Open Access Article
Open Access ArticleShrimp lectin (Md-Lec) conjugated copper sulfide nanoparticles enhance the elimination of aquatic pathogens in infected Nile tilapia (Oreochromis niloticus)†
Abdul Salam Rubeenaa,
Sreeja Lakshmia,
Digi Georgea,
Siva Bala Subramaniyanb,
Anbazhagan Veerappan *b and
Elumalai Preetham
*b and
Elumalai Preetham *ac
*ac
aSchool of Ocean Science and Technology, Kerala University of Fisheries and Ocean Studies, Panangad, Kerala, India. E-mail: preetham@kufos.ac.in
bSchool of Chemical and Biotechnology, SASTRA Deemed University, Thanjavur, Tamil Nadu 613401, India. E-mail: anbazhagan@scbt.sastra.edu
cDepartment of Fish Processing Technology (Biochemistry), Kerala University of Fisheries and Ocean Studies, Panangad, Kerala, India
First published on 15th December 2020
Abstract
Lectins are known for their ability to bind to cell surface glycans, and are useful to develop a glycan-targeted drug delivery system. This study aimed to evaluate the capacity of pectin capped copper sulfide nanoparticles (pCuS NPs) to modulate the antibacterial activity of a lectin, Md-Lec, purified from the shrimp, Metapenaeus dobsoni. Fluorescence spectroscopy revealed that Md-Lec has the ability to form a complex with pCuS NPs. Haemagglutination assay showed that the carbohydrate binding site of the lectin was preserved even after complexing with pCuS. The minimum inhibitory concentrations (MICs) obtained for Md-Lec and pCuS NPs against the tested aquatic pathogens were 50 μg ml−1 and 12.5 μM, respectively. Interestingly, the MIC of Md-Lec–pCuS NPs complex was four fold lower than that of pCuS, which was attributed to the bacterial cell surface glycan recognization activity of Md-Lec. Zone of inhibition assay showed that the zone size was highest for the lectin conjugated nanoparticles. Mechanistic study revealed that Md-Lec–pCuS NPs affect the bacterial membrane integrity and produce a large volume of reactive oxygen species to kill the bacteria. The practical aspect of using this lectin–pCuS NPs complex was evaluated by treating bacteria infected Nile tilapia (Oreochromis niloticus). The bacterial load was much less in the lectin–pCus NPs complex treated fish; moreover, the fish fully recovered from the infection. It was concluded that the conjugate of antibacterial lectin and NPs is more effective than the individual components.
1. Introduction
As the aquaculture industry has headed to new heights in a short span of time, the problems related to fish farming, including infectious diseases, are also increasing.1 In order to combat this scenario, there has been extensive and unethical use of antibiotics by farmers which has eventually led to the emergence of multiple antibiotic resistances.2 In order to resolve this situation, scientists have been looking for alternatives that have minimal side effects. The introduction of nanoparticle-conjugated drugs has increased the opportunities and possibilities to treat and address a lot of infectious diseases.3,4 Metal nanoparticles such as Au, Ag, Cu, Pt and Pd and metal oxide nanoparticles such as ZnO, TiO2, Bi2O3, CuO, and Fe2O3 in particular are used as they have promising antibacterial properties.5–9 Functionalized NPs are expected to be more active than NPs alone. For example, Butea monosperma lectin functionalized AgNPs were better than AgNPs in inhibiting the biofilms formed by E. coli.10 Parkia platycephala seed lectin enhances the antibiotic activity of gentamicin against the multi-drug resistant E. coli and S. aureus.11Lectins are glycoproteins that possess one or more Carbohydrate Recognition Domains (CRD) which can reversibly and specifically bind to certain sugar molecules.12–15 Lectins are present ubiquitously in nature, that is in all living beings, from bacteria and viruses to higher animals, fish and plants.16,17 Lectins are classified into different groups, on the basis of their binding and structural properties. They are C-type lectins, galectins, rhamnose binding lectins, fucose binding lectins, intelectins, lily-type lectins etc., and among these, C-type lectins comprise the majority of the marine lectins.18,19 Previously, the important roles and therapeutic potential of different types of fish lectins were described by Preetham et al.20 Herein, Md-Lec, a C-type lectin purified from the haemolymph of the shrimp, Metapenaeus dobsoni, was evaluated for its antibacterial activity against common aquatic pathogens.21 This study aims to analyse the complex formation ability of Md-Lec with pectin capped copper sulfide nanoparticles (pCuS NPs), and further to study the extent of the antibacterial activity of the complex in vitro and in vivo. The results indicate that the Md-Lec–pCuS NPs complex has a better or enhanced antibacterial activity than the pCuS NPs alone. In vivo testing revealed that Md-Lec–pCuS NPs are highly effective in reducing the bacterial load in the infected Nile tilapia. This study concludes that lectin-NPs conjugates can be considered as a useful alternative to antibiotics for the treatment of bacterial infection in aquatic animals.
