5 eq.) |
MeCN |
28 |
14 |
20 |
— |
| 17 |
KAuCl4 (10), FeCl3 (2.0 eq.) |
MeCN |
80 |
05 |
81 |
11 |
| 18 |
FeCl3 (2.0 eq.) |
MeCN |
80 |
12 |
20 |
6 |
| 19 |
KAuCl4 (10), FeCl3 (2.0 eq.) |
Toluene |
80 |
12 |
20 |
32 |
| 20 |
KAuCl4 (10), FeCl3 (2.0 eq.) |
MeOH |
80 |
12 |
30 |
35 |
| 21 |
KAuCl4 (10), FeCl3 (2.0 eq.) |
THF |
80 |
12 |
48 |
20 |
| 22 |
KAuCl4 (10), FeCl3 (2.0 eq.) |
DMF |
80 |
12 |
46 |
25 |
| 23 |
KAuCl4 (10), FeCl3 (2.0 eq.) |
DCE |
80 |
12 |
41 |
28 |
| 24 |
KAuCl4 (5), FeCl3 (1.0 eq.) |
MeCN |
80 |
12 |
52 |
21 |
| 25 |
KAuCl4 (7), FeCl3 (1.5 eq.) |
MeCN |
80 |
08 |
58 |
18 |
| 26d |
AgSbF6 (10) |
MeCN |
80 |
48 |
12 |
8 |
| 27d |
AuCl3 (10) |
MeCN |
80 |
36 |
47 |
— |
The above experiments concludes that Table 1, entry 17 is the best suitable reaction conditions. Then substrate scope was tested by utilizing different enynones (1a–k) with anthranilamide 2a under the optimal conditions. These results are incorporated in the Table 2.
Table 2 Scope of substituted dihydroquinazolinones (3)a
| Reaction conditions: all reactions were carried out at 80 °C under nitrogen atmosphere with 1 (1.0 equiv.), and 2a (1.5 equiv.) in the presence of KAuCl4 (10 mol%), FeCl3 (2.0 equiv.) and solvent (3 mL) in oil bath; yields are for isolated products. |
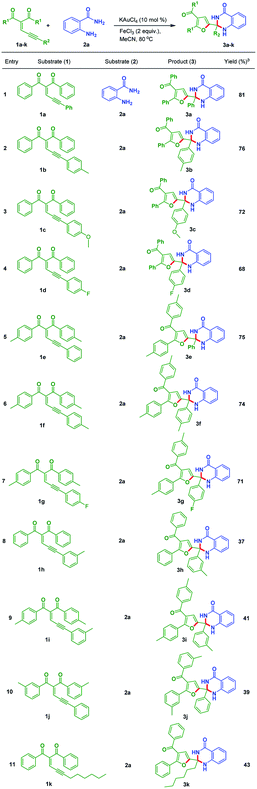 |
The substrates which are bearing electron-donating groups such as 1b and 1c were tested with 2a to provide 76% and 72% yields of corresponding products 3b and 3c, respectively. Electron-withdrawing functional group containing enynone such as 1d react with 2a to give the corresponding dihydroquinazolinone derivative 3d in 68% yield. Substrates bearing electron-donating groups like 1e and 1f reacted with 2a to produce 75% and 74% yields of the corresponding dihydroquinazolinone derivatives 3e and 3f, respectively. Both electron-donating and electron-withdrawing functional groups containing enynone like 1g reacted with 2a to generate the corresponding product 3g in 71% yield. The substrates which are having electron-donating groups at ortho position of R2 like 1h (R2 = 3-Me-C6H4), 1i (R2 = 3-Me-C6H4, R1 = 4-Me-C6H4) and 1j (R1 = 3-Me-C6H4) reacted with 2a to provide the corresponding products 3h, 3i and 3j in moderate yields, respectively (Table 2, entries 8–10). Alkyl substitution at R2 position containing substrate like 1k produced the product 3k in 43% yield (Table 2, entry 11).
Further, experiments were conducted to check the scope of dihydroquinazolinone derivatives by utilizing different substituted anthranilamides (2b–e) with enynones (1a–f). These results were included in the Table 3. The enynone 1a was tested with electron-donating functional group containing anthranilamide 2b to give 3l in 72% yield. Electron-withdrawing functional group containing anthranilamides such as 2c, 2d, and 2e reacted with 1a to produce the corresponding dihydroquinazolinone derivatives 3m, 3n, and 3o in 65%, 63% and 60% yields, respectively (Table 3, entries 2–4). Enynones bearing electron donating groups such as 1b and 1c reacted with 2b to provide corresponding products 3p and 3q in 72% and 70% yields, respectively (Table 3, entries 5 and 6). Fluorine substituted enynone such as 1d reacted with 2b, 2c, 2d and 2e to provide the corresponding dihydroquinazolinone derivatives such as 3r, 3s, 3t and 3u in 69%, 63%, 61% and 62% yields, respectively (Table 3, entries 7 and 10). Electron-donating substitutions containing enynones such as 1e and 1f reacted with 2b under optimized reaction conditions to give the corresponding products 3v and 3w in 70% and 68% yields, respectively (Table 3, entries 11 and 12).
Table 3 Scope of substituted dihydroquinazolinones (3)a
| Reaction conditions: all reactions were carried out at 80 °C under nitrogen atmosphere with 1 (1.0 equiv.), and 2 (1.5 equiv.) in the presence of KAuCl4 (10 mol%), FeCl3 (2.0 equiv.) and solvent (3 mL) in oil bath; yields are for isolated products 3. |
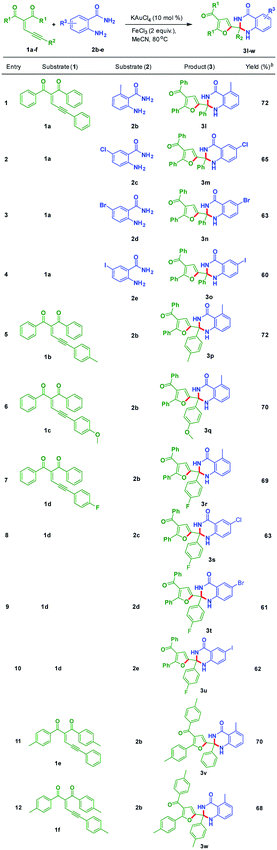 |
2-Amino-6-phenyl-4-(trifluoromethyl)nicotinamide 2f reacted with 1a to produce the corresponding product 3t in 58% yield (Scheme 3, eqn (1)). An experiment was conducted by employing a phosphorus substituted enynone like 1l with 2a to provide the corresponding dihydroquinazolinone derivative 3y in 68% yield (Scheme 3, eqn (2)). Further, one more experiment was conducted in gram scale by utilizing 1a and 2a under optimized reaction conditions to give the corresponding product 3a in 74% yield (Scheme 3, eqn (3)).
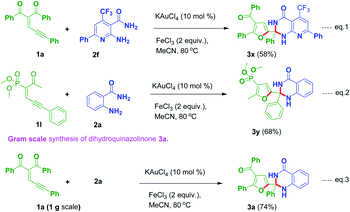 |
| | Scheme 3 (a) Reaction of 1a with heteroaryl amine 2f (eqn (1)); (b) reaction of dimethyl-(2-oxo-6-phenylhex-3-en-5-yn-3-yl)phosphonate 1l with 2a (eqn (2)); (c) gram scale synthesis of product 3a (eqn (3)). | |
Control experiments were conducted to clarify the reaction mechanism (Scheme 4). The substrate 1a was tested under optimized conditions to produce good yields of product 3a′ (Scheme 4, eqn (1)). A reaction was conducted by utilizing 3a′ with anthranilamide 2a in the presence of gold-catalyst to provide 32% yield of product 3a (Scheme 4, eqn (2)). Without using catalyst one reaction was conducted by using 3a′ and 2a, in this case product 3a was not observed (Scheme 4, eqn (3)). Then 1a was tested with 2-(prop-2-yn-1-yloxy)benzohydrazide 4 to give 62% yield of product 5. The structure of the compound 5a further charaecterized by single crystal X-ray analysis.22
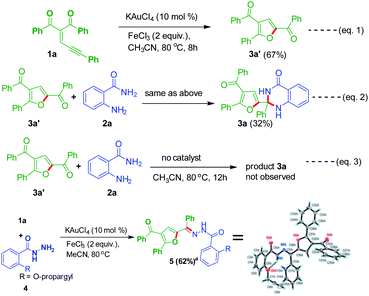 |
| | Scheme 4 Control experiments. aCompound 5 CCDC 1898773.22 | |
Formation of dihydroquinazolinones can be proposed by the reaction mechanism as shown in Scheme 5. Gold catalyst would coordinate with enynone 1a may form complex-A, which would further generate 2-furyl gold carbene complex-I via intramolecular of 5-exo-dig cyclised zwitterionic complex-B.24 Then, it would produce ketone (3a′),13,14 which would coordinate with ferric chloride as a lewis acid in a regioselective fashion then it would reacts with anthranilamide (2a) may generate IM-I. Subsequent activation of IM-I by metal catalyst may lead to cyclization to form intermediate IM-II, which would finally afford the product 3a.
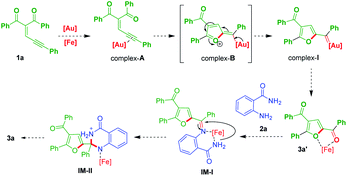 |
| | Scheme 5 A plausible reaction mechanism. | |
Conclusion
In conclusion, we have established gold-catalyzed reaction of enynones with dual insertion of anthranilamides to produce a novel approach for synthesis of dihydroquinazolinones. It is significant that in this organic transformation new C–O and two C–N bonds were formed with a quaternary centre with good functional group tolerance.
