Open Access Article
Open Access ArticlePyridine-2-carboxylic acid as an effectual catalyst for rapid multi-component synthesis of pyrazolo[3,4-b]quinolinones†
Mayank G. Sharma ,
Ruturajsinh M. Vala
,
Ruturajsinh M. Vala and
Hitendra M. Patel
and
Hitendra M. Patel *
*
Department of Chemistry, Sardar Patel University, University Campus, Vallabh Vidyanagar, 388 120, Gujarat, India. E-mail: hm_patel@spuvvn.edu
First published on 25th September 2020
Abstract
Green synthesis of pyrazolo[3,4-b]quinolinones was designed using bioproduct pyridine-2-carboxylic acid (P2CA) as a green and efficient catalyst. The multi-component reaction of aldehydes, 1,3-cyclodiones and 5-amino-1-phenyl-pyrazoles regioselectively produced pyrazolo[3,4-b]quinolinones in excellent yield (84–98%). Recyclization of the catalyst was also investigated. The electronic effect of the various substituents in aromatic rings indicated that the reaction proceeded through the carbocation intermediate. This newly designed protocol very quickly constructed products conventionally under milder conditions.
Introduction
Environmental challenges demand cost-effective syntheses that proceed through fast routes and generate less chemical waste. Multicomponent synthesis is more suitable than linear-type synthesis because single-step conversion reduces chemical waste, reaction time, work-up procedure and cost.1 Multicomponent reactions (MCRs) have emerged as valuable tools for the development of diverse heterocyclic scaffolds.2–12 Pyrazolo[3,4-b]quinolinones are also a product of multicomponent synthesis and show important biological properties. They are GSK3 inhibitors,13 tubulin inhibitors,14 phosphoinositide 3-kinase inhibitors15 and potassium channel openers.16 Besides, they show antileishmanial activity against amastigotes,17 antimicrobial activity and antiproliferative activity.18 These therapeutic properties make them effective and inspire development in their synthesis.Various methods were reported for the synthesis of pyrazolo[3,4-b]quinolinones from aldehyde, 5-amino pyrazole and 1,3-dicarbonyl compounds. Ionic liquid such as [bmim]Br,19 [bmim]BF4,20 and HEAAc18 are used as a solvent and catalytic medium for the synthesis. Other catalysts like InCl3,21 PEG2000 (ref. 22) and nickel nanoparticle23 are also used for the synthesis. Most of them suffered from either longer reaction time or low product yield.
Biomass derived chemicals provide the renewable sources in chemicals with low cost and it considerably enhance the sustainability of organic synthesis.24 In this work, we explored the catalytical efficiency of versatile compound pyridine-2-carboxylic acid (P2CA) in the synthesis of pyrazolo[3,4-b]quinolinones. Pyridine-2-carboxylic acid is an endogenous metabolite of L-tryptophan that has been reported to possess a wide range of neuroprotective, immunological, and anti-proliferative affects within the body.25 Enzymatic oxidation of 3-hydroxyanthranilic acid by 3-hydroxyanthranilic acid oxygenase is a biosynthesis route to produce pyridine-2-carboxylic acid (P2CA).26 P2CA and its derivatives are a chelating agent in coordination complexes of metal ions.27–29 Its lead salt (PbPyA2) does the efficient passivation and generates high-performance and stable perovskite solar cells.30
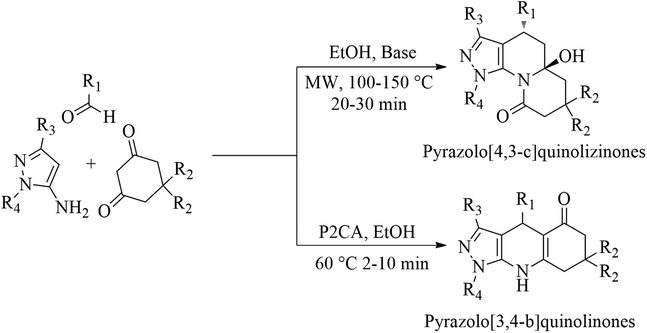
Catalyst has a crucial role during synthetic transformation. It speeds up the transformation and modulates selectivity of the transformation same as steric effect.31 MCRs of aldehydes, 5-amino pyrazoles and 1,3-cyclodiones promoted by bases like NaOEt, KOH and t-BuOK generate pyrazolo[4,3-c]quinolizinones.32,33 Whereas MCRs of same substrates promoted by pyridine-2-carboxylic acid (P2CA) regioselectively generate pyrazolo[3,4-b]quinolinones (Scheme 1). Furthermore, P2CA also reported as a catalyst with a metal salt or complex for some reactions. P2CA with Mn(II) catalysed oxidation of alkane, alkene and alcohol with H2O2.34 P2CA with Cu(I) also catalyses N-arylation of hydrazides.35 Here, we report catalytic efficiency of P2CA without any use of metal salt or complex and expand its utility towards MCRs with significant impact.