2. Materials and methods
2.1 Purification of Md-Lec
The shrimp lectin, Md-Lec was previously purified by affinity chromatography using mannose-sepharose CL 4B22 from the haemolymph of the Kadal shrimp, Metapenaeus dobsoni, by our group.21 The mannose acted as a ligand to which the lectin in the haemolymph samples could bind, and later the lectin was eluted by passing through elution buffer (10 mM Tris HCl, 140 mM NaCl, 3 mM EDTA, pH 8.0). Then the eluted fractions were tested for purity and molecular mass by SDS-PAGE.2.2 Interaction of CuS NPs with Md-Lec
The CuS NPs were prepared using the procedure reported in Subramaniyan et al.23 Briefly, pectin (50 mg) and copper chloride (1 mM) were dissolved in 50 ml double distilled water. To this mixture, 200 μl ammonium hydroxide was added, which was followed by 400 μl hydrazine hydride. In 3 h, the solution color changed from blue to a wine red color, suggesting the formation of copper nanoparticles. The addition of 1 mM sodium sulphide to the copper nanoparticles changed the color from wine red to olive green in 3 h, suggesting the formation of pectin stabilized copper sulfide nanoparticles (pCuS NPs). The obtained pCuS NPs was purged with nitrogen gas for 15 min and then used in the study. The interaction between lectin and pCuS NPs was studied using a Jasco-FP8200 spectrophotometer. Typically, small aliquots of 1 mM pCuS NPs were added to 0.1 OD280 nm of Md-Lec and allowed to equilibrate for 2 min. Then, the fluorescence emission spectra were measured with an excitation wavelength of 280 nm. The addition of NPs drastically affected lectin fluorescence. A blank experiment was performed by titrating Md-Lec with small aliquots of buffer. The addition of buffer did not affect the lectin fluorescence significantly.2.3 Lectin activity assay
To find out whether there was any alteration in the activity of Md-Lec after complexing with the pCuS NPs, haemagglutination assay was performed according to the method described by Correia and Coelho24 with slight modifications. Briefly, 50 μl of sample preparations of 50 μg ml−1 concentration were added to PBS before addition of 50 μl 2% (v/v) suspension of human erythrocytes in a 96 well plate. In the control, the sample was replaced by BSA.2.4 Antibacterial studies
2.5 In vivo antibacterial studies
All experiments were performed in compliance with the guidelines as prescribed by the Institutional Animal Ethics Committee (SOST/PhD001/2019) of Kerala University of Fisheries and Ocean Studies, India. Optimizations of bacterial dosage for establishment of infection were done previously.40 Infection was done intramuscularly and treatment was given from the opposite side to that of the infection.23 The in vivo bacterial infection and the treatment with Md-Lec–pCuS NPs (1× MIC) were performed in Nile tilapia that was divided into 3 groups; group I: bacteria infected control, group II: bacterial infected + treated with Md-Lec–pCuS NPs, group III: control, uninfected fish. Each group consisted of 10 fish. In a typical experiment, 10 μl of 0.1 OD660 culture of A. hydrophila was used for infection. The infection dose was optimized previously. The fish were fed normally and after 3 h of infection with bacteria, 10 μl of samples were injected as described above. The fish were monitored for mortality due to bacterial infection for a period of 24 h. At regular time points, (3 h, 6 h, 12 h) one fish from each group was removed, anesthetized and sacrificed. Approximately 100 mg of muscle tissue was dissected and homogenized in sterile PBS. The homogenate was serially diluted using sterile PBS and plated on sterile LB agar plates. The plates were incubated for 24 h at 37 °C. After 24 h, bacterial colonies were counted and reported. All of the experiments were performed in triplicate.2.6 Statistical analysis