Experimental section
General information
Reactions were carried out in oven dried reaction flasks under nitrogen atmosphere and also solvents and reagents were transferred by oven-dried syringes to ambient temperature. TLC was performed on Merck silica gel aluminium sheets using UV as a visualizing agent. Solvents were removed under reduced pressure. Columns were packed as slurry of silica gel in hexane and ethyl acetate solvent mixture. The elution was assisted by applying pressure with an air pump. 13C NMR spectra were recorded on 75, 100 and 125 MHz spectrometers. 1HNMR spectra were recorded on 300, 400 and 500 MHz spectrometers in appropriate solvents using TMS as internal standard. The following abbreviations were used to explain multiplicities: s = singlet, d = doublet, dd = double doublet, dt = doublet of triplet, td = triplet of doublet, t = triplet, m = multiplet, br s = broad singlet. All reactions were performed under nitrogen atmosphere with freshly distilled and dried solvents. All solvents were distilled using standard procedures. Unless otherwise noted, reagents were obtained from Aldrich, Alfa Aesar, and TCI used without further purification. Synthesis of enynones (1a–l) were prepared by following reported procedures.25
General procedure for synthesis of dihydroquinazolinone derivatives (3a)
To a 10 mL round-bottomed flask equipped with magnetic stir bar the substrate 2-aminobenzamide 2a (0.45 mmol, 61 mg, 1.5 equiv.) was taken and dissolved in dry CH3CN (3 mL) at 80 °C (oil bath) after that 1,3-diphenyl-2-(3-phenylprop-2-yn-1-ylidene)propane-1,3-dione 1a (0.3 mmol, 100 mg, 1 equiv.) was added. To this reaction mixture KAuCl4 (10 mol%, 11 mg) and FeCl3 (0.6 mmol, 97 mg, 2.0 equiv.) was added and stirred at 80 °C for 5 h under nitrogen atmosphere. Progress of the reaction was monitored by using TLC. After completion of the reaction, the reaction mixture was filtered through celite plug and washed with ethyl acetate. The ethyl acetate layer was concentrated under reduced pressure to get crude residue which was purified by column chromatography through silica gel using hexane and ethyl acetate as eluent (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5) to give 113 mg of the product 2-(4-benzoyl-5-phenylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 3a (81% yield). The same reaction was conducted on a gram scale by utilizing 1a (1 g) and 2a (0.61 g) produced the corresponding product 3a in 74% yield (1.03 g). A similar experimental procedure was adopted for the synthesis of all the furan containing dihydroquinazolinones (3b–y) and 5.
3.5) to give 113 mg of the product 2-(4-benzoyl-5-phenylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 3a (81% yield). The same reaction was conducted on a gram scale by utilizing 1a (1 g) and 2a (0.61 g) produced the corresponding product 3a in 74% yield (1.03 g). A similar experimental procedure was adopted for the synthesis of all the furan containing dihydroquinazolinones (3b–y) and 5.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3a). Rf: 0.5; hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate mixture (10
ethyl acetate mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5); yellow solid with 113 mg (81%) yield; melting point: 188–190 °C; 1H NMR (500 MHz, CDCl3): δ 7.93 (dd, J = 7.7, 1.2 Hz, 1H), 7.73–7.66 (m, 2H), 7.63–7.56 (m, 4H), 7.52–7.48 (m, 1H), 7.47–7.42 (m, 3H), 7.37–7.31 (m, 3H), 7.30–7.26 (m, 3H), 6.97–6.87 (m, 1H), 6.79–6.70 (m, 2H), 6.35 (s, 1H), 5.09 (br s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.0, 156.3, 153.1, 145.2, 140.4, 137.5, 134.4, 133.0, 129.7, 129.6, 129.3, 129.0, 128.8, 128.4, 128.3, 128.2, 127.4, 126.9, 120.9, 119.8, 115.1, 114.9, 114.0, 72.7 ppm; IR(KBr): ν = 3368, 3057, 2922, 1654, 1613, 1485, 1367, 1262 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H21N2O3H 471.1703, found 471.1705.
3.5); yellow solid with 113 mg (81%) yield; melting point: 188–190 °C; 1H NMR (500 MHz, CDCl3): δ 7.93 (dd, J = 7.7, 1.2 Hz, 1H), 7.73–7.66 (m, 2H), 7.63–7.56 (m, 4H), 7.52–7.48 (m, 1H), 7.47–7.42 (m, 3H), 7.37–7.31 (m, 3H), 7.30–7.26 (m, 3H), 6.97–6.87 (m, 1H), 6.79–6.70 (m, 2H), 6.35 (s, 1H), 5.09 (br s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.0, 156.3, 153.1, 145.2, 140.4, 137.5, 134.4, 133.0, 129.7, 129.6, 129.3, 129.0, 128.8, 128.4, 128.3, 128.2, 127.4, 126.9, 120.9, 119.8, 115.1, 114.9, 114.0, 72.7 ppm; IR(KBr): ν = 3368, 3057, 2922, 1654, 1613, 1485, 1367, 1262 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H21N2O3H 471.1703, found 471.1705.
Crystal data for 3a. C31H22N2O3 (M =470.50 g mol−1): triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 8.2197(2) Å, b = 10.4608(2) Å, c = 14.4752(3) Å, α = 77.0948(8)°, β = 79.6924(8)°, γ = 81.5655(9)°, V = 1186.21(4) Å3, Z = 2, T = 294.15 K, μ(MoKα) = 0.085 mm−1, Dcalc = 1.317 g cm−3, 35
(no. 2), a = 8.2197(2) Å, b = 10.4608(2) Å, c = 14.4752(3) Å, α = 77.0948(8)°, β = 79.6924(8)°, γ = 81.5655(9)°, V = 1186.21(4) Å3, Z = 2, T = 294.15 K, μ(MoKα) = 0.085 mm−1, Dcalc = 1.317 g cm−3, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 reflections measured (4.464° ≤ 2Θ ≤ 61.018°), 7216 unique (Rint = 0.0618, Rsigma = 0.0518) which were used in all calculations. The final R1 was 0.0634 (I > 2σ(I)) and wR2 was 0.1669 (all data). CCDC 1863534.
683 reflections measured (4.464° ≤ 2Θ ≤ 61.018°), 7216 unique (Rint = 0.0618, Rsigma = 0.0518) which were used in all calculations. The final R1 was 0.0634 (I > 2σ(I)) and wR2 was 0.1669 (all data). CCDC 1863534.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(p-tolyl)-2,3-dihydroquinazolin-4(1H)-one (3b). Following the general procedure, 100 mg (0.285 mmol, 1.0 equiv.) of 1b, 58 mg (0.428 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 92 mg (0.571 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.5; hexane/ethyl acetate mixture 10/3.5), 105 mg of 3b was obtained in 76% yield as a yellow solid. Mp: 166–168 °C; 1H NMR (500 MHz, CDCl3): δ 7.92 (dd, J = 7.8, 1.2 Hz, 1H), 7.74–7.65 (m, 2H), 7.64–7.56 (m, 2H), 7.53–7.43 (m, 3H), 7.39–7.31 (m, 3H), 7.30–7.21 (m, 5H), 6.94–6.87 (m, 1H), 6.75–6.70 (m, 2H), 6.31 (s, 1H), 5.06 (br s, 1H), 2.38 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 163.8, 156.2, 153.4, 145.3, 139.7, 137.6, 137.5, 134.2, 132.9, 129.6, 129.4, 129.2, 129.0, 128.37, 128.31, 128.2, 127.4, 126.8, 120.9, 119.7, 115.1, 114.8, 113.8, 72.5, 21.0 ppm; IR(KBr): ν = 3376, 3068, 2922, 1654, 1612, 1484, 1368, 1267 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H23N2O3H 485.1859, found 485.1862.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(4-methoxyphenyl)-2,3-dihydroquinazolin-4(1H)-one (3c). Following the general procedure, 100 mg (0.273 mmol, 1.0 equiv.) of 1c, 55 mg (0.409 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 88 mg (0.546 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.5; hexane/ethyl acetate mixture 10/3.5), 98 mg of 3c was obtained in 72% yield as a yellow solid. Mp: 111–113 °C; 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 7.3 Hz, 1H), 7.75–7.46 (m, 6H),7.39–7.23 (m, 7H), 7.01–6.88 (m, 3H), 6.79–6.65 (m, 2H), 6.28 (s, 1H), 5.04 (br s, 1H), 3.83 (s, 3H), ppm; 13C NMR (100 MHz, CDCl3): δ191.1, 163.9, 160.5, 156.3, 153.3, 145.3, 137.5, 134.3, 133.0, 132.5, 129.1, 128.4, 128.3, 128.2, 127.4, 120.9, 119.8, 115.2, 114.8, 114.0, 113.9, 72.4, 55.3 ppm; IR(KBr): ν = 3285, 3058, 2925, 1655, 1609, 1507, 1368, 1254 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H23N2O4H 501.1808, found 501.1812.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(4-fluorophenyl)-2,3-dihydroquinazolin-4(1H)-one (3d). Following the general procedure, 100 mg (0.282 mmol, 1.0 equiv.) of 1d, 57 mg (0.423 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 91 mg (0.564 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.5; hexane/ethyl acetate mixture 10/3.5), 95 mg of 3d was obtained in 68% yield as a yellow solid. Mp: 138–140 °C; 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 7.4 Hz, 1H), 7.73–7.47 (m, 7H), 7.39–7.24 (m, 6H), 7.17–7.06 (m, 2H), 6.98–6.87 (m, 1H), 6.80–6.69 (m, 2H), 6.45 (s, 1H), 5.07 (br s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 191.0, 163.8, 162.2 (d, 3JC–F = 250.148 Hz), 156.4, 152.9, 145.1, 137.4, 136.4, 134.4, 133.0, 129.6, 129.4, 129.12 (d, 2JC–F = 8.069 Hz), 128.9, 128.3, 128.2, 127.4, 120.9, 120.0, 115.7 (d, 1JC–F = 21.274 Hz), 115.1, 114.9, 114.0, 72.3 ppm; IR(KBr): ν = 3283, 3060, 2922, 1656, 1609, 1493, 1367, 1229 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H20FN2O3H 489.1609, found 489.1607.