Results and discussions
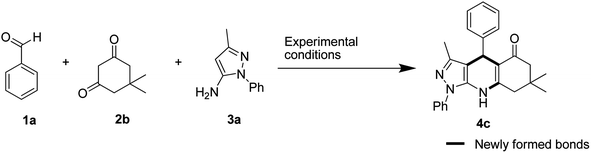
In our initial endeavour to synthesise pyrazolo[3,4-b]quinolinones, the model reactions of 1 mmol benzaldehyde 1a, 1 mmol dimedone 2b, and 1 mmol 5-amino-3-methyl-1-phenyl-1H-pyrazole 3a were carried out in different reaction conditions as mention in Table 1. The reactions were investigated with different amount of catalyst, different temperature and two green solvents water and ethanol.| Entry | Catalyst | Catalyst (mol%) | Solvent | Temp. | Time (minutes) | Consumption of aldehydeb |
|---|---|---|---|---|---|---|
| a 1 mmol benzaldehyde 1a, 1 mmol dimedone 2b, and 1 mmol 5-amino-3-methyl-1-phenyl-1H-pyrazole 3a stirred under various experimental conditions.b Monitored by TLC. | ||||||
| 1 | — | — | Water | RT | 300 | NIL |
| 2 | — | — | Ethanol | RT | 300 | NIL |
| 3 | — | — | Water | 60 °C | 240 | NIL |
| 4 | — | — | Ethanol | 60 °C | 240 | Incomplete |
| 5 | P2CA | 10 | Water | RT | 180 | Incomplete |
| 6 | P2CA | 10 | Ethanol | RT | 180 | Incomplete |
| 7 | P2CA | 10 | Water | 60 °C | 10 | 100% |
| 8 | P2CA | 10 | Ethanol | 60 °C | 5 | 100% |
| 9 | P2CA | 10 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1 ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C | 10 | 100% |
| 10 | P2CA | 10 | Water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1 ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
60 °C | 10 | 100% |
| 11 | P2CA | 5 | Ethanol | 60 °C | 10 | Incomplete |
| 12 | P2CA | 20 | Ethanol | 60 °C | 5 | 100% |
| 13 | P3CA | 10 | Ethanol | 60 °C | 12 | 100% |
| 14 | P4CA | 10 | Ethanol | 60 °C | 8 | 100% |
| 15 | CH3COOH | 10 | Ethanol | Reflux | 60 | 100% |
| 16 | FeCl3 | 10 | Ethanol | Reflux | 120 | 100% |
| 17 | Betaine–oxalic acid | 20 | Ethanol | Reflux | 90 | 100% |
| 18 | Betaine–succinic acid | 20 | Ethanol | Reflux | 90 | 100% |
The obtained results indicated that ethanol gave better product yield over water as a solvent. Additionally, when the reaction was carried out with water and 10% mol P2CA at 60 °C, 74% product yield was obtained (Entry 7). But the product became sticky at the completion of the reaction and we have to follow some tedious workup procedures. The same results were obtained when the mixture of water and ethanol was used as a solvent (Entry 9–10). The best result was obtained when the reaction carried out with 10 mol% of P2CA in ethanol at 60 °C (Entry 8). With this condition, the product fallout within 5 minutes. After a simple work-up process, 98% isolated yield of product was obtained. Pyridine-3-carboxylic acid (P3CA) and pyridine-4-carboxylic acid (P4CA) have almost similar catalytic efficiency to pyridine-2-carboxylic acid. Acetic acid, ferric chloride, betaine–oxalic acid and betaine–succinic acid was also used as a catalyst in the reaction which take more time to complete the reaction as compare to pyridine carboxylic acids.