All experiments were performed in triplicate (n = 3). Data is presented as mean ± standard deviation (S.D.) of control and treated samples. The data were subjected to one way analysis of variance (ANOVA) and the significance of differences between mean were calculated by Tukey’s HSD test and the significance accepted at P < 0.05.3. Results and discussion
Being a lectin, Md-Lec has the ability to bind to the sugar moieties present on membrane surfaces.21,30 Meanwhile, the pCuS nanoparticles were reported to have an antibacterial property.31,32 Thus, it was assumed that the antibacterial properties of the Md-Lec and pCuS nanoparticles would be enhanced through conjugation.3.1 Antimicrobial activity of Md-Lec
Infections caused by aquatic pathogens are the leading reason for the mortality of fish in the aquaculture industry.1 Herein, we evaluated the antimicrobial activity of Md-Lec against the common fish pathogens, A. hydrophila, E. fecalis and S. iniae. Md-Lec was isolated from the haemolymph of Metapenaeus dobsoni using affinity chromatography and their purity was judged by SDS-PAGE.213.2 Interaction of purified Md-Lec with CuS nanoparticles
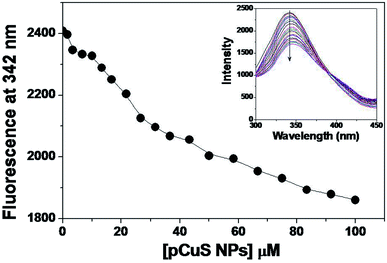
Of late, nanoparticles have been widely considered for antimicrobial and drug delivery applications. The copper sulphide nanoparticles used in this study were prepared from the precursor copper chloride and sodium sulphide, where pectin was used as stabilizing agent. The morphology and size of the prepared NPs were determined from transmission electron microscopy (Fig. S1†). It is clear from Fig. S1,† the pCuS NPs are spherical in shape with size range from 3 to 14 nm, which is consistent with previous reports. Having observed the antibacterial activity of Md-Lec, we set out to evaluate the antibacterial activity of Md-Lec in combination with pCuS NPs. Therefore, the complex formation ability of pCuS NPs and Md-Lec was judged by fluorescence spectroscopy. The tryptophan emission spectra of Md-Lec were observed between 300–450 nm with an emission maximum at 342 nm (Fig. 1).33,34 The titration of Md-Lec with pCuS NPs affected the fluorescence intensity, suggesting a possible interaction between Md-Lec and pCuS NPs. The intrinsic fluorescence of Md-Lec was quenched to 22.8% at 100 μM pCuS NPs, which could be attributed to the energy transfer between Md-Lec and pCuS NPs. The fluorescence quenching data was analyzed by Stern–Volmer plot, which gives the information about the mechanism of quenching [Fig. S2†].| F0/Fc = 1 + Ksv[pCuS NPs] = 1 + Kqτ0[pCuS NPs] | (1) |
| Kq = Ksv/τ0 | (2) |
3.3 Lectin activity assay
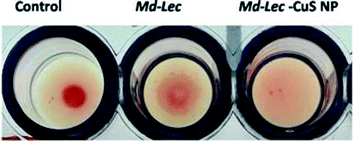
The sugar binding site of Md-Lec is important for recognizing bacterial cell surface glycans. Therefore, the sugar binding activity of Md-Lec complexed with pCuS NPs was evaluated by haemagglutintion assay. In the control well, a negative result was observed as button-like formation at the bottom due to the settling of erythrocytes. In the wells containing Md-Lec alone and Md-Lec–pCuS NPs, a positive result was observed as a fluffy cotton-like formation at the bottom due to clumping of the erythrocytes (Fig. 2). The formation of clumps or aggregates (haemagglutination) was caused by the Md-Lec as it can bind to the carbohydrate molecules present on the cell surfaces of the erythrocytes. Even after conjugating with the nanoparticles, the Md-Lec could retain its haemagglutination activity and it was obvious that the carbohydrate binding site of lectin was conserved. This also reveals that the immunological activity of lectin is retained in the Md-Lec–pCuS NPs complex. As this property is retained, this lectin-nanoconjugate can be used for specific identification and binding to pathogens or tumour cells.353.4 Antibacterial activity of the Md-Lec–pCuS NPs complex