2-(4-(4-Methylbenzoyl)-5-p-tolylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3e). Following the general procedure, 100 mg (0.274 mmol, 1.0 equiv.) of 1e, 56 mg (0.412 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 89 mg (0.549 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.5; hexane/ethyl acetate mixture 10/3.5), 103 mg of 3e was obtained in 75% yield as a yellow solid. Mp: 223–225 °C; 1H NMR (500 MHz, CDCl3): δ 7.90 (d, J = 7.4 Hz, 1H), 7.62–7.56 (m, 4H), 7.51 (d, J = 8.0 Hz, 2H), 7.44–7.39 (m, 3H), 7.36–7.30 (m, 1H), 7.14 (d, J = 7.3 Hz, 2H), 7.06 (d, J = 7.1 Hz, 2H), 6.93–6.87 (m, 1H), 6.73 (d, J = 7.9 Hz, 1H), 6.69 (s, 1H), 6.55–6.27 (m, 1H), 5.28–5.02 (br s, 1H), 2.37 (s, 3H), 2.30 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.8, 163.8, 156.2, 152.6, 145.3, 143.8, 140.6, 139.4, 135.0, 134.2, 129.7, 129.5, 129.0, 128.9, 128.6, 128.3, 127.2, 126.9, 126.3, 120.4, 119.6, 115.1, 114.8, 114.0, 72.6, 21.5, 21.2 ppm; IR(KBr): ν = 3357, 3050, 2918, 1653, 1612, 1507, 1372, 1271, 1179 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H25N2O3H 499.2016, found 499.2023.
2-(4-(4-Methylbenzoyl)-5-p-tolylfuran-2-yl)-2-p-tolyl-2,3-dihydroquinazolin-4(1H)-one (3f). Following the general procedure, 100 mg (0.264 mmol, 1.0 equiv.) of 1f, 54 mg (0.396 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 85 mg (0.529 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.5; hexane/ethyl acetate mixture 10/3.5), 100 mg of 3f was obtained in 74% yield as a yellow solid. Mp: 210–212 °C; 1H NMR (500 MHz, CDCl3): δ 7.91 (dd, J = 7.4, 1.0 Hz, 1H), 7.60 (d, J = 8.0 Hz, 2H), 7.51 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 7.35–7.31 (m, 1H), 7.22 (d, J = 8.0 Hz, 2H), 7.14 (d, J = 8.0 Hz, 2H), 7.07 (d, J = 8.2 Hz, 2H), 6.92–6.88 (m, 1H), 6.71 (d, J = 8.0 Hz, 1H), 6.68 (s, 1H), 6.27 (s, 1H), 5.06 (br s, 1H), 2.38 (s, 6H), 2.30 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.8, 163.9, 156.1, 152.8, 145.4, 143.7, 139.5, 139.3, 137.7, 135.0, 134.1, 129.7, 129.3, 128.9, 128.8, 128.2, 127.2, 126.8, 126.4, 120.4, 119.5, 115.1, 114.8, 113.9, 72.5, 21.5, 21.2, 21.0 ppm; IR(KBr): ν = 3346, 3033, 2918, 1660, 1643, 1503, 1374, 1179 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C34H27N2O3H 513.2172, found 513.2176.
2-(4-Fluorophenyl)-2-(4-(4-methylbenzoyl)-5-p-tolylfuran-2-yl)-2,3-dihydroquinazolin-4(1H)-one (3g). Following the general procedure, 100 mg (0.261 mmol, 1.0 equiv.) of 1g, 53 mg (0.392 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 85 mg (0.523 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.5; hexane/ethyl acetate mixture 10/3.5), 96 mg of 3g was obtained in 71% yield as a yellow solid. Mp: 223–225 °C; 1H NMR (400 MHz, CDCl3): δ 7.91 (dd, J = 7.7, 1.2 Hz, 1H), 7.63–7.55 (m, 4H), 7.50 (d, J = 8.1 Hz, 2H), 7.38–7.31 (m, 1H), 7.16–7.06 (m, 6H), 6.94–6.88 (m, 1H), 6.73 (d, J = 8.0 Hz, 1H), 6.70 (s, 1H), 6.39 (s, 1H), 5.06 (br s, 1H), 2.38 (s, 3H), 2.31 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.8, 163.8, 163.2 (d, 3JC–F = 249.4 Hz), 156.3, 152.3, 145.1, 143.9, 139.6, 136.6, 134.9, 134.3, 129.7, 129.1, 129.0 (d, 2JC–F = 7.3 Hz), 128.3, 127.2, 126.3, 120.5, 119.9, 115.7 (d, 1JC–F = 22.0 Hz), 115.2, 114.9, 114.1, 72.3, 21.6, 21.3 ppm; IR(KBr): ν = 3343, 3051, 2918, 1662, 1643, 1507, 1373, 1234, 893 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H24FN2O3H 517.1922, found 517.1931.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(m-tolyl)-2,3-dihydroquinazolin-4(1H)-one (3h). Following the general procedure, 100 mg (0.285 mmol, 1.0 equiv.) of 1h, 58 mg (0.427 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 92 mg (0.571 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 52 mg of 3h was obtained in 37% yield as a light yellow solid. Mp: 224–226 °C; 1H NMR (400 MHz, CDCl3): δ 7.93 (dd, J = 7.8, 1.4 Hz, 1H), 7.71–7.67 (m, 2H), 7.63–7.57 (m, 2H), 7.53–7.46 (m, 2H), 7.37–7.23 (m, 9H), 6.94–6.89 (m, 1H), 6.76–6.72 (m, 2H), 6.33 (s, 1H), 5.08 (br s, 1H), 2.39 (s, 3H). ppm; 13C NMR (100 MHz, DMSO-d6 & CDCl3): δ 189.1, 162.5, 153.7, 153.0, 145.2, 140.2, 136.0, 132.0, 131.4, 127.9, 127.8, 127.7, 127.6, 126.8, 126.7, 126.5, 126.2, 125.8, 122.7, 119.2, 116.4, 113.4, 113.2, 111.6, 70.6, 19.9. ppm; IR(KBr): ν = 3335, 3061, 2919, 1668, 1487, 1265, 1148, 756 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H24N2O3H 485.1865, found 485.1864.
2-(4-(4-Methylbenzoyl)-5-(p-tolyl)furan-2-yl)-2-(m-tolyl)-2,3-dihydroquinazolin-4(1H)-one (3i). Following the general procedure, 100 mg (0.264 mmol, 1.0 equiv.) of 1i, 54 mg (0.396 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 85 mg (0.529 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 56 mg of 3i was obtained in 41% yield as a light yellow solid. Mp: 204–206 °C; 1H NMR (400 MHz, CDCl3): δ 7.93 (dd, J = 7.8, 1.3 Hz, 1H), 7.60 (d, J = 8.1 Hz, 2H), 7.52 (d, J = 8.1 Hz, 2H), 7.46 (s, 1H), 7.37–7.23 (m, 4H), 7.15 (d, J = 7.9 Hz, 2H), 7.08 (d, J = 8.0 Hz, 2H), 6.94–6.89 (m, 1H), 6.72 (d, J = 7.9 Hz, 1H), 6.68 (s, 1H), 6.24 (s, 1H), 5.04 (br s, 1H), 2.38 (d, J = 1.5 Hz, 6H), 2.31 (s, 3H). ppm; 13C NMR (75 MHz, CDCl3): δ 190.9, 163.8, 156.2, 152.7, 145.3, 143.8, 140.4, 139.5, 138.7, 135.1, 134.3, 130.5, 129.8, 129.0, 128.9, 128.6, 128.4, 127.6, 127.3, 126.4, 123.9, 120.5, 119.8, 115.2, 114.9, 114.1, 72.7, 21.6, 21.5, 21.3. ppm; IR(KBr): ν = 3448, 3034, 2919, 1663, 1498, 1271, 1155, 754 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C34H28N2O3H 513.2178, found 513.2180.
