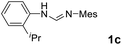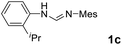Open Access Article
Open Access ArticleSyntheses of tetrahydroquinoline-based chiral carbene precursors and the related chiral NHC–Au(I) complex†
Licheng Zhan,
Gengtao Zhang,
Jiwei Wang and
Jun Zhang *
*
Key Laboratory for Advanced Materials, Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, East China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China. E-mail: zhangj@ecust.edu.cn
First published on 23rd September 2020
Abstract
Four tetrahydroquinoline-based chiral carbene precursors were synthesized using unsymmetrical N,N′-diarylformamidines and chiral 2-allyloxiranes as starting materials. A representative NHC–gold complex has been prepared and fully characterized, the crystal structure of which reveals an intramolecular Au⋯H–C(sp3) interaction between Au(I) and the hydrogen atom of the isopropyl moiety in the N-aryl group.
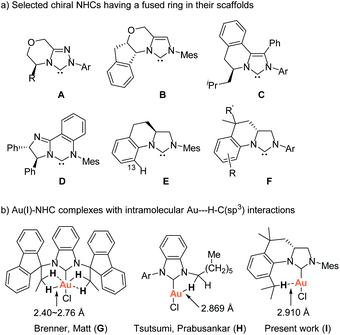
Imidazolidin-2-ylidene carbenes are among the most important N-heterocyclic carbenes (NHCs) due to their widespread and spectacular applications as organocatalysts and as ligands for organometallic catalysis.1 Over the past twenty years, a great deal of effort has been made to employ chiral NHCs as organocatalysts and as ligands in asymmetric catalysis.2 Despite the successful applications of a variety of chiral NHCs in asymmetric catalysis, the development of facile methodologies for structurally-specific chiral NHCs with structural diversity is highly desirable and a challenging issue in asymmetric catalysis. Several types of chiral backbone-unsaturated NHCs having a fused ring in their scaffolds, such as A,3 B,4 C,5 and D,6 have been successfully developed for organocatalysts and/or as ligands for organometallic catalysis (Scheme 1a). In their scaffolds, chiral moiety is directly linked to the N-atom and embedded in a fused ring. Due to the rigidity of the fused ring, the rotation of chiral moiety about the N–C bond is restricted, thereby enhancing the asymmetric induction of the NHCs in controlling the stereochemistry of the asymmetric catalytic reaction.
 | ||
| Scheme 1 Chiral NHCs having a fused ring in their scaffolds and related NHC–Au(I) complexes with intramolecular Au⋯H–C(sp3) interaction. | ||
With different design strategy, Blechert et al. have developed chiral imidazolidin-2-ylidene E having a fused ring in the scaffold, which exhibited high efficiency in Ru-catalyzed asymmetric ring-opening cross-metathesis.7 The fused ring in E twists the framework, hampers rotation of the N-aryl substituent, and thus reaches the optimal transfer of chirality, while at the same time second N-mesityl substituent adopts a planar orientation. However, the approach for the synthesis of the carbene precursors, imidazolinium salts requires uncommon starting material and a kinetic enzymatic resolution, which limit modification of NHC ligands. Additionally, only the imidazolinium salt of type E having no C13 substituent was prepared by the method.8 Varying the N-aryl substituents with different steric bulkiness in E might create a tunable chiral environment closer to the reactive site. Until very recently, a consecutive intermolecular reductive amination/asymmetric hydrogenation has been developed for the synthesis of the precursors of E.8
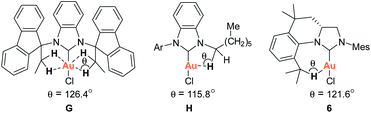
As part of our studies on the design and synthesis of various novel NHC ligands for carbene chemistry and catalysis,9 we herein wish to report a new synthetic strategy for the facile preparation of various tetrahydroquinoline-based chiral carbene precursors of NHCs F using unsymmetrical N,N′-diarylformamidines and chiral 2-allyloxiranes as starting materials. A representative NHC–gold complex I has been prepared and structurally characterized. The crystal structure of the NHC–gold reveals an intramolecular Au(I)⋯H–C(sp3) interaction between Au(I) and the hydrogen atom of isopropyl moiety (Scheme 1b). Recently, the Au(I)⋯H–C(sp3) hydrogen-bonding interaction have been observed in NHC–Au(I) complexes G and H, which are believed to either stabilise the out-of-plane conformation (G)10a or make partial contributions to the luminescence properties of the NHC–Au(I) complex (H)10b (Scheme 1b). Additionally, Au⋯H–C hydrogen-bonding interactions in NHC–Au complexes have been proposed as key intermediates in the mechanistic studies of NHC–Au catalyzed C–H activation.11