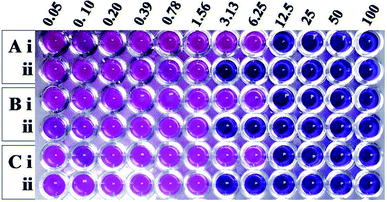
Having learnt that the complex formation between Md-Lec and pCuS NPs could occur without affecting the cell surface sugar recognition site, we expanded the investigation of Md-Lec–pCuS NPs complex against A. hydrophila, E. fecalis and S. iniae. Fig. 4 shows the REMA of pCuS NPs with and without Md-Lec. In REMA, the respiring viable cells reduce resazurin to resorufin and appear pink in color, whereas the cells lacking respiration appear blue in color.23 It is noted from Fig. 3, the MIC of pCuS NPs is 12.5 μM against the tested pathogens as they appear blue in colour, suggesting that the cells are inactive; below that, the cells are able to respire and convert the resazurin to resorufin. Strikingly, the MIC of pCuS NPs in the presence of 25 μg ml−1 Md-Lec was drastically reduced from 12.5 μM to 3.13 μM. The four fold reduction in the MIC of pCuS was attributed to Md-Lec functionalization, because Md-Lec has the ability to bind to the bacterial cell surface, as indicated by the bacterial agglutination study.3.5 Zone of inhibition assay (ZOI)
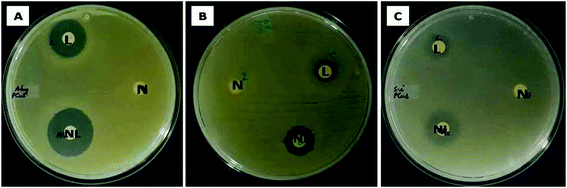
The ability of the test samples to inhibit the growth of bacteria was evident from the zone of inhibition (ZOI) assay. In zone of inhibition assay, filter paper discs loaded with Md-Lec, pCuS NPs and Md-Lec–pCuS NPs were used against A. hydrophila, E. fecalis and S. iniae. The test sample diffuses out from the filter paper discs into the media, such that the bacteria susceptible to the test sample are inhibited from growing around the disc and the bacteria which are more susceptible will exhibit a wider zone of clearance. From Fig. 4, it is evident that the ZOI was smaller for lectin and nanoparticles. Interestingly, the ZOI was largest around the filter paper disc loaded with Md-Lec–pCuS NPs (25 μg ml−1) confirming the intensified antibacterial activity of Md-Lec when conjugated with pCuS NPs. However, the zone size differs with respect to the tested bacterium, which can be attributed to the difference in the bacterial membrane composition.3.6 Effect of Md-Lec–pCuS NPs complex on membrane integrity
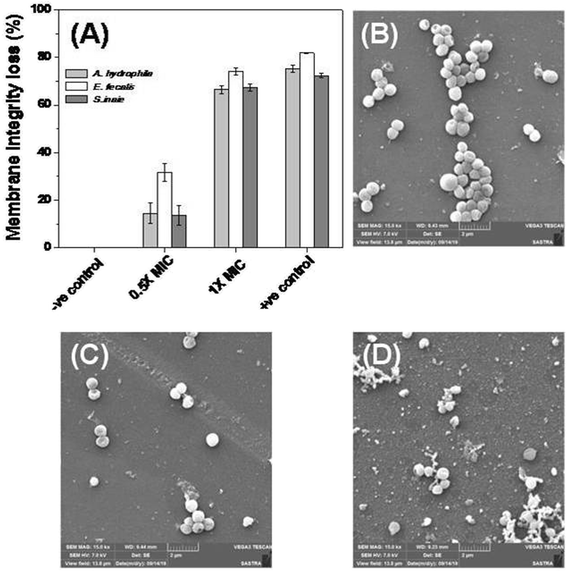
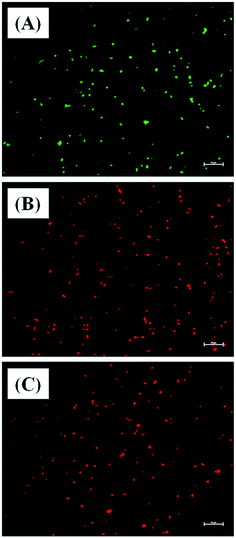
The bacterial membrane serves as a first line of defense for the bacteria’s survival. The effect of Md-Lec–pCuS NPs complex was investigated using the fluorescence probe, NPN for A. hydrophila and E. fecalis, and PI for S. iniae. These probes enter into cells with compromised membranes, showing strong fluorescence. The bacterial cells were treated with 0.5× and 1× MIC for 2 h, and then stained with the fluorescent probe. After staining, the fluorescence of the cells was measured and data is shown in Fig. 5. The untreated cells were used as negative control, 3.13 μM CTAB, a membrane damaging agent, was used as positive control for Gram negative bacteria, and 3.13 μM Triton X 100 was used as positive control for Gram positive bacteria. It is noted from Fig. 5A, the cells treated at 1× MIC show strong fluorescence, as good as the positive control, suggesting the loss of membrane integrity. The extent of membrane damage by Md-Lec–pCuS NPs was further analyzed by scanning electron microscopy. The SEM of S. iniae was used to represent the membrane damaging activity of Md-Lec–pCuS NPs. Fig. 5B and C show that the untreated cells and the cells treated at 0.5× MIC of Md-Lec–pCuS NPs are spherical in morphology with intact cell wall. Interestingly, the cells treated at 1× MIC of Md-Lec–pCuS NPs exhibited cell wall damage, as