2-(4-(3-Methylbenzoyl)-5-(m-tolyl)furan-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3j). Following the general procedure, 100 mg (0.274 mmol, 1.0 equiv.) of 1j, 56 mg (0.412 mmol, 1.5 equiv.) of 2a, 10 mg (10 mol%) of KAuCl4 and 89 mg (0.549 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 54 mg of 3j was obtained in 39% yield as a light yellow solid. Mp: 102–104 °C; 1H NMR (400 MHz, CDCl3): δ 7.93 (dd, J = 7.7, 1.3 Hz, 1H), 7.61–7.56 (m, 2H), 7.51–7.42 (m, 5H), 7.40–7.28 (m, 4H), 7.21 (t, J = 7.6 Hz, 1H), 7.17–7.12 (m, 1H), 7.09 (d, J = 7.6 Hz, 1H), 6.94–6.89 (m, 1H), 6.76–6.72 (m, 2H), 6.48 (s, 1H), 5.09 (br s, 1H), 2.29 (s, 3H), 2.25 (s, 3H). ppm; 13C NMR (75 MHz, CDCl3): δ 191.3, 164.0, 156.6, 152.9, 145.2, 140.4, 138.1, 137.9, 137.6, 134.4, 133.7, 130.1, 129.7, 129.0, 128.8, 128.4, 128.2, 128.1, 126.9, 126.8, 124.7, 121.0, 119.9, 115.1, 114.9, 114.0, 72.7, 21.2, 21.1. ppm; IR(KBr): ν = 3318, 3056, 2920, 1657, 1485, 1270, 1144, 754 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H26N2O3H 499.2022, found 499.2027.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-hexyl-2,3-dihydroquinazolin-4(1H)-one (3k). Following the general procedure, 100 mg (0.29 mmol, 1.0 equiv.) of 1k, 59 mg (0.436 mmol, 1.5 equiv.) of 2a, 11 mg (10 mol%) of KAuCl4 and 94 mg (0.581 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 60 mg of 3k was obtained in 43% yield as a light yellow semi solid. 1H NMR (400 MHz, CDCl3): δ 7.87 (dd, J = 7.8, 1.3 Hz, 1H), 7.68–7.65 (m, 2H), 7.60–7.57 (m, 2H), 7.49–7.44 (m, 1H), 7.33–7.24 (m, 6H), 7.20 (s, 1H), 6.88–6.83 (m, 1H), 6.68 (d, J = 7.9 Hz, 1H), 6.52 (s, 1H), 4.75 (br s, 1H), 2.26–2.13 (m, 2H), 1.42–1.23 (m, 8H), 0.91–0.85 (m, 3H). ppm; 13C NMR (100 MHz, CDCl3): δ 191.2, 165.0, 155.6, 154.7, 145.6, 137.5, 134.2, 132.9, 129.6, 129.2, 128.2, 127.3, 126.1, 121.0, 119.4, 114.8, 114.6, 111.1, 70.2, 39.9, 31.5, 29.0, 23.1, 22.4, 13.9. ppm; IR(KBr): ν = 3301, 3060, 2927, 1661, 1486, 1270, 1150, 756 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H30N2O3H 479.2335, found 479.2339.
2-(4-Benzoyl-5-phenylfuran-2-yl)-5-methyl-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3l). Following the general procedure, 100 mg (0.3 mmol, 1.0 equiv.) of 1a, 67 mg (0.45 mmol, 1.5 equiv.) of 2b, 11 mg (10 mol%) of KAuCl4 and 97 mg (0.6 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.7; hexane/ethyl acetate mixture 10/2.5), 104 mg of 3l was obtained in 72% yield as a yellow solid. Mp: 228–230 °C; 1H NMR (400 MHz, CDCl3): δ 7.72–7.68 (m, 2H), 7.64–7.57 (m, 3H), 7.55–7.48 (m, 1H), 7.46–7.42 (m, 3H), 7.39–7.33 (m, 2H), 7.30–7.25 (m, 4H), 7.17 (t, J = 7.8 Hz, 1H), 6.75 (s, 1H), 6.71 (d, J = 7.5 Hz, 1H), 6.59 (d, J = 7.9 Hz, 1H), 6.21 (s, 1H), 5.03 (d, J = 1.4 Hz, 1H), 2.68 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.3, 156.2, 153.2, 146.5, 142.3, 140.4, 137.6, 133.1, 133.0, 129.7, 129.6, 129.3, 129.1, 128.8, 128.3, 128.2, 127.4, 127.0, 123.6, 120.9, 114.2, 113.8, 113.2, 72.1, 22.2 ppm; IR(KBr): ν = 3341, 3060, 2927, 1659, 1636, 1519, 1368, 1265 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H23N2O3H 485.1859, found 485.1873.
2-(4-Benzoyl-5-phenylfuran-2-yl)-6-chloro-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3m). Following the general procedure, 100 mg (0.3 mmol, 1.0 equiv.) of 1a, 76 mg (0.45 mmol, 1.5 equiv.) of 2c, 11 mg (10 mol%) of KAuCl4 and 97 mg (0.6 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 98 mg of 3m was obtained in 65% yield as a yellow solid. Mp: 148–150 °C; 1H NMR (400 MHz, CDCl3): δ 7.89 (s, 1H), 7.69 (d, J = 7.4 Hz, 2H), 7.61–7.41 (m, 8H), 7.38–7.33 (m, 1H), 7.31–7.23 (m, 5H), 6.75–6.67 (m, 2H), 6.48 (s, 1H), 5.16 (s, 1H), ppm; 13C NMR (75 MHz, DMSO-d6 & CDCl3): δ 190.6, 162.7, 155.6, 153.1, 144.5, 140.5, 137.1, 133.3, 132.6, 129.1, 128.9, 128.8, 128.7, 128.1, 127.9, 127.7, 127.1, 126.9, 126.7, 123.0, 120.5, 116.2, 115.5, 113.4, 72.1 ppm; IR(KBr): ν = 3283, 3060, 2924, 1658, 1610, 1486, 1265, 891 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H20ClN2O3H 505.1313, found 505.1322.
2-(4-Benzoyl-5-phenylfuran-2-yl)-6-bromo-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3n). Following the general procedure, 100 mg (0.3 mmol, 1.0 equiv.) of 1a, 86 mg (0.45 mmol, 1.5 equiv.) of 2d, 11 mg (10 mol%) of KAuCl4 and 97 mg (0.6 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 103 mg of 3n was obtained in 63% yield as a yellow solid. Mp: 186–188 °C; 1H NMR (400 MHz, CDCl3): δ 8.02 (d, J = 2.0 Hz, 1H), 7.73–7.67 (m, 2H), 7.61–7.48 (m, 5H), 7.47–7.40 (m, 4H), 7.38–7.33 (m, 1H), 7.30–7.22 (m, 4H), 6.73 (s, 1H), 6.64 (d, J = 8.5 Hz, 1H), 6.49 (s, 1H), 5.18 (br s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 191.0, 162.7, 156.4, 152.7, 144.2, 140.0, 137.4, 136.9, 133.1, 130.8, 129.8, 129.6, 129.4, 128.9, 128.8, 128.3, 128.2, 127.4, 126.9, 120.9, 116.7, 114.1, 111.8, 72.7 ppm; IR(KBr): ν = 3325, 3064, 2923, 1651, 1605, 1490, 1319, 1144 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H20BrN2O3H 549.0808, found 549.0824.
2-(4-Benzoyl-5-phenylfuran-2-yl)-6-iodo-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3o). Following the general procedure, 100 mg (0.3 mmol, 1.0 equiv.) of 1a, 117 mg (0.45 mmol, 1.5 equiv.) of 2e, 11 mg (10 mol%) of KAuCl4 and 96 mg (0.6 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 107 mg of 3o was obtained in 60% yield as a yellow solid. Mp: 200–202 °C; 1H NMR (400 MHz, CDCl3): δ 8.21 (s, 1H), 7.70 (d, J = 7.4 Hz, 2H), 7.62–7.23 (m, 14H), 6.73 (s, 1H), 6.53 (d, J = 8.3 Hz, 1H), 6.41 (s, 1H), 5.16 (br s, 1H) ppm; 13C NMR (75 MHz, DMSO-d6 & CDCl3): δ 189.4, 161.5, 154.3, 152.7, 144.9, 140.3, 140.2, 136.3, 134.5, 131.7, 128.1, 128.0, 127.7, 127.1, 127.0, 126.9, 126.2, 125.9, 119.5, 116.2, 115.7, 112.2, 70.9 ppm; IR(KBr): ν = 3324, 3062, 1646, 1602, 1489, 1318, 1146, 890 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H20IN2O3H 597.0669, found 597.0696.