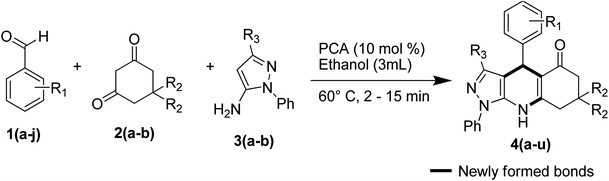
With the optimal condition in hand, the reaction of substituted aldehydes 1(a–j), cyclic 1,3-diones 2(a–b), and 5-amino-pyrazole derivatives 3(a–b) was carried out to check the scope of this protocol. We studied the effect of the substituents R1, R2, R3 and the outcomes are summarized in Table 2.
| Sr. no. | Product | (R1) | (R2) | (R3) | Time (min) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a MCRs of 1 mmol of aldehydes 1(a–j), 1 mmol of cyclic 1,3-diones 2(a–b), and 5-amino-pyrazole derivatives 3(a–b) carried out in 3 ml ethanol at 60 °C.b Isolated yield. | ||||||
| 1 | 4a | -H | -H | -Me | 5 | 96 |
| 2 | 4b | -H | -H | -Ph | 5 | 98 |
| 3 | 4c | -H | -Me | -Me | 5 | 94 |
| 4 | 4d | 4-Br | -H | -Me | 5 | 94 |
| 5 | 4e | 4-Br | -Me | -Me | 5 | 98 |
| 6 | 4f | 4-Br | -Me | -Ph | 5 | 92 |
| 7 | 4g | 3-Cl | -H | -Me | 5 | 94 |
| 8 | 4h | 3-Cl | -Me | -Me | 5 | 95 |
| 9 | 4i | 4-OMe | -H | -Me | 3 | 94 |
| 10 | 4j | 4-OMe | -Me | -Me | 3 | 96 |
| 11 | 4k | 3-OMe | -H | -Me | 3 | 95 |
| 12 | 4l | 3-OMe | -Me | -Me | 3 | 94 |
| 13 | 4m | 2-5-OMe | -H | -Me | 2 | 92 |
| 14 | 4n | 2-5-OMe | -Me | -Me | 2 | 88 |
| 15 | 4o | 3-4-OMe | -H | -Me | 3 | 96 |
| 16 | 4p | 3-4-OMe | -Me | -Me | 3 | 97 |
| 17 | 4q | 2-NO2 | -H | -Me | 10 | 87 |
| 18 | 4r | 2-NO2 | -Me | -Me | 10 | 89 |
| 19 | 4s | 3-OH | -H | -Me | 4 | 84 |
| 20 | 4t | 3-OH | -Me | -Me | 4 | 88 |
| 21 | 4u | 4-Ph | -Me | -Me | 5 | 84 |
Pyrazolo[3,4-b]quinolinones 4(a–u) successfully synthesised with a wide range of substrates using the optimised protocol. The excellent yield of products was obtained with short reaction time range (2–10 min) by the help of P2CA. Variation in reaction time was observed because the reaction was affected by the electronic effects of substituents in the aromatic ring. The substrates bearing activating group (ERG) increased the reactivity. Whereas, the substrates bearing deactivating group (EWG) decreased reactivity. For example, the reaction of 2,5-dimethoxy benzaldehyde 1e completed only in 2 minutes and reaction of 4-nitro benzaldehyde 1g completed in 10 minutes. This is only possible when reaction proceeded through electron-deficient carbocation as a reaction centre. Because ERG stabilised carbocation by releasing electron towards the reaction centre which lowered the energy level of the transition state. Due to this reason, rate of reaction increased. The opposite effect was observed due to EWG.