evident from the damaged and shrunken cells (Fig. 5D). The maximum damage to membrane at 1× MIC was considered as an important mechanism for cell death induced by Md-Lec–pCuS NPs.Further evidence for Md-Lec–pCuS NPs induced cell death was analyzed by live and dead staining using fluorescence microscopy. In this study, acridine orange (AO), and propidium iodide (PI) were used as a dual probe. AO has the ability to cross the intact membrane and stains the nucleic acid to give green fluorescence from live cells, whereas PI can only cross the compromised membrane and shows red fluorescence from dead cells. The fish specific pathogens were treated with 1× MIC of Md-Lec–pCuS NPs for 2 h and stained with the dual probe, AO and PI. Fig. 6 shows the fluorescence microscopy image of untreated cells and cells treated with 1× MIC Md-Lec–pCuS NPs. The green fluorescent cells observed in Fig. 6A suggest the cells are stained only with AO, thus, these cells are considered as live cells. Whereas the cells treated with 1× MIC show red fluorescence. Similarly, the cells treated with membrane damaging agent, Triton X 100 also show red fluorescence. Of particular note, the treated cells visualized with a green filter showed no green cells, suggesting all the cells were dead in the treated group. This result suggests that Md-Lec–pCuS NPs damaged the bacterial membrane, which resulted in cell death, supporting the result obtained from the membrane integrity study.
3.7 Lipid peroxidation assay
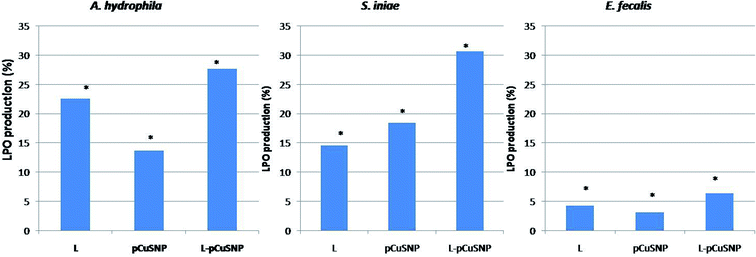
The lipid peroxidation was estimated for evaluating the oxidative damage caused to the bacterial membrane. The reactive oxygen species produced leads to the formation of lipid peroxide radical which is then converted into malondialdehyde. The formation of the lipid peroxidation product was estimated by thiobarbituric acid reactive substances assay (TBARS assay). It is noted from Fig. 7, that the LPO production was high when the lectin was conjugated with nanoparticles. The increased production of malondialdehyde as a result of membrane damage caused by reactive oxygen species confirmed the enhanced antibacterial activity of the lectin conjugated nanoparticles.36–38 Noteworthy was that the activity of the conjugate is different against the different bacteria tested (Fig. 7). pCuS NPs conjugated with lectin (25 μg ml−1) exhibited higher LPO activity against S. iniae than A. hydrophila and comparatively lesser activity against E. fecalis. The difference in the LPO activity can be attributed to the differential membrane composition of the tested bacterium. These results go in parallel with the LPO activity reported earlier.23,333.8 In vivo antibacterial studies
The in vivo antibacterial studies show that the pathogenic bacterial load was decreased drastically when they were injected with Md-Lec–pCuS NPs (Fig. 8). Each treatment group consisted of 10 fish each and all of them, except the last group that served as uninfected control, were intramuscularly infected with a dose of 10 μl of 0.1 OD660 cultures of A. hydrophila which was optimized previously. After 3 h of infection, the second group was injected with 10 μl of Md-Lec–pCuS NPs (3.13 μM). The fish in the infected control group died within 12 h while the fish in the other two groups survived. The bacterial load in the muscle tissue of the fish was analyzed at different time points by sacrificing each one of them. The muscle tissue (100 mg) was homogenized and serially diluted, and then plated in LB agar plates (10−4 dilution) and incubated (25 °C for 24 h) to obtain a viable bacterial count. The infected control group showed the highest number of colonies at 3 h which increased at 6 h and finally all of the fish in the group died by 12 h. Meanwhile the treated group showed a rapid decline in the viable bacterial count with the progression of time. This study suggests that treatment of fish with Md-Lec–pCuS NPs could inhibit the proliferation of infectious bacteria, thus preventing the fish from suffering and spreading the disease.31,39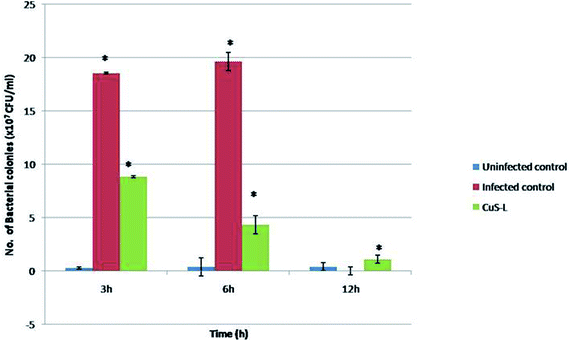 | ||