2-(4-Benzoyl-5-phenylfuran-2-yl)-5-methyl-2-(p-tolyl)-2,3-dihydroquinazolin-4(1H)-one (3p). Following the general procedure, 100 mg (0.285 mmol, 1.0 equiv.) of 1b, 64 mg (0.428 mmol, 1.5 equiv.) of 2b, 10 mg (10 mol%) of KAuCl4 and 92 mg (0.571 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.7; hexane/ethyl acetate mixture 10/2.5), 103 mg of 3p was obtained in 72% yield as a yellow solid. Mp: 202–204 °C; 1H NMR (400 MHz, CDCl3): δ 7.69 (d, J = 7.3 Hz, 2H), 7.66–7.60 (m, 2H), 7.54–7.43 (m, 3H), 7.38–7.32 (m, 2H), 7.29–7.21 (m, 5H), 7.18–7.13 (m, 1H), 6.73 (s, 1H), 6.69 (d, J = 7.4 Hz, 1H), 6.58 (d, J = 7.9 Hz, 1H), 6.29 (s, 1H), 5.03 (br s, 1H), 2.67 (s, 3H), 2.38 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.4, 156.1, 153.4, 146.6, 142.2, 139.6, 137.67, 137.61, 133.0, 132.9, 129.6, 129.4, 129.2, 129.1, 128.3, 128.2, 127.4, 126.8, 123.5, 120.8, 114.0, 113.7, 113.2, 71.9, 22.1, 21.1 ppm; IR(KBr): ν = 3321, 3037, 2919, 1652, 1601, 1507, 1373, 1269 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H25N2O3H 499.2016, found 499.2021.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(4-methoxyphenyl)-5-methyl-2,3-dihydroquinazolin-4(1H)-one (3q). Following the general procedure, 100 mg (0.273 mmol, 1.0 equiv.) of 1c, 61 mg (0.409 mmol, 1.5 equiv.) of 2b, 10 mg (10 mol%) of KAuCl4 and 88 mg (0.546 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.7; hexane/ethyl acetate mixture 10/2.5), 98 mg of 3q was obtained in 70% yield as a yellow solid. Mp: 223–225 °C; 1H NMR (400 MHz, CDCl3): δ 7.69 (d, J = 7.3 Hz, 2H), 7.66–7.61 (m, 2H), 7.54–7.46 (m, 3H), 7.39–7.33 (m, 2H), 7.30–7.25 (m, 3H), 7.19–7.13 (m, 1H), 6.92 (d, J = 8.8 Hz, 2H), 6.73 (s, 1H), 6.69 (d, J = 7.4 Hz, 1H), 6.58 (d, J = 7.8 Hz, 1H), 6.25 (s, 1H), 5.01 (br s, 1H), 3.83 (s, 3H), 2.67 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.4, 160.4, 156.1, 153.4, 146.6, 142.2, 137.6, 133.1, 132.9, 132.5, 129.6, 129.3, 129.1, 128.4, 128.3, 128.2, 127.4, 123.6, 120.8, 114.1, 113.9, 113.7, 113.2, 71.8, 55.3, 22.2 ppm; IR(KBr): ν = 3326, 3034, 2920, 1652, 1601, 1506, 1371, 1251 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C33H25N2O4H 515.1965, found 515.1974.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(4-fluorophenyl)-5-methyl-2,3-dihydroquinazolin-4(1H)-one (3r). Following the general procedure, 100 mg (0.282 mmol, 1.0 equiv.) of 1d, 63 mg (0.423 mmol, 1.5 equiv.) of 2b, 10 mg (10 mol%) of KAuCl4 and 91 mg (0.564 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.7; hexane/ethyl acetate mixture 10/2.5), 98 mg of 3r was obtained in 69% yield as a yellow solid. Mp: 170–172 °C; 1H NMR (400 MHz, CDCl3): δ 7.68 (d, J = 7.4 Hz, 2H), 7.63–7.49 (m, 4H), 7.38–7.25 (m, 6H), 7.21–7.07 (m, 3H), 6.77–6.69 (m, 2H), 6.59 (d, J = 7.9 Hz, 1H), 6.38 (s, 1H), 5.02 (br s, 1H), 2.66 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): 191.0, 164.3, 163.2 (d, 3JC–F = 250.1 Hz), 156.3, 153.0, 146.4, 142.3, 137.5, 136.4, 133.2, 133.0, 129.6, 129.4, 129.17 (d, 2JC–F = 8.8 Hz), 129.0, 128.36, 128.30, 127.4, 123.8, 120.9, 115.7 (d, 1JC–F = 21.2 Hz), 114.2, 113.7, 113.2, 72.3, 22.1 ppm; IR(KBr): ν = 3397, 3062, 2923, 1661, 1601, 1504, 1360, 1224 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C32H22FN2O3H 503.1765, found 503.1766.
2-(4-Benzoyl-5-phenylfuran-2-yl)-6-chloro-2-(4-fluorophenyl)-2,3-dihydroquinazolin-4(1H)-one (3s). Following the general procedure, 100 mg (0.282 mmol, 1.0 equiv.) of 1d, 72 mg (0.423 mmol, 1.5 equiv.) of 2c, 10 mg (10 mol%) of KAuCl4 and 91 mg (0.564 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 93 mg of 3s was obtained in 63% yield as a yellow solid. Mp: 130–132 °C; 1H NMR (400 MHz, CDCl3): δ 7.85 (d, J = 2.2 Hz, 1H), 7.68 (d, J = 7.4 Hz, 2H), 7.59–7.47 (m, 5H), 7.37–7.22 (m, 6H), 7.15–7.06 (m, 2H), 6.76–6.67 (m, 3H), 5.19 (br s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.9, 163.3 (d, 3JC–F = 250.8 Hz), 162.8, 156.5, 152.4, 143.5, 137.3, 136.0, 134.3, 133.1, 129.6, 129.5, 129.13 (d, 2JC–F = 8.1 Hz), 128.8, 128.4, 128.3, 127.9, 127.5, 125.2, 120.9, 116.5, 116.4, 115.8 (d, 1JC–F = 22.0 Hz), 114.2, 72.3 ppm; IR(KBr): ν = 3279, 3062, 2923, 1660, 1607, 1486, 1347, 1231 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H19ClFN2O3H 523.1219, found 523.1230.
2-(4-Benzoyl-5-phenylfuran-2-yl)-6-bromo-2-(4-fluorophenyl)-2,3-dihydroquinazolin-4(1H)-one (3t). Following the general procedure, 100 mg (0.282 mmol, 1.0 equiv.) of 1d, 91 mg (0.423 mmol, 1.5 equiv.) of 2d, 10 mg (10 mol%) of KAuCl4 and 91 mg (0.564 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 98 mg of 3t was obtained in 61% yield as a yellow solid. Mp: 198–200 °C; 1H NMR (400 MHz, CDCl3): δ 8.02 (d, J = 2.0 Hz, 1H), 7.72–7.66 (m, 2H), 7.59–7.49 (m, 4H), 7.46–7.41 (m, 1H), 7.38–7.23 (m, 7H), 7.15–7.09 (m, 1H), 6.74 (s, 1H), 6.65 (d, J = 8.5 Hz, 1H), 6.56 (s, 1H), 5.13 (br s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.9, 163.3 (d, 3JC–F = 250.1 Hz), 162.7, 156.5, 152.4, 144.0, 137.3, 137.0, 135.9, 133.1, 130.9, 129.6, 129.5, 129.12 (d, 2JC–F = 8.8 Hz), 128.8, 128.4, 128.3, 127.5, 120.9, 116.820, 116.820, 115.8 (d, 1JC–F = 22.0 Hz), 112.1, 72.3 ppm; IR(KBr): ν = 3319, 3064, 1654, 1604, 1499, 1319, 1230, 1144 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H19BrFN2O3H 567.0714, found, 567.0732.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2-(4-fluorophenyl)-6-iodo-2,3-dihydroquinazolin-4(1H)-one (3u). Following the general procedure, 100 mg (0.282 mmol, 1.0 equiv.) of 1d, 111 mg (0.423 mmol, 1.5 equiv.) of 2e, 10 mg (10 mol%) of KAuCl4 and 91 mg (0.564 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 107 mg of 3u was obtained in 62% yield as a yellow solid. Mp: 194–196 °C; 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 1.5 Hz, 1H), 7.72–7.67 (m, 2H), 7.62–7.49 (m, 6H), 7.40–7.33 (m, 2H), 7.30–7.24 (m, 4H), 7.16–7.08 (m, 1H), 6.74 (s, 1H), 6.54 (d, J = 8.4 Hz, 1H), 6.49 (s, 1H), 5.13 (br s, 1H) ppm; 13C NMR (75 MHz, DMSO-d6 & CDCl3): δ 188.8, 160.95, 160.93 (d, 3JC–F = 247.0 Hz), 153.8, 152.1, 144.4, 140.0, 136.0, 135.8, 134.0, 131.4, 127.7, 127.6, 127.5, 126.7 (d, 2JC–F = 11.0 Hz), 125.8, 119.1, 115.9, 115.3, 113.4 (d, 1JC–F = 22.0 Hz), 111.7, 70.0 ppm; IR(KBr): ν = 3319, 3062, 1650, 1601, 1494, 1319, 1231, 1153 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H19FIN2O3H 615.0575, found, 615.0602.
5-Methyl-2-(4-(4-methylbenzoyl)-5-p-tolylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (3v). Following the general procedure, 100 mg (0.274 mmol, 1.0 equiv.) of 1e, 62 mg (0.412 mmol, 1.5 equiv.) of 2b, 10 mg (10 mol%) of KAuCl4 and 89 mg (0.549 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.7; hexane/ethyl acetate mixture 10/2.5), 99 mg of 3v was obtained in 70% yield as a yellow solid. Mp: 180–182 °C; 1H NMR (400 MHz, CDCl3): δ 7.64–7.52 (m, 6H), 7.46–7.40 (m, 3H), 7.19–7.13 (m, 3H), 7.08 (d, J = 8.0 Hz, 2H), 6.73–6.66 (m, 2H), 6.58 (d, J = 7.9 Hz, 1H), 6.24 (s, 1H), 5.04 (br s, 1H), 2.68 (s, 3H), 2.39 (s, 3H), 2.31 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.8, 164.3, 156.2, 152.6, 146.6, 143.8, 142.3, 140.6, 139.4, 135.2, 133.0, 129.7, 129.6, 129.0, 128.9, 128.7, 127.2, 127.0, 126.5, 123.6, 120.4, 114.3, 113.7, 113.2, 72.1, 22.2, 21.6, 21.3 ppm; IR(KBr): ν = 3326, 3056, 2922, 1652, 1602, 1499, 1368, 1269 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C34H27N2O3H 513.2172, found 513.2177.