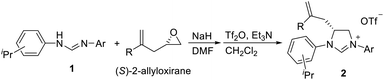
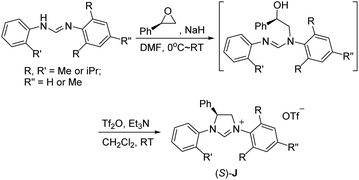
We have previously developed a versatile and modular method for the preparation of various backbone-substituted imidazolinium salts from the reaction of formamidines with alkene oxides.9a The methodology exhibits high regiochemistry. When reacting styrene oxide with the unsymmetrical N,N′-diarylformamidines bearing a mono-o-substituted aryl group and a di-o-substituted aryl group, only one regioisomer in which the backbone-substituted phenyl group is on the carbon atom close to the mono-substituted aryl ring was formed. More importantly, chiral monosubstituted imidazolinium salts, (S)-J could be obtained when using (R)-styrene oxide, indicating that inversion of the configuration of (R)-styrene oxide occurred in the two-step synthesis (Scheme 2).9a Therefore, we decide to use (S)-2-allyloxirane and the unsymmetrical N,N′-diarylformamidines to synthesize chiral backbone-allyl-substituted imidazolinium salts, which is supposed to undergo intramolecular Friedel–Crafts alkylation12 to afford chiral imidazolinium salts as the precursors of the chiral NHCs of type F.
 | ||
| Scheme 2 Synthesis of chiral backbone-monosubstituted imidazolinium salts from the reaction of formamidines with alkene oxide.9a | ||
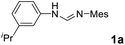
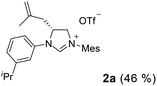
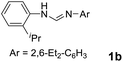
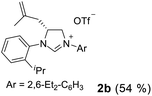
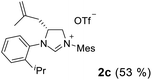
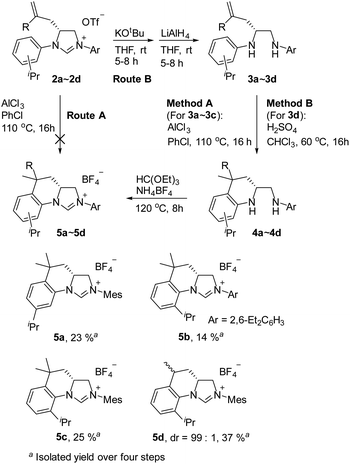
As expected, the ring opening reaction of unsymmetrical N,N′-diarylformamidines 1a–1d with (S)-2-allyloxiranes followed by cyclization afforded four backbone-allyl-substituted imidazolinium salts 2a–2d, respectively (Table 1). However, attempts to direct Friedel–Crafts alkylation of 2a–2d failed (Route A, Scheme 3). Therefore, an alternative route was investigated. In the presence of KOtBu as base, the ring opening of 2a–2d followed by reduction using LiAlH4 afforded diamines 3a–3d (Route b, Scheme 3).13 Delightfully, in the presence of either AlCl3 (for 3a–3c) or H2SO4 (3d), the resulting amines could smoothly undergo intramolecular Friedel–Crafts alkylation to give diamines 4a–4d. Finally, cyclization of the diamines 4a–4d with HC(OEt)3 in the presence of NH4BF4 generated the desired chiral imidazolidinium salts 5a–5d. During the transformations, a partial racemization was detected. The ee value of 4a was determined as 88% on the basis of chiral HPLC analysis (p. S8, see ESI†).
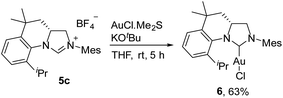
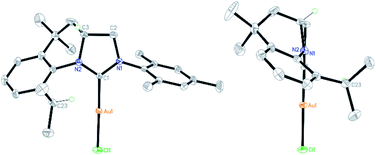
The ability of the novel tetrahydroquinoline-based chiral imidazolidin-2-ylidene carbene to ligate a transition metal fragment was also examined. Treatment of the in situ generated free carbene with AuCl·Me2S gave the chiral NHC gold complex 6 in 63% yield (Scheme 4). The structure of 6 was determined by single-crystal X-ray diffraction, which exhibits the expected linear coordination geometry, and also shows that the chiral NHC in 6 has the R configuration (Fig. 1). In 6, the Au–C bond length at the normal C2 position (1.972(6) Å) is typical of NHC–Au complexes.14 The crystal structure also reveals that the C2 bridge at the chirality center leads to a dihedral angle of 58° between the N-aryl group and the imidazoline plane and enforces a close approach of isopropyl group to the coordination sphere of the gold center. The Au⋯H–C distance found in 6 (2.910 Å) is comparable with that of a NHC–Au(I) complex ligated by N-(9-anthracenyl)-N′-(heptyl)benzimidazol-2-ylidene (2.869 Å), and van der Waals radii (2.86 Å),10 suggesting the presence of a rare Au⋯H–C(sp3) interaction in gold complex 6 (Scheme 1b). The Au⋯H–C(sp3) angle in 6 (121.6°) is similar to those observed in G (126.4°) and H (115.8°) (Fig. 2). The 1H NMR resonance for the hydrogen atom H23 in 6 appears at 4.10 ppm downfield relative to that for its precursor, imidazolidinium salt 5c.