| Fig. 8 In vivo assay for antibacterial activity. In the fish treated with Md-Lec–pCuS NPs, the number of viable bacterial cells present is reported to be declining with increasing time. | ||
4. Conclusion
In the present study, the antibacterial activity of the complex formed between Md-Lec and pCuS NPs was reported by REMA, and zone of inhibition assay. The mechanism of action was judged by lipid peroxidase assay, and membrane integrity study. The in vivo efficacy of the Md-Lec–pCuS NPs complex was demonstrated through treating Nile tilapia infected with bacteria. The results revealed that the nanoparticles and lectin, when administered individually, are proved to have a limited activity, while their combination is reported to have a very high antibacterial activity. This sheds light on the possibilities of these combinations to be used as promising therapeutic agents after further proper investigations.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by KUFOS PhD fellowship to A. S. R and by the DST-SERB project ECR/2015/000554 to E. P. S. L. deeply acknowledges the Department of Health Research, ICMR, New Delhi, for the financial assistance (No. 12013/04/2017-HR) towards HRD fellowship-Women Scientist with break in career. S. B. S. earnestly acknowledges the teaching assistantship from SASTRA Deemed University. The Central Research Facility (R&M/0021/SCBT 007/2012-13), SASTRA Deemed University, and the DST-FIST grant (SR/FST/ETI-331/2013) to SCBT, SASTRA Deemed University, are gratefully acknowledged.References
- D. J. Alderman and T. S. Hastings, Antibiotic use in aquaculture: development of antibiotic resistance – potential for consumer health risks, Int. J. Food Sci. Technol., 1998, 33, 139–155 Search PubMed.
- L. O. Chuah, M. E. Effarizah, A. M. Goni and G. Rusul, Antibiotic Application and Emergence of Multiple Antibiotic Resistance (MAR) in Global Catfish Aquaculture, Current Environmental Health Reports, 2016, 3, 118–127 Search PubMed.
- S. A. A. Rizvi and A. M. Saleh, Applications of nanoparticle systems in drug delivery technology, Saudi Pharm. J., 2018, 26, 64–70 Search PubMed.
- D. Mandal, S. K. Dash, B. Das, S. Chattopadhyay, T. Ghosh, D. Dasc and S. Roy, Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge, Biomed. Pharmacother., 2016, 83, 548–558 Search PubMed.
- K. B. A. Ahmed, T. Raman and A. Veerappan, Future prospects of antibacterial metal nanoparticles as enzyme inhibitor, Mater. Sci. Eng., C, 2016, 68, 939–947 Search PubMed.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Antimicrobial activity of metals: mechanisms, molecular targets and applications, Nat. Rev. Microbiol., 2013, 11, 371–384 Search PubMed.
- D. Liang, Z. Lu, H. Yang, J. Gao and R. Chen, Novel Asymmetric Wettable AgNPs/Chitosan Wound Dressing: In Vitro and In Vivo Evaluation, ACS Appl. Mater. Interfaces, 2016, 8, 3958–3968 Search PubMed.
- S. Wang, Q. Li, F. Chen, J. Ke and R. Chen, HEPES-mediated controllable synthesis of hierarchical CuO nanostructures and their analogous photo-Fenton and antibacterial performance, Adv. Powder Technol., 2017, 28, 1332–1339 Search PubMed.
- F. Qin, H. Zhao, G. Li, H. Yang, J. Li, R. Wang, Y. Liu, J. Hu, H. Sun and R. Chen, Size-tunable fabrication of multifunctional Bi2O3 porous nanospheres for photocatalysis, bacteria inactivation and template-synthesis, Nanoscale, 2014, 6, 5402 Search PubMed.