5-Methyl-2-(4-(4-methylbenzoyl)-5-p-tolylfuran-2-yl)-2-p-tolyl-2,3-dihydroquinazolin-4(1H)-one (3w). Following the general procedure, 100 mg (0.264 mmol, 1.0 equiv.) of 1f, 60 mg (0.396 mmol, 1.5 equiv.) of 2b, 10 mg (10 mol%) of KAuCl4 and 85 mg (0.529 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.7; hexane/ethyl acetate mixture 10/2.5), 95 mg of 3w was obtained in 68% yield as a yellow solid. Mp: 228–230 °C; 1H NMR (400 MHz, CDCl3): δ 7.60 (d, J = 8.1 Hz, 2H), 7.54 (d, J = 8.3 Hz, 2H), 7.44 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 7.9 Hz, 2H), 7.19–7.12 (m, 3H), 7.08 (d, J = 8.0 Hz, 2H), 6.71–6.67 (m, 2H), 6.56 (d, J = 7.9 Hz, 1H), 6.24–6.17 (m, 1H), 5.04–4.98 (br s, 1H), 2.68 (s, 3H), 2.38 (d, J = 4.8 Hz, 6H), 2.31 (s, 3H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.9, 164.4, 156.1, 152.8, 146.7, 143.7, 142.2, 139.6, 139.3, 137.7, 135.2, 133.0, 129.7, 129.3, 129.0, 128.9, 127.2, 126.9, 126.5, 123.5, 120.4, 114.1, 113.7, 113.2, 71.9, 22.1, 21.6, 21.3, 21.0 ppm; IR(KBr): ν = 3328, 3040, 2922, 1648, 1602, 1501, 1368, 1269, 891 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C35H29N2O3H 527.2329, found 527.2346.
2-(4-Benzoyl-5-phenylfuran-2-yl)-2,7-diphenyl-5-(trifluoromethyl)-2,3-dihydropyrido[2,3-d]pyrimidin-4(1H)-one (3x). Following the general procedure, 100 mg (0.3 mmol, 1.0 equiv.) of 1a, 112 mg (0.45 mmol, 1.5 equiv.) of 2f, 10 mg (10 mol%) of KAuCl4 and 97 mg (0.6 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 106 mg of 3x was obtained in 58% yield as a yellow solid. Mp: 140–142 °C; 1H NMR (500 MHz, CDCl3): δ, 8.03–7.96 (m, 2H), 7.79–7.73 (m, 2H), 7.64–7.57 (m, 4H), 7.54–7.45 (m, 7H), 7.40–7.35 (m, 2H), 7.29–7.25 (m, 5H), 6.83 (s, 1H), 6.39 (s, 1H) ppm; 13C NMR (100 MHz, CDCl3): δ 190.9, 161.5, 160.7, 157.7, 156.7, 152.2, 139.7 (q, 2JC–F = 33.7 Hz), 139.3, 137.4, 136.8, 133.1, 130.7, 129.8, 129.5, 129.4, 128.9, 128.8, 128.3, 128.2, 127.4, 127.2, 126.6, 122.3 (q, 1JC–F = 272.1 Hz), 120.9, 114.3, 110.78, 110.73, 104.4, 71.2 ppm; IR(KBr): ν = 3196, 3063, 2922, 1677, 1579, 1447, 1263, 1156 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C37H23F3N3O3H 616.1842, found 616.1869.
Dimethyl(2-methyl-5-(4-oxo-2-phenyl-1,2,3,4-tetrahydroquinazolin-2-yl)furan-3-yl)phosphonate (3y). Following the general procedure, 100 mg (0.359 mmol, 1.0 equiv.) of 1l, 73 mg (0.539 mmol, 1.5 equiv.) of 2a, 14 mg (10 mol%) of KAuCl4 and 116 mg (0.719 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.8; hexane/ethyl acetate mixture 10/7.0), 101 mg of 3y was obtained in 68% yield as a light yellow solid. Mp: 226–228 °C; 1H NMR (300 MHz, CDCl3): δ 7.88 (d, J = 7.7 Hz, 1H), 7.50–7.30 (m, 6H), 6.87 (t, J = 7.7 Hz, 1H), 6.71 (d, J = 7.9 Hz, 1H), 6.50 (d, J = 3.3 Hz, 1H), 6.27 (s, 1H), 4.99 (br s, 1H), 3.75–3.63 (m, 6H), 2.45 (d, J = 1.9 Hz, 3H). ppm; 13C NMR (75 MHz, DMSO-d6 & CDCl3): δ 162.2, 158.8, 158.4, 153.0 (d, 2JC,P = 14.8 Hz), 144.9, 140.4, 131.9, 127.0, 126.5, 125.7, 125.5, 116.1, 113.2, 112.9, 110.2(d, 2JC,P = 11.5 Hz), 106.0, 103.2, 70.2, 50.6 (d, 2JC,P = 4.9 Hz), 11.9. ppm; 31P NMR (162 MHz, CDCl3 & DMSO-d6) 21.287 (m); IR(KBr): ν = 3443, 3252, 2953, 1659, 1521, 1242, 1018, 830 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C21H21N2O5PH 413.1266, found 413.1266.
N′-((4-Benzoyl-5-phenylfuran-2-yl)(phenyl)methylene)-2-(prop-2-yn-1-yloxy)benzohydrazide (5). Following the general procedure, 100 mg (0.297 mmol, 1.0 equiv.) of 1a, 85 mg (0.446 mmol, 1.5 equiv.) of 7, 10 mg (10 mol%) of KAuCl4 and 96 mg (0.595 mmol, 2.0 equiv.) of FeCl3 was used and the reaction time was 12 h. After flash column chromatography on silica gel (eluted with Rf: 0.6; hexane/ethyl acetate mixture 10/3.0), 97 mg of 8 was obtained in 62% yield as a yellow solid. Mp: 73–75 °C; 1H NMR (400 MHz, CDCl3): δ 10.81 (s, 1H), 8.34 (dd, J = 7.8 Hz, 1.7 Hz, 1H), 7.83–7.78 (m, 2H), 7.75–7.70 (m, 2H), 7.69–7.62 (m, 3H), 7.55–7.41 (m, 4H), 7.39–7.33 (m, 2H), 7.30–7.26 (m, 3H), 7.18–7.11 (m, 1H), 7.01 (d, J = 8.3 Hz, 1H), 6.72 (s, 1H), 4.18 (d, J = 2.2 Hz, 2H), 2.50 (t, J = 2.2 Hz, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 191.4, 161.2, 157.1, 154.9, 149.8, 145.0, 137.4, 133.2, 133.0, 131.4, 130.2, 129.69, 129.62, 129.4, 128.9, 128.6, 128.3, 128.2, 127.9, 122.5, 122.4, 120.8, 116.9, 112.7, 55.8. ppm; IR(KBr): ν = 3357, 3297, 3200, 3058, 2923, 2853, 1655, 1598, 1476, 1231, 1012, 752 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C34H24N2O4H 525.1814, found 525.1808.
Crystal data 5. C70H53N4O9 (M =1066.17 g mol−1): triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 9.1182(6) Å, b = 13.8291(10) Å, c = 23.4126(16) Å, α = 103.581(1)°, β = 91.205(2)°, γ = 98.758(2)°, V = 2831.3(3) Å3, Z = 2, T = 294.15 K, μ(Mo Kα) = 0.084 mm−1, Dcalc = 1.2505 g cm−3, 35
(no. 2), a = 9.1182(6) Å, b = 13.8291(10) Å, c = 23.4126(16) Å, α = 103.581(1)°, β = 91.205(2)°, γ = 98.758(2)°, V = 2831.3(3) Å3, Z = 2, T = 294.15 K, μ(Mo Kα) = 0.084 mm−1, Dcalc = 1.2505 g cm−3, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 reflections measured (1.8° ≤ 2Θ ≤ 50°), 9961 unique (Rint = 0.0944, Rsigma = 0.1499) which were used in all calculations. The final R1 was 0.1271 (I > 2σ(I)) and wR2 was 0.3437 (all data). CCDC 1898773.
814 reflections measured (1.8° ≤ 2Θ ≤ 50°), 9961 unique (Rint = 0.0944, Rsigma = 0.1499) which were used in all calculations. The final R1 was 0.1271 (I > 2σ(I)) and wR2 was 0.3437 (all data). CCDC 1898773.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
We thank Department of Science and Technology (DST) India grant no: DST-SB/EMEQ-257/2014 and CSIR, New Delhi for financial support. We are thankful to Director Dr S. Chandrasekhar CSIR-IICT for his support. We thank Dr S. Suresh for his suggestions on this work. We acknowledge our colleagues from CSIR-IICT Dr K. Srinivas and Dr Rajesh for their encouragement. V. K thanks to UGC-SRF, K. S thanks to Dr Ch. Raji Reddy and CSIR grant HCP-0011. P. B. K and C. E. R thanks to DST for Inspire fellowship and also thanks to AcSIR, manuscript communication number:IICT/pubs.2019/176.
Notes and references
- G. M. Cragg and D. J. Newman, Expert Opin. Invest. Drugs, 2000, 9, 2783 CrossRef CAS.
-
(a) E. G. Brown, Ring Nitrogen and Key Biomolecules, The Biochemistry of N-heterocycles, Kluwer Academic, Boston, 1998 CrossRef;
(b) K. C. Majumdar, G. V. Karunakar and B. Sinha, Synthesis, 2012, 44, 2475 CrossRef CAS;
(c) Z. Ye, L. Shi, X. Shao, X. Xu, Z. Xu and Z. Li, J. Agric. Food Chem., 2013, 61, 312 CrossRef CAS.
