Open Access Article
Open Access ArticleSynthesis and evaluation of eco-friendly carboxymethyl cellulose/polyvinyl alcohol/CuO bionanocomposites and their use in coating processed cheese
Ahmed M. Youssef a,
Fayza M. Assemb,
Hoda S. El-Sayedb,
Samah M. El-Sayed*b,
Mostafa Elaaserb and
Mohamed H. Abd El-Salamb
a,
Fayza M. Assemb,
Hoda S. El-Sayedb,
Samah M. El-Sayed*b,
Mostafa Elaaserb and
Mohamed H. Abd El-Salamb
aPackaging Materials Department, National Research Centre, 33 El Bohouth St. (former El Tahrir St.), Dokki, Giza, P.O. 12622, Egypt. E-mail: drahmadyoussef1977@gmail.com; Fax: +202 33370931; Tel: +202 33322418
bDairy Science Department, National Research Centre, 33 El Bohouth St. (former El Tahrir St.), Dokki, Giza, P.O. 12622, Egypt
First published on 14th October 2020
Abstract
In the present study, we formulated and characterized CMC/PVA/CuO bionanocomposites to evaluate their use in coating processed cheese. Copper oxide nanoparticles (CuO-NPs) were prepared and added to a mixed solution of carboxymethyl cellulose (CMC)/polyvinyl alcohol (PVA) using compositions of 0.3, 0.6 and 0.9% (w/v). The CMC/PVA/CuO bionanocomposites were prepared by a solution casting method and used for coating processed cheese. The fabricated bionanocomposite films and CuO-NPs were characterized by TEM, SEM, EDEX, XRD, DLS, and FT-IR analysis. Inclusion of CuO-NPs decreased the gas transmission rate (GTR) and water vapor transmission rate (WVTR) of the prepared film. Also, the bionanocomposite suspensions exhibited high but variable inhibitory effects against several pathogenic bacteria and fungi. The impact of coating of processed cheese surfaces with the prepared bionanocomposite films on microbiological, physicochemical, textural and sensory properties of the processed cheese were assessed during 6 months of cold storage. Coating cheese with film containing CuO-NPs eliminated mould growth on the cheese surface and decreased significantly (P < 0.05) the total bacterial count of the cheese. Furthermore, coating of cheese decreased the moisture losses and retarded the increase in the cheese hardness during storage. The highest acceptability at the end of the storage period was given for processed cheese coated with the bionanocomposite containing 0.9% CuO-NPs. Thus, the obtained CMC/PVA/CuO bionanocomposite films could be a promising candidate for cheese packaging applications.
1. Introduction
In the past years, nanotechnology has been increasingly used to meet the consumer's demand for quality foods and in developing antimicrobial agents to prolong the food lifetime during storage and distribution. Food producers continuously evaluate the benefits and disadvantages of packaging materials in order to choose suitable packaging materials for their food products.1,2 The application of nanomaterials has a wide range in various fields, such as physical, chemical, electronic, biomedical and materials sciences. Carboxymethyl cellulose (CMC) is an anionic decomposable and biocompatible polymer derivative from natural cellulose. CMC is usually blended with other stabilizers and gums to retain moisture, and to improve the structural consistency of bakery products due to its high water-absorbing capacity.3 Polyvinyl alcohol (PVA) is a biocompatible polymer rich in hydroxyl groups, non-toxic, semi-crystalline and soluble in water.4 It is broadly used in food packaging, construction sectors and household, due to its properties, such as biocompatibility, mechanical performance and solvent resistance.5 The major disadvantages of using PVA in food packaging are its low biodegradation rate and its high moisture absorption. So, it is commonly mixed with biopolymers and/or bio-based materials to improve its performance and environmental properties. Also the limitations in both mechanical properties and water permeability of PVA films can be overcome via the addition of different nanomaterials or blending with other biopolymers.6Copper oxide (CuO) is widely used for various applications in a number of areas such as catalyst, ceramic, thermoelectric sensors, glass, superconducting materials and antimicrobial agents.7 Fabrication of CuO-NPs is less expensive when compared to gold and silver nanoparticles. In addition, CuO-NPs possess excellent potent antimicrobial action because of its characteristic crystal structure. The physical and chemical properties of NPs, such as size, agglomeration state in liquids and surface charge, play a significant role in the final interactions of NPs with target cells. CuO-NPs were reported to have potent antimicrobial activity against Escherichia coli and Staphylococcus depending on the concentration and particle size.8 Harikumar & Aravind9 found that copper nanocomposites can inhibit E. coli and that its effect increased with the increase of concentration and contact time. The antimicrobial property of film containing heat treated CuO-NPs against the growth of E. coli were higher than the film containing NaBH4-treated CuO-NPs.10 Almasi, Jafarzadeh & Mehryar11 found that CuO-NPs exhibit antibacterial act against Gram negative and Gram positive bacteria and that its inclusion in bacterial cellulose nanofiber reduced its antibacterial activity.