- S. B. Subramaniyan, R. Senthilnathan, J. Arunachalam and V. Anbazhagan, Revealing the Significance of the Glycan Binding Property of Butea monosperma Seed Lectin for Enhancing the Antibiofilm Activity of Silver Nanoparticles against Uropathogenic Escherichia coli, Bioconjugate Chem., 2020, 31(1), 139–148 Search PubMed.
- R. R. S. Silva, C. R. Silva, V. F. Santos, C. R. S. Barbosa, D. F. Muniz, A. L. E. Santos, M. H. C. Santos, B. A. M. Rocha, K. L. R. Batista, L. M. Costa-Júnior, H. D. M. Coutinho and C. S. Teixeira, Parkia platycephala lectin enhances the antibiotic activity against multi-resistant bacterial strains and inhibits the development of Haemonchus contortus, Microb. Pathog., 2019, 135, 103629 Search PubMed.
- C. L. Nilsson, Chapter 1 – lectins: analytical tools from nature, in In Lectins, ed. C. L. Nilsson, Elsevier Science B.V, Amsterdam, 2007, pp. 1–13 Search PubMed.
- R. Loris, Principles of structures of animal and plant lectins, Biochim. Biophys. Acta, Gen. Subj., 2002, 1572, 198–208 Search PubMed.
- M. Veelders, S. Brückner, D. Ott, C. Unverzagt, H. U. Mösch and L. O. Essen, Structural basis of flocculin-mediated social behavior in yeast, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 22511–22516 Search PubMed.
- R. S. Ferreira, T. H. Napoleao, A. F. S. Santos, R. A. Sa, M. G. Carneiro-da-Cunha, M. M. C. Morais, R. A. Silva-Lucca, M. L. V. Oliva, L. C. B. B. Coelho and P. M. G. Paiva, Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera, Lett. Appl. Microbiol., 2011, 53, 186–192 Search PubMed.
- E. S. Nunes, M. A. A. Souza, A. F. M. Vaz, T. G. Silva, J. S. Aguiar, A. M. Batista, M. M. P. Guerra, M. C. Guarnieri, L. C. B. B. Coelho and M. T. S. Correia, Cytotoxic effect and apoptosis induction by Bothrops leucurus venom lectin on tumor cell lines, Toxicon, 2012, 59, 667–671 Search PubMed.
- M. A. Lino, R. F. Bezerra, C. D. C. Silva, E. V. M. M. Carvalho and L. C. B. B. Coelho. Fish lectins: a brief review, Advances in Zoology Research, Nova Science Publishers, Inc., 2013, vol. 5, pp. 95–114 Search PubMed.
- T. Ogawa, M. Watanabe, T. Naganuma and K. Muramoto, Diversified carbohydrate binding lectins from marine resources, J. Amino Acids, 2011, 2011, 838–914 Search PubMed.
- G. R. Vasta and H. Ahmed, Animal Lectins: A Functional View, CRC Press, 2008 Search PubMed.
- E. Preetham, A. S. Rubeena, R. Wongpanya, M. Cammarata, E. Ringo and B. Vaseeharan, Lectins in finfishes: a review, Reviews in Fisheries Science & Aquaculture, 2019, 27, 152–169 Search PubMed.
- A. S. Rubeena and E. Preetham, Antimicrobial properties and phenoloxidase activation of the lectin isolated from kadal shrimp (Metapenaeus dobsoni), Fish Shellfish Immunol., 2019, 90, 118–125 Search PubMed.
- A. S. Rubeena, M. Divya, B. Vaseeharan, S. Karthikeyan, E. Ringø and E. Preetham, Antimicrobial and biochemical characterization of a C-type lectin isolated from pearl spot (Etroplus suratensis), Fish Shellfish Immunol., 2019, 87, 202–211 Search PubMed.
- S. B. Subramaniyan, S. Vijayakumar, S. Megarajan, R. K. Kamlekar and V. Anbazhagan, Remarkable Effect of Jacalin in Diminishing the Protein Corona Interference in the Antibacterial Activity of Pectin-Capped Copper Sulfide Nanoparticles, ACS Omega, 2019, 4, 14049–14056 Search PubMed.
- M. T. S. Correia and L. C. B. B. Coelho, Purification of a glucose/mannose specific lectin, isoform 1, from seeds of Cratyliamollis mart. (Camaratu Bean), Appl. Biochem. Biotechnol., 1995, 55, 261–273 Search PubMed.
- F. G. Witebsky, J. D. Maclowry and S. S. French, Broth dilution minimum inhibitory concentrations: rationale for use of selected antimicrobial concentrations, J. Clin. Microbiol., 1979, 9, 589–595 Search PubMed.