-
(a) V. Mühlbauer, D. Dallmeier, S. Brefka, C. Bollig, S. Voigt-Radloff and M. Denkinger, Dtsch. Arztebl. Int., 2019, 116, 23 Search PubMed;
(b) A. A. Kamble, R. R. Kamble, L. S. Chougala, J. S. Kadadevarmath, S. R. Maidur, P. S. Patil, M. N. Kumbar and S. B. Marganakop, ChemistrySelect, 2017, 2, 6882 CrossRef CAS.
- A. Y. Chen, R. N. Adamek, B. L. Dick, C. V. Credille, C. N. Morrison and S. M. Cohen, Chem. Rev., 2019, 119, 1323 CrossRef CAS.
-
(a) X.-F. Shang, S. L. Morris-Natschke, Y.-Q. Liu, X. Guo, X.-S. Xu, M. Goto, J.-C. Li, G.-Z. Yang and K.-H. Lee, Med. Res. Rev., 2018, 38, 775 CrossRef CAS;
(b) M. R. Luth, P. Gupta, S. Ottilie and E. A. Winzeler, ACS Infect. Dis., 2018, 4, 301 CrossRef CAS.
-
(a) A. S. El-Azab and K. E. H. Eltahir, Bioorg. Med. Chem. Lett., 2012, 22, 327 CrossRef CAS;
(b) D. C. White, T. D. Greenwood, A. L. Downey, J. R. Bloomquist and J. F. Wolfe, Bioorg. Med. Chem., 2004, 12, 5711 CrossRef CAS.
-
(a) T. Ishihara, K. Kohno, S. Ushio, K. Iwaki, M. Ikeda and M. Kurimoto, Eur. J. Pharmacol., 2000, 407, 197 CrossRef CAS;
(b) P. P. Bandekar, K. A. Roopnarine, V. J. Parekh, T. R. Mitchell, M. J. Novak and R. R. Sinden, Escherichia coli, J. Med. Chem., 2010, 53, 3558 CrossRef CAS;
(c) L. Gao, Z. Xu, Y. Rao, Y.-T. Lu, Y.-T. Hu, H. Yu, Y.-H. Xu, Q.-Q. Song, J.-M. Ye and Z.-S. Huang, Eur. J. Med. Chem., 2018, 147, 90 CrossRef CAS;
(d) M. G. Nathalie, D. C. B. Craig and K. L. Evelyn, J. Neurosci., 2012, 32, 5804 CrossRef;
(e) M. J. Hour, L. J. Huang, S. C. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel and K. H. Lee, J. Med. Chem., 2000, 43, 4479 CrossRef CAS;
(f) G. M. Chinigo, M. Paige, S. Grindrod, E. Hamel, S. Dakshanamurthy, M. Chruszcz, W. Minor and M. L. Brown, J. Med. Chem., 2008, 51, 4620 CrossRef CAS.
-
(a) E. Shirakawa, D. Ikeda, S. Masui, M. Yoshida and T. Hayashi, J. Am. Chem. Soc., 2012, 134, 272 CrossRef CAS;
(b) L. Yifan and W. Jérôme, Beilstein J. Org. Chem., 2013, 9, 1763 CrossRef;
(c) D.-S. Kim, W.-J. Park and C.-H. Jun, Chem. Rev., 2017, 117, 8977 CrossRef CAS.
-
(a) D. Sudarshan, S. Sougata, J. Sourav, V. Z. Grigory, M. Adinath and H. Alakananda, Eur. J. Org. Chem., 2017, 4955 Search PubMed;
(b) A. Dutta, D. Krishnaiah, A. Kumar, S. J. Prakash and D. Sarma, Tetrahedron Lett., 2020, 61, 151587 CrossRef CAS;
(c) Q. Liu, Y. Sui, Y. Zhang, K. Zhang, Y. Chen and H. Zhou, Synlett, 2020, 31, 275 CrossRef CAS;
(d) T. Tamoradi, S. M. Mousavi and M. Mohammadi, New J. Chem., 2020, 44, 3012 RSC;
(e) H. Kothayer, M. S. Ibrahim, K. S. Moustafa, R. Samar and S. M. Shireen, Drug Dev. Res., 2019, 80, 343 CrossRef CAS;
(f) J. Mou, N. Chen, Y. Zhao, H. Qi, S. Meng, R. Xiang and D. Pei, Front. Chem., 2020, 8, 239 CrossRef CAS;
(g) S. J. Wu, Z. Q. Zhao, G. J. Shuo, H. B. Chen and G. F. Chen, Res. Chem. Intermed., 2019, 45, 2327 CrossRef CAS;
(h) A. Dutta, D. Krishnaiah, B. Ankur, A. Kumar and D. Sarma, Tetrahedron Lett., 2019, 60, 1614 CrossRef CAS;
(i) G. Yashwantrao, P. J. Valmik, K. Rajpratap and S. Saha, ACS Sustainable Chem. Eng., 2019, 7, 13551 CrossRef CAS;
(j) Z. Almarhoon, K. A. Dahlous, R. Abd Alhameed, H. A. Ghabbour and A. El-Faham, Molecules, 2019, 24, 4052 CrossRef.
-
(a) E. Jimenez-Nunez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326 CrossRef CAS;
(b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS;
(c) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS;
(d) A. Arcadi, Chem. Rev., 2008, 108, 3266 CrossRef CAS;
(e) A. Fürstner and P. W. Davis, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef;
(f) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef;
(g) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395 CrossRef CAS.
-
(a) A. S. K. Hashmi and F. D. Toste, Modern gold catalyzed Synthesis. Wiley-VCH, Weinheim, 2012 CrossRef;
(b) R. Dorel and A. M. Echavarren, Chem. Rev., 2015, 115, 9028 CrossRef CAS;
(c) A. M. Asiria and A. S. K. Hashmi, Chem. Rev., 2016, 45, 4471 Search PubMed;
(d) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS;
(e) M. Rudolph and A. S. K. Hashmi, Chem. Commun., 2011, 47, 6536 RSC.
-
(a) Y. Zhou, J. Ma, K. Chen, H. Jiang and S. Zhu, Chem. Commun., 2016, 52, 13345 RSC;
(b) R. S. Sheridan, Chem. Rev., 2013, 113, 7179 CrossRef CAS;
(c) J. Ma, K. Chen, H. Fu, L. Zhang, W. Wu, H. Jiang and S. Zhu, Org. Lett., 2016, 18, 1322 CrossRef CAS;
(d) D. Zhu, J. Ma, K. Luo, H. Fu, L. Zhang and S. Zhu, Angew. Chem., Int. Ed., 2016, 55, 8452 CrossRef CAS;
(e) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365 CrossRef CAS;
(f) M. Brookhar and W. B. Studabaker, Chem. Rev., 1987, 87, 2411 Search PubMed;
(g) D. Zhang, Z. Kang, J. Liu and W. Hu, iScience, 2019, 14, 292 CrossRef CAS and references cited there in.
-
(a) H. Kusama and N. Iwasawa, Chem. Lett., 2006, 35, 1082 CrossRef CAS;
(b) J. Barluenga, L. Riesgo, R. Vicente, L. A. López and M. Tomás, J. Am. Chem. Soc., 2008, 130, 13528 CrossRef CAS.
-
(a) M. J. Gonzalez, E. Lopez and R. Vicente, Chem. Commun., 2014, 50, 5379 RSC;
(b) R. J. Harris and R. A. Widenhoefer, Chem. Soc. Rev., 2016, 45, 4533 RSC;
(c) Y. Xia, L. Chen, P. Qu, G. Ji, S. Feng, Q. Xiao, Y. Zhang and J. Wang, J. Org. Chem., 2016, 81, 10484 CrossRef CAS;
(d) J. M. Yang, Z. Q. Li, M. L. Li, Q. He, S. F. Zhu and Q. L. Zhou, J. Am. Chem. Soc., 2017, 139, 3784 CrossRef CAS;
(e) H. Keipour, V. Carreras and T. Ollevier, Org. Biomol. Chem., 2017, 15, 5441 RSC.
-
(a) S. Gonzalez-Pelayo and L. A. Lopez, Adv. Synth. Catal., 2016, 358, 4114 CrossRef CAS;
(b) Y. Yu, Y. Chen, W. Wu and H. Jiang, Chem. Commun., 2017, 53, 640 RSC;
(c) Y. Ren, L. G. Meng, T. Peng and L. Wang, Org. Lett., 2018, 20, 4430 CrossRef CAS.
- J. González, J. González, C. Pérez-Calleja, L. A. López and R. Vicente, Angew. Chem., Int. Ed., 2013, 52, 5853 CrossRef.
-
(a) D. Zhu, L. Chen, H. Zhang, Z. Ma, H. Jiang and S. Zhu, Angew. Chem., Int. Ed., 2018, 57, 12405 CrossRef CAS;
(b) Y. Xia, S. Qu, Q. Xiao, Z. Zhi-Xiang Wang, P. Qu, L. Chen, Z. Liu, L. Tian, Z. Huang, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2013, 135, 13502 CrossRef CAS;
(c) J. Yang, Z. Li, M. Li, Q. He, S. Zhu and Q. Zhou, J. Am. Chem. Soc., 2017, 139, 3784 CrossRef CAS;
(d) M. Huang, J. Yang, Y. Zhao and S. Zhu, ACS Catal., 2019, 9, 5353 CrossRef CAS;
(e) L. Chen, K. Chen and S. Zhu, Chem, 2018, 4, 1208 CrossRef CAS;
(f) R. Vicente, J. González, L. Riesgo, J. González and L. A. López, Angew. Chem., Int. Ed., 2012, 51, 8063 CrossRef CAS;
(g) S. Mata, L. A. López and R. Vicente, Synlett, 2015, 26, 2685 CrossRef CAS;
(h) L. N. S. Comprido, J. E. M. N. Klein, G. Knizia, J. Kästner and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2015, 54, 10336 CrossRef;
(i) H. Luo, K. Chen, H. Jiang and S. Zhu, Org. Lett., 2016, 18, 5208 CrossRef CAS;
(j) B. Song, L.-H. Li, X.-R. Song, Y.-F. Qiu, M.-J. Zhong, P.-X. Zhou and Y.-M. Liang, Chem.–Eur. J., 2014, 20, 5910 CrossRef CAS.