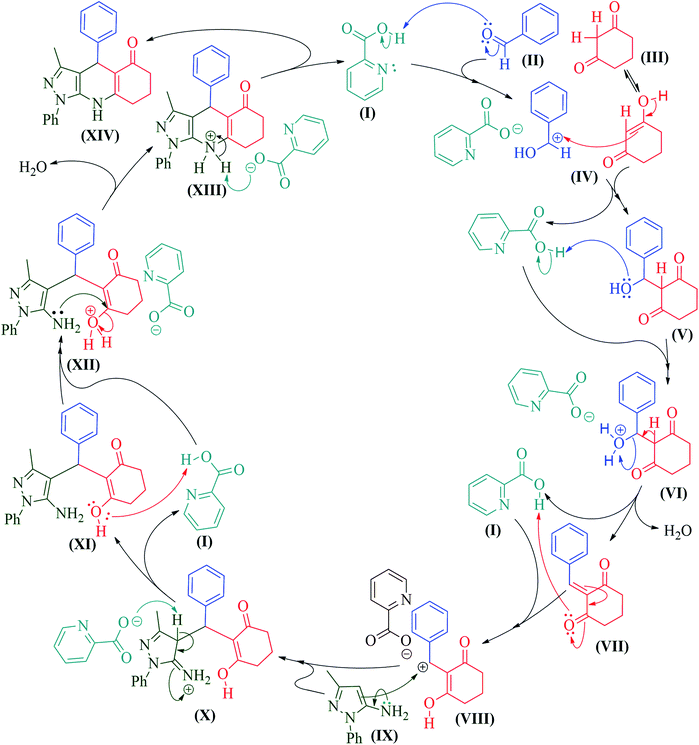
Based on this fact, a plausible mechanism for this reaction is proposed (Scheme 2). P2CA (I) has dual nature i.e. acidic and basic. Initially, Knoevenagel condensation was carried out between aldehydes (II) and 1,3-cyclohexanones (III) to produce Knoevenagel adduct (VI) through the simultaneous generation of carbocation and carbanion. In the next step, again carbocation (VIII) was generated from adduct (VII) by P2CA. Nucleophilic attack by 5-amino pyrazoles (IX) on carbocation (VIII) gave stable intermediate (XI) which is further cyclised by P2CA and condensed to give final product (XIV). Here, reaction forwarded through two carbocations (IV & VIII) which was affected by the electronic effect of the substituent on an aromatic ring.
Recyclization of the catalyst was also carried out for making our protocol more eco-compatible and cost-effective. We check recyclability of P2CA for the synthesis of 4c. A mixture of 2 mmol benzaldehyde 1a, 2 mmol dimedone 2b, 2 mmol 5-amino-3-methyl-1-phenyl-1H-pyrazole 3a and 20% mol P2CA was stirred in 3 ml ethanol at 60 °C for 5 minutes. The reaction mixture was cooled and 9 ml water was added with stirring. The precipitated product was separated by simple filtration. Collected filtrate was heated at 60 °C under reduced pressure. P2CA was recovered and used for the next cycle (Table 3). Obtained results indicated that P2CA can be reused up to 4th cycle.
| Run | % yield |
|---|---|
| 1st | 98 |
| 2nd | 95 |
| 3rd | 86 |
| 4th | 80 |
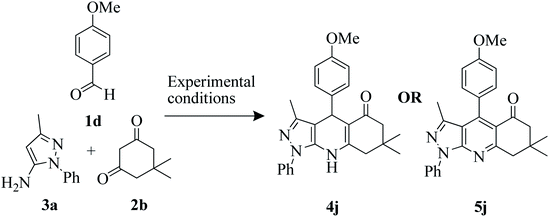
Further, to check the efficiency of newly selected catalyst P2CA, we compared the results with the previously reported work for the same product 4j synthesised from 4-methoxy benzaldehyde 1d, dimedone 2b and 5-amino-3-methyl-1-phenyl-1H-pyrazole 3a (Table 4). Reported work with SDS and PEG-400 catalyst showed that reaction of 1d, 2b and 3a produced 5j (pyrazolopyridine) instead of our desired product 4j (pyrazolodihydropyridine). The overall comparison indicates that our protocol produced desired product 4j conventionally as good as microwave irradiation method. This shows that our protocol is green, eco-compatible and cost-effective.
| Entry | Reaction condition | Time (minutes) | Yield (%) | Reference | |
|---|---|---|---|---|---|
| 4j | 5j | ||||
| a Multicomponent reaction of 4-methoxy benzaldehyde, 1d, dimedone 2b and 5-amino-3-methyl-1-phenyl-1H-pyrazole 3a in a various experimental condition. | |||||
| 1 | SDS, H2O, 90 °C | 720 | — | 97 | 36 |
| 2 | PEG-400, 100–110 °C | 240 | — | 90 | 37 |
| 3 | MWI, EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 50 °C H2O, 50 °C |
5 | 94 | — | 38 |
| 4 | [bmim]Br, 90 °C | 180 | 85 | — | 19 |
| 5 | P2CA, ethanol, 60 °C | 3 | 96 | — | This work |
Conclusion
The newly selected catalyst P2CA showed good efficiency towards the reaction of pyrazolo[3,4-b]quinolinones. The reaction completed rapidly (in 2–10 minutes). The electronic effect of aromatic substituent was observed in the form of reactivity of the substrate. Recyclization of catalyst showed that P2CA can be reused up to 4th cycle. Comparison of our protocol with reported work indicated that P2CA produced pyrazolo[3,4-b]quinolinones conventionally as efficient as microwave irradiation. We are in the process of applying PAC as a green and sustainable catalyst for the other multicomponent reactions which involves C–C and C–N bond formation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful to SERB, New Delhi, India for providing financial assistance from the major research project (file no. EEQ/2016/000376, dated 07/02/2017). We thank UGC, New Delhi for UGC-CPEPA Phase-II program sponsored under award letter no. F. No. 1–14/2002-2016(NS/PE) dated 28th April 2016 and UGC-CAS, Phase-II sponsored under award letter no. F-540/5/CASII/2018 (SAP-I) dated July 25, 2018, for the assistance in general and NMR facility in particular. MGS thankful to SERB, New Delhi for SRF.References
- T. Shi, S. Teng, Y. Wei, X. Guo and W. Hu, Green Chem., 2019, 21, 4936–4940 RSC.