In conclusion, we present a method for the synthesis of tetrahydroquinoline-based chiral carbene precursors using unsymmetrical N,N′-diarylformamidines and 2-allyloxiranes as starting materials. Treatment of unsymmetrical N,N′-diarylformamidines with 2-allyloxirane followed by cyclization gave backbone-allyl-substituted imidazolinium salts, which could be transformed into the desired tetrahydroquinoline-based chiral carbene precursors through a key intramolecular Friedel–Crafts alkylation. A representative chiral NHC–gold complex has been prepared by the reaction of the in situ generated free carbene with AuCl·Me2S. The crystal structure of the NHC–gold complex reveals a rare intramolecular Au⋯H–C(sp3) interaction between Au(I) and the hydrogen atom of isopropyl moiety. We are currently exploring the application of the resulting chiral carbene metal complexes in transition metal-catalyzed asymmetric synthetic transformations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the National Natural Science Foundation of China for financial support (21671066) is greatly acknowledged.Notes and references
- (a) D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS; (b) P. L. Arnold and I. J. Casely, Chem. Rev., 2009, 109, 3599–3611 CrossRef CAS; (c) C. Samojłowicz, M. Bieniek and K. Grela, Chem. Rev., 2009, 109, 3708–3742 CrossRef; (d) J. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Hwang and I. J. B. Lin, Chem. Rev., 2009, 109, 3561–3598 CrossRef CAS; (e) G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746–1787 CrossRef CAS; (f) T. Dröge and F. Glorius, Angew. Chem., Int. Ed., 2010, 49, 6940–6952 CrossRef; (g) Q. Zhao, G. Meng, S. P. Nolan and M. Szostak, Chem. Rev., 2020, 120, 1981–2048 CrossRef CAS; (h) M. J. Benedikter, F. Ziegler, J. Groos, P. M. Hauser, R. Schowner and M. R. Buchmeiser, Coord. Chem. Rev., 2020, 415, 213315 CrossRef CAS; (i) T. Ishii, K. Nagao and H. Ohmiya, Chem. Sci., 2020, 11, 5630–5636 RSC.
- (a) F. Wang, L. Liu, W. Wang, S. Li and M. Shi, Coord. Chem. Rev., 2012, 256, 804–853 CrossRef CAS; (b) D. Janssen-Müller, C. Schlepphorst and F. Glorius, Chem. Soc. Rev., 2017, 46, 4845–4854 RSC; (c) L. Prieto, E. Sánchez-Díez, U. Uria, E. Reyes, L. Carrillo and J. L. Vicario, Adv. Synth. Catal., 2017, 359, 1678–1683 CrossRef CAS; (d) J. Thongpaen, R. Manguin and O. Baslé, Angew. Chem., Int. Ed., 2020, 59, 10242–10251 CrossRef CAS; (e) X. Chen, Z. Gao and S. Ye, Acc. Chem. Res., 2020, 53, 690–702 CrossRef CAS.
- L. Zhou, X. Wu, X. Yang, C. Mou, R. Song, S. Yu, H. Chai, L. Pan, Z. Jin and Y. R. Chi, Angew. Chem., Int. Ed., 2020, 59, 1557–1561 CrossRef CAS.
- (a) C. Guo, D. Janssen-Müller, M. Fleige, A. Lerchen, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2017, 139, 4443–4451 CrossRef CAS; (b) S. Singha, E. Serrano, S. Mondal, C. G. Daniliuc and F. Glorius, Nat. Catal., 2020, 3, 48–54 CrossRef CAS.
- D. Hirsch-Weil, K. A. Abboud and S. Hong, Chem. Commun., 2010, 46, 7525–7527 RSC.