- M. M. Hansmann, F. Rominger and A. S. K. Hashmi, Chem. Sci., 2013, 4, 1552 RSC.
- F. Li, P. Hu, M. Sun, C. Li, X. Jia and J. Li, Chem. Commun., 2018, 54, 6412 RSC.
- D. Purnachandar, K. Suneel, B. Sridhar and G. V. Karunakar, Org. Biomol. Chem., 2019, 19, 4856 RSC.
-
(a) K. Goutham, D. A. Kumar, S. Suresh, B. Sridhar, R. Narender and G. V. Karunakar, J. Org. Chem., 2015, 80, 11162 CrossRef CAS;
(b) N. V. S. M. R. Mangina, K. Veerabhushanam, G. Ravinder, K. Goutham, B. Sridhar and G. V. Karunakar, Org. Lett., 2017, 19, 282 CrossRef CAS;
(c) K. Goutham, V. Kadiyala, B. Sridhar and G. V. Karunakar, Org. Biomol. Chem., 2017, 15, 7813 RSC;
(d) K. Veerabhushanam, P. B. Kumar, B. Sridhar and G. V. Karunakar, J. Org. Chem., 2019, 84, 12228 CrossRef;
(e) V. Nagaraju, C. E. Raju, D. Purnachandar, V. Jayathirtha Rao and G. V. Karunakar, ChemistrySelect, 2019, 4, 2053 CrossRef CAS;
(f) K. Sunil, Y. Thummala, P. Dalovai, B. Sridhar and G. V. Karunakar, Org. Biomol. Chem., 2019, 17, 6015 RSC;
(g) G. Ravinder, N. S. V. M. Rao, B. Sridhar and G. V. Karunakar, Org. Biomol. Chem., 2019, 17, 2809 RSC.
- See the ESI† for X-ray crystallographic data for compound 3a and 5.
- Authors thank the reviewers suggestions
(a) J. Schießl, J. Schulmeister, A. Doppiu, E. Wörner, M. Rudolph, R. Karch and A. S. K. Hashmi, Adv. Synth. Catal., 2018, 360, 2493 CrossRef;
(b) J. Schießl, J. Schulmeister, A. Doppiu, E. Wörner, M. Rudolph, R. Karch and A. S. K. Hashmi, Adv. Synth. Catal., 2018, 360, 3949 CrossRef;
(c) A. S. K. Hashmi, Angew. Chem., Int. Ed. Engl., 2000, 39, 3590 CrossRef CAS;
(d) A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553 CrossRef CAS.
-
(a) J. Ma, H. Jiang and S. Zhu, Org. Lett., 2014, 16, 4472 CrossRef CAS;
(b) P. Liu and J. Sun, Org. Lett., 2017, 19, 3482 CrossRef CAS;
(c) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS;
(d) T. Lauterbach, A. M. Asiri and A. S. K. Hashmi, Adv. Organomet. Chem., 2014, 62, 261 CrossRef CAS.
- B. Song, L.-H. Li, X.-R. Song, Y.-F. Qiu, M.-J. Zhong, P.-X. Zhou and Y.-M. Liang, Chem.–Eur. J., 2014, 20, 5910 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, 1H, 13C NMR spectra and mass for the compounds. CCDC 1863534 (3a) and 1898773 (5). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra06537d |
|
| This journal is © The Royal Society of Chemistry 2020 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *ab
*ab


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5) to give 113 mg of the product 2-(4-benzoyl-5-phenylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 3a (81% yield). The same reaction was conducted on a gram scale by utilizing 1a (1 g) and 2a (0.61 g) produced the corresponding product 3a in 74% yield (1.03 g). A similar experimental procedure was adopted for the synthesis of all the furan containing dihydroquinazolinones (3b–y) and 5.
3.5) to give 113 mg of the product 2-(4-benzoyl-5-phenylfuran-2-yl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 3a (81% yield). The same reaction was conducted on a gram scale by utilizing 1a (1 g) and 2a (0.61 g) produced the corresponding product 3a in 74% yield (1.03 g). A similar experimental procedure was adopted for the synthesis of all the furan containing dihydroquinazolinones (3b–y) and 5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate mixture (10
ethyl acetate mixture (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5); yellow solid with 113 mg (81%) yield; melting point: 188–190 °C; 1H NMR (500 MHz, CDCl3): δ 7.93 (dd, J = 7.7, 1.2 Hz, 1H), 7.73–7.66 (m, 2H), 7.63–7.56 (m, 4H), 7.52–7.48 (m, 1H), 7.47–7.42 (m, 3H), 7.37–7.31 (m, 3H), 7.30–7.26 (m, 3H), 6.97–6.87 (m, 1H), 6.79–6.70 (m, 2H), 6.35 (s, 1H), 5.09 (br s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.0, 156.3, 153.1, 145.2, 140.4, 137.5, 134.4, 133.0, 129.7, 129.6, 129.3, 129.0, 128.8, 128.4, 128.3, 128.2, 127.4, 126.9, 120.9, 119.8, 115.1, 114.9, 114.0, 72.7 ppm; IR(KBr): ν = 3368, 3057, 2922, 1654, 1613, 1485, 1367, 1262 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H21N2O3H 471.1703, found 471.1705.
3.5); yellow solid with 113 mg (81%) yield; melting point: 188–190 °C; 1H NMR (500 MHz, CDCl3): δ 7.93 (dd, J = 7.7, 1.2 Hz, 1H), 7.73–7.66 (m, 2H), 7.63–7.56 (m, 4H), 7.52–7.48 (m, 1H), 7.47–7.42 (m, 3H), 7.37–7.31 (m, 3H), 7.30–7.26 (m, 3H), 6.97–6.87 (m, 1H), 6.79–6.70 (m, 2H), 6.35 (s, 1H), 5.09 (br s, 1H), ppm; 13C NMR (100 MHz, CDCl3): δ 191.1, 164.0, 156.3, 153.1, 145.2, 140.4, 137.5, 134.4, 133.0, 129.7, 129.6, 129.3, 129.0, 128.8, 128.4, 128.3, 128.2, 127.4, 126.9, 120.9, 119.8, 115.1, 114.9, 114.0, 72.7 ppm; IR(KBr): ν = 3368, 3057, 2922, 1654, 1613, 1485, 1367, 1262 cm−1; HRMS (ESI-TOF) m/z: [M + H]+ calcd for C31H21N2O3H 471.1703, found 471.1705.![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 8.2197(2) Å, b = 10.4608(2) Å, c = 14.4752(3) Å, α = 77.0948(8)°, β = 79.6924(8)°, γ = 81.5655(9)°, V = 1186.21(4) Å3, Z = 2, T = 294.15 K, μ(MoKα) = 0.085 mm−1, Dcalc = 1.317 g cm−3, 35
(no. 2), a = 8.2197(2) Å, b = 10.4608(2) Å, c = 14.4752(3) Å, α = 77.0948(8)°, β = 79.6924(8)°, γ = 81.5655(9)°, V = 1186.21(4) Å3, Z = 2, T = 294.15 K, μ(MoKα) = 0.085 mm−1, Dcalc = 1.317 g cm−3, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 683 reflections measured (4.464° ≤ 2Θ ≤ 61.018°), 7216 unique (Rint = 0.0618, Rsigma = 0.0518) which were used in all calculations. The final R1 was 0.0634 (I > 2σ(I)) and wR2 was 0.1669 (all data). CCDC 1863534.
683 reflections measured (4.464° ≤ 2Θ ≤ 61.018°), 7216 unique (Rint = 0.0618, Rsigma = 0.0518) which were used in all calculations. The final R1 was 0.0634 (I > 2σ(I)) and wR2 was 0.1669 (all data). CCDC 1863534.![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 9.1182(6) Å, b = 13.8291(10) Å, c = 23.4126(16) Å, α = 103.581(1)°, β = 91.205(2)°, γ = 98.758(2)°, V = 2831.3(3) Å3, Z = 2, T = 294.15 K, μ(Mo Kα) = 0.084 mm−1, Dcalc = 1.2505 g cm−3, 35
(no. 2), a = 9.1182(6) Å, b = 13.8291(10) Å, c = 23.4126(16) Å, α = 103.581(1)°, β = 91.205(2)°, γ = 98.758(2)°, V = 2831.3(3) Å3, Z = 2, T = 294.15 K, μ(Mo Kα) = 0.084 mm−1, Dcalc = 1.2505 g cm−3, 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814 reflections measured (1.8° ≤ 2Θ ≤ 50°), 9961 unique (Rint = 0.0944, Rsigma = 0.1499) which were used in all calculations. The final R1 was 0.1271 (I > 2σ(I)) and wR2 was 0.3437 (all data). CCDC 1898773.
814 reflections measured (1.8° ≤ 2Θ ≤ 50°), 9961 unique (Rint = 0.0944, Rsigma = 0.1499) which were used in all calculations. The final R1 was 0.1271 (I > 2σ(I)) and wR2 was 0.3437 (all data). CCDC 1898773.