- D. M. Patel and Hs. M. Patel, ACS Sustainable Chem. Eng., 2019, 7, 18667–18676 CrossRef CAS.
- D. M. Patel, H. J. Patel, J. M. Padrón and H. M. Patel, RSC Adv., 2020, 10, 19600–19609 RSC.
- J. Xu, W. Huang, R. Bai, Y. Queneau, F. Jérôme and Y. Gu, Green Chem., 2019, 21, 2061–2069 RSC.
- G. C. Brahmbhatt, T. R. Sutariya, H. D. Atara, N. J. Parmar, V. K. Gupta, I. Lagunes, J. M. Padrón, P. R. Murumkar and M. R. Yadav, Mol. Diversity, 2020, 24, 355–377 CrossRef CAS.
- D. M. Patel, R. M. Vala, M. G. Sharma, D. P. Rajani and H. M. Patel, ChemistrySelect, 2019, 4, 1031–1041 CrossRef CAS.
- S. Chen, P. Ravichandiran, A. El-Harairy, Y. Queneau, M. Li and Y. Gu, Org. Biomol. Chem., 2019, 17, 5982–5989 RSC.
- M. G. Sharma, J. Pandya, D. M. Patel, R. M. Vala, V. Ramkumar, R. Subramanian, V. K. Gupta, R. L. Gardas, A. Dhanasekaran and H. M. Patel, Polycyclic Aromat. Compd., 2019 DOI:10.1080/10406638.2019.1686401.
- A. El-Harairy, Y. Qi, M. Yue, W. Fan, F. Popowycz, Y. Queneau, M. Li and Y. Gu, ChemCatChem, 2019, 11, 4403–4410 CrossRef CAS.
- H. Patel, D. Rajani, M. Sharma and H. Bhatt, Lett. Drug Des. Discovery, 2019, 16, 119–126 CrossRef CAS.
- H. M. Patel, K. D. Patel and H. D. Patel, Curr. Bioact. Compd., 2017, 13, 47–58 CrossRef CAS.
- H. M. Patel, Curr. Bioact. Compd., 2018, 14, 278–288 CrossRef CAS.
- F. F. Wagner, J. A. Bishop, J. P. Gale, X. Shi, M. Walk, J. Ketterman, D. Patnaik, D. Barker, D. Walpita, A. J. Campbell, S. Nguyen, M. Lewis, L. Ross, M. Weïwer, W. F. An, A. R. Germain, P. P. Nag, S. Metkar, T. Kaya, S. Dandapani, D. E. Olson, A.-L. Barbe, F. Lazzaro, J. R. Sacher, J. H. Cheah, D. Fei, J. Perez, B. Munoz, M. Palmer, K. Stegmaier, S. L. Schreiber, E. Scolnick, Y.-L. Zhang, S. J. Haggarty, E. B. Holson and J. Q. Pan, ACS Chem. Biol., 2016, 11, 1952–1963 CrossRef CAS.
- G. F. Mangiatordi, D. Trisciuzzi, D. Alberga, N. Denora, R. M. Iacobazzi, D. Gadaleta, M. Catto and O. Nicolotti, Eur. J. Med. Chem., 2017, 139, 792–803 CrossRef CAS.
- B. Drees, L. Chakravarty, G. Prestwich, G. Dorman, M. Kavecz, A. Lukacs, L. Urge, F. Darvas, P. Rzepecki and C. Ferguson, US Pat., US 2007/0010548 A1, 2007.