- (a) J. K. Park, H. H. Lackey, M. D. Rexford, K. Kovnir, M. Shatruk and D. T. McQuade, Org. Lett., 2010, 12, 5008–5011 CrossRef CAS; (b) S. Budagumpi, R. S. Keri, G. Achar and K. N. Brinda, Adv. Synth. Catal., 2020, 362, 970–997 CrossRef CAS.
- A. Kannenberg, D. Rost, S. Eibauer, S. Tiede and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 3299–3302 CrossRef CAS.
- Y. Chen, Y. Pan, Y. He and Q. Fan, Angew. Chem., Int. Ed., 2019, 58, 16831–16834 CrossRef CAS.
- (a) J. Zhang, X. Su, J. Fu and M. Shi, Chem. Commun., 2011, 47, 12541–12543 RSC; (b) J. Zhang, X. Su, J. Fu, X. Qin, M. Zhao and M. Shi, Chem. Commun., 2012, 48, 9192–9194 RSC; (c) J. Zhang, J. Fu, X. Su, X. Qin, M. Zhao and M. Shi, Chem. Commun., 2012, 48, 9625–9627 RSC; (d) J. Zhang, S. Song, X. Wang, J. Jiao and M. Shi, Chem. Commun., 2013, 49, 9491–9493 RSC; (e) S. Lv, J. Wang, C. Zhang, S. Xu, M. Shi and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14941–14946 CrossRef CAS; (f) J. Wang, X. Cao, S. Lv, C. Zhang, S. Xu, M. Shi and J. Zhang, Nat. Commun., 2017, 8, 14625 CrossRef; (g) H. Chen, J. Wang, Z. Hu, S. Xu, M. Shi and J. Zhang, Chem. Commun., 2017, 53, 10835–10838 RSC.
- (a) M. Teci, E. Brenner, D. Matt, C. Gourlaouen and L. Toupet, Chem.–Eur. J., 2015, 21, 10997–11000 CrossRef CAS; (b) M. Vaddamanu, A. Sathyanarayana, Y. Masaya, S. Sugiyama, O. Kazuhisa, K. Velappan, K. Subramaniyam, K. Hisano, O. Tsutsumi and G. Prabusankar, Organometallics, 2020, 39, 2202–2206 CrossRef CAS.
- (a) Q. Zhao, G. Meng, S. P. Nolan and M. Szostak, Chem. Rev., 2020, 120, 1981–2048 CrossRef CAS; (b) W.-P. To, G. S.-M. Tong, W. Lu, C. Ma, J. Liu, A. L.-F. Chow and C.-M. Che, Angew. Chem., Int. Ed., 2012, 51, 2654–2657 CrossRef CAS; (c) M. R. Fructos, T. R. Belderrain, P. de Frémont, N. M. Scott, S. P. Nolan, M. M. Díaz-Requejo and P. J. Pérez, Angew. Chem., Int. Ed., 2005, 44, 5284–5288 CrossRef CAS; (d) M. R. Fructos, P. de Frémont, S. P. Nolan, M. M. Díaz-Requejo and P. J. Pérez, Organometallics, 2006, 25, 2237–2241 CrossRef CAS.
- The intramolecular Friedel–Crafts alkylation approach was also previously used to construct chiral NHC ligands having a 2,2′-bisquinoline-based C2 symmetric skeleton: L. Liu, N. Ishida, S. Ashida and M. Murakami, Org. Lett., 2011, 13, 1666–1669 CrossRef CAS.
- (a) S. Bulut and W. L. Queen, J. Org. Chem., 2018, 83, 3806–3818 CrossRef CAS; (b) O. Hollóczki, P. Terleczky, D. Szieberth, G. Mourgas, D. Gudat and L. Nyulászi, J. Am. Chem. Soc., 2011, 133, 780–789 CrossRef; (c) B. J. van Lierop, A. M. Reckling, J. A. M. Lummiss and D. E. Fogg, ChemCatChem, 2012, 4, 2020–2025 CrossRef CAS; (d) M. K. Denk, J. M. Rodezno, S. Gupta and A. J. Lough, J. Organomet. Chem., 2001, 617–618, 242–253 CrossRef CAS.
- (a) P. de Frémont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 2411–2418 CrossRef; (b) P. Ai, M. Mauro, L. De Cola, A. A. Danopoulos and P. Braunstein, Angew. Chem., Int. Ed., 2016, 55, 3338–3341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization details of new compounds, crystallographic details, NMR spectra. CCDC 2011334 for (6). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra07271k |
| This journal is © The Royal Society of Chemistry 2020 |