- N. G. Heatley, A method for the assay of penicillin, Biochemical Journal, 1944, 38, 61–65 Search PubMed.
- K. B. A. Ahmed and V. Anbazhagan, Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria infected zebrafish, RSC Adv., 2017, 7, 36644–36652 Search PubMed.
- S. B. Subramaniyan, S. Megarajan, S. Vijayakumar, M. Mariappan and V. Anbazhagan, Evaluation of the toxicities of silver and silver sulfide nanoparticles against Gram-positive and Gram-negative bacteria, IET Nanobiotechnol., 2019, 13, 326–331 Search PubMed.
- S. V. Jenkinsa, D. A. Nedosekinb, E. K. Millerc, V. P. Zharovb, R. P. Dings, J. Chenc and R. J. Griffin, Galectin-1-based tumour-targeting for gold nanostructure-mediated photothermal therapy, Int. J. Hyperthermia, 2018, 34, 19–29 Search PubMed.
- H. Lis and N. Sharon, Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition, Chem. Rev., 1998, 98, 637–674 Search PubMed.
- K. B. A. Ahmed and V. Anbazhagan, Synthesis of copper sulfide nanoparticles and evaluation of in vitro antibacterial activity and in vivo therapeutic effect in bacteria-infected zebrafish, RSC Adv., 2017, 7, 36644–36652 Search PubMed.
- C. Malarkodi and S. Rajeshkumar, In vitro bactericidal activity of biosynthesized CuS nanoparticles against UTI-causing pathogens, Inorganic and Nano-Metal Chemistry, 2017, 47, 1290–1297 Search PubMed.
- G. Pandey, T. Fatma, S. M. Cowsik and S. S. Komath, Specific interaction of jacalin with phycocyanin, a fluorescent phycobiliprotein, J. Photochem. Photobiol., B, 2009, 97, 87–93 Search PubMed.
- R. Liu, A. Siemiarczuk and F. J. Sharom, Intrinsic Fluorescence of the P-glycoprotein Multidrug Transporter: Sensitivity of Tryptophan Residues to Binding of Drugs and Nucleotides, Biochemistry, 2000, 39, 14927–14938 Search PubMed.
- K. B. A. Ahmed, T. Raman and A. Veerappan, Jacalin capped platinum nanoparticles confer persistent immunity against multiple Aeromonas infection in zebrafish, Sci. Rep., 2018, 8, 2200 Search PubMed.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Antimicrobial activity of metals: mechanism, molecular targets and applications, Nat. Rev. Microbiol., 2013, 11, 371–384 Search PubMed.
- V. Kalyanaraman, K. J. Darley-Usmar, P. A. Davies, H. J. Dennery, M. B. Forman, G. E. Grisham, K. Mann, L. J. Moore, I. I. Roberts and H. Ischiropoulos, Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations, Free Radical Biol. Med., 2012, 52, 1–6 Search PubMed.
- K. B. A. Ahmed, S. B. Subramaniyan, S. F. Banu, N. Paramasivam and V. Anbazhagan, Jacalin-copper sulfide nanoparticles complex enhance the antibacterial activity against drug resistant bacteria via cell surface glycan recognition, Colloids Surf., B, 2018, 163, 209–217 Search PubMed.
- K. B. A. Ahmed, R. Thiagarajan and V. Anbazhagan, Platinum nanoparticles inhibit bacteria proliferation and rescue zebrafish from bacterial infection, RSC Adv., 2016, 6, 44415–44424 Search PubMed.
- P. Elumalai, P. Prakash, M. S. Musthafa and C. Faggio, Effect of alkoxy glycerol on growth performance, immune response and disease resistance in Nile Tilapia (Oreochromis niloticus), Res. Vet. Sci., 2019, 123, 298–304 Search PubMed.
- K. B. A. Ahmed, A. S. Mohammed and A. Veerappan, Interaction of sugar stabilized silver nanoparticles with the T-antigen specific lectin, jacalin from Artocarpus integrifolia, Spectrochim. Acta, Part A, 2015, 145, 110–116 Search PubMed.
- M. F. Alam, S. Varshney, M. A. Khan, A. A. Laskar and H. Younus, In vitro DNA binding studies of therapeutic and prophylactic drug citral, Int. J. Biol. Macromol., 2018, 113, 300–308 Search PubMed.
- S. S. Komath, M. Kavitha and M. J. Swamy, Beyond carbohydrate binding: new directions in plant lectin research, Org. Biomol. Chem., 2006, 4, 973–988 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra06853e |
| This journal is © The Royal Society of Chemistry 2020 |