- I. Drizin, R. J. Altenbach and W. A. Carroll, US Pat., US 6538004 B2, 2003.
- D. Anand, P. K. Yadav, O. P. S. Patel, N. Parmar, R. K. Maurya, P. Vishwakarma, K. S. R. Raju, I. Taneja, M. Wahajuddin, S. Kar and P. P. Yadav, J. Med. Chem., 2017, 60, 1041–1059 CrossRef CAS.
- D. M. Patel, M. G. Sharma, R. M. Vala, I. Lagunes, A. Puerta, J. M. Padrón, D. P. Rajani and H. M. Patel, Bioorg. Chem., 2019, 86, 137–150 CrossRef CAS.
- D.-Q. Shi and F. Yang, J. Heterocycl. Chem., 2011, 48, 308–311 CrossRef CAS.
- X. Zhang, D. Li, X. Fan, X. Wang, X. Li, G. Qu and J. Wang, Mol. Diversity, 2010, 14, 159–167 CrossRef CAS.
- J. M. Khurana, A. Chaudhary, B. Nand and A. Lumb, Tetrahedron Lett., 2012, 53, 3018–3022 CrossRef CAS.
- L.-Y. Zeng, T. Liu, J. Yang, Y. Yang, C. Cai and S. Liu, ACS Comb. Sci., 2017, 19, 437–446 CrossRef CAS.
- N. G. Singh, R. Nagarajaprakash, J. W. S. Rani, C. Kathing, R. Nongrum and R. Nongkhlaw, New J. Chem., 2015, 39, 3908–3915 RSC.
- L. Yang and J.-P. Wan, Green Chem., 2020, 22, 3074–3078 RSC.
- R. S. Grant, S. E. Coggan and G. A. Smythe, Int. J. Tryptophan Res., 2009, 2, 71–79 CAS.
- D. Datta and S. Kumar, J. Chem. Eng. Data, 2015, 60, 2709–2716 CrossRef CAS.
- O. N. Shishilov, N. S. Akhmadullina and V. R. Flid, Russ. Chem. Bull., 2020, 69, 289–294 CrossRef CAS.
- B. Li, J. Wang, H. Song, H. Wu, X. Chen and X. Ma, J. Coord. Chem., 2019, 72, 2562–2573 CrossRef CAS.
- Ö. Tamer, H. Mahmoody, K. F. Feyzioğlu, O. Kılınç, D. Avci, O. Orun, N. Dege and Y. Atalay, Appl. Organomet. Chem., 2020, 34, e5416 CrossRef.
- S. Fu, X. Li, L. Wan, Y. Wu, W. Zhang, Y. Wang, Q. Bao and J. Fang, Adv. Energy Mater., 2019, 9, 1901852 CrossRef.
- R. M. Vala, D. M. Patel, M. G. Sharma and H. M. Patel, RSC Adv., 2019, 9, 28886–28893 RSC.
- V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, S. V. Shishkina, O. V. Shishkin, K. M. Kobzar and C. O. Kappe, Org. Lett., 2007, 9, 1691–1694 CrossRef CAS.
- V. A. Chebanov, V. E. Saraev, S. M. Desenko, V. N. Chernenko, I. V. Knyazeva, U. Groth, T. N. Glasnov and C. O. Kappe, J. Org. Chem., 2008, 73, 5110–5118 CrossRef CAS.
- P. Saisaha, J. J. Dong, T. G. Meinds, J. W. de Boer, R. Hage, F. Mecozzi, J. B. Kasper and W. R. Browne, ACS Catal., 2016, 6, 3486–3495 CrossRef CAS.
- M. S. Lam, H. W. Lee, A. S. C. Chan and F. Y. Kwong, Tetrahedron Lett., 2008, 49, 6192–6194 CrossRef CAS.
- H.-Y. Wang and D.-Q. Shi, J. Heterocycl. Chem., 2012, 49, 212–216 CrossRef CAS.
- K. Karnakar, S. Narayana Murthy, K. Ramesh, G. Satish, J. B. Nanubolu and Y. V. D. Nageswar, Tetrahedron Lett., 2012, 53, 2897–2903 CrossRef CAS.
- M. Robert Khumalo, S. N. Maddila, S. Maddila and S. B. Jonnalagadda, RSC Adv., 2019, 9, 30768–30772 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06738e |
| This journal is © The Royal Society of Chemistry 2020 |