Nanomaterials have been used in food packaging in various forms including nanocomposites polymers with high barrier properties, smart packaging, nano-coatings, surface biocides effect, active packaging and as antimicrobial agents.12–14 CuO-NPs have been incorporated in different composites used in food packaging. It is included in two-layer bags made of heat-resistant casein protein layer coated with sodium alginate–pectin.10 These pouches were used to package coconut edible oil which was found to retard oil oxidation. Low density polyethylene/CuO-NPs composite film was used to package UF cheese15 whereas it exhibited high antimicrobial activity and UV-light barrier. In addition, the metallic nanoparticles could be utilize in different applications16–19 the release of metallic nanoparticles into a food matrix was in the safe acceptable level. Also, CuO-NPs composite film was used successfully in the packing of an Indian fermented milk product (Peda) whereas it exhibited high antimicrobial activity against several pathogenic and spoilage microorganisms.20
Processed cheeses are gaining worldwide continuous popularity due to its diverse composition and wide applications.21 The diversity of ingredients used for its formulation permits the production of a wide variety of processed cheeses with different flavours, textures and functions. Recently,22 more attention has been paid to processed cheese as a promising food vehicle able to deliver specific nutrients into human body. Packaging is a crucial step in processed cheese packaging due to its long shelf life at ambient temperatures.21 Improper packaging and storage conditions results on surface growth of yeasts and moulds conventionally overcome by adding mould inhibitor to the formulation. The presence of spoilage microorganisms is more critical to the safety of processed cheese. The use of packaging material containing antimicrobial agents may be a good alternative ensuring the safety of the product.
In the current work, novel CMC/PVA/CuO bionanocomposite materials containing different levels of CuO-NPs have been prepared, characterized and evaluated as surface coating film for processed cheese. Changes in the composition, textural and microbiological properties and acceptability of processed cheese from different treatments were followed during storage period.
2. Materials and methods
2.1. Materials
Carboxymethyl cellulose (CMC) polymer was gotten from Kelong Chemical Factory, China. Polyvinyl alcohol (PVA), copper nitrate and sodium hydroxide were obtained from (Sigma Aldrich). Ras cheese (27.50% fat, 61.00% total solids) and Cheddar cheese (25.50% fat, and 60.00% total solids) were bought from Cairo Market. Soft cheese (13.00% fat, 40.00% total solids) and butter oil were gotten from the Dairy Department, Cairo University. Joha S9 emulsifying salts (BK Giulini Chemie GmbH, Ladenburg, Germany) and citric acid were obtained from the local market. Pathogenic strains: All test strains Yersinia enterocolitica, Aspergillus niger, Bacillus cereus B-3711, Aspergillus flavus 3357, Listeria monocytogenes 598, Escherichia coli and Salmonella typhimurium were collected from Dairy Microbiology Lab., National Research Centre, Egypt.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v). The prepared CuO-NPs were added at the ratios of 0.0, 0.3, 0.6 and 0.9% (w/v) to different aliquots of the prepared mixed CMC/PVA solution. The bionanocomposites were prepared by gradual addition of CuO-NPs suspension (1 mg mL−1 in water) to the mixed polymer solution and sonicated for 3 h at 25 °C, 500 W, frequency, 20 kHz and amplitude 50% using ultrasonic homogenizer (Q500 Sonicator, Qsonica, USA). The CMC/PVA/CuO bionanocomposites solutions were transferred into transparent glass Petri dish and left at room temperature for 72 h in order to vaporize the solvent then create the CMC/PVA/CuO bionanocomposites film.
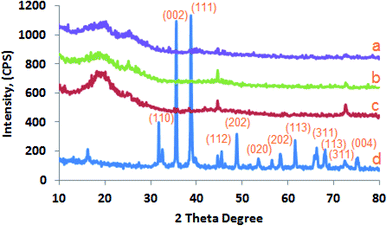
1 (v/v). The prepared CuO-NPs were added at the ratios of 0.0, 0.3, 0.6 and 0.9% (w/v) to different aliquots of the prepared mixed CMC/PVA solution. The bionanocomposites were prepared by gradual addition of CuO-NPs suspension (1 mg mL−1 in water) to the mixed polymer solution and sonicated for 3 h at 25 °C, 500 W, frequency, 20 kHz and amplitude 50% using ultrasonic homogenizer (Q500 Sonicator, Qsonica, USA). The CMC/PVA/CuO bionanocomposites solutions were transferred into transparent glass Petri dish and left at room temperature for 72 h in order to vaporize the solvent then create the CMC/PVA/CuO bionanocomposites film.2.2.3.1. X-ray diffraction pattern (XRD). The prepared CuO-NPs powder was evaluated via Philips X-ray diffractometer (PW 1930 generator, PW 1820 goniometer) using Cu Kα radiation (45 kV, 40 mA, with λ = 0.15418 nm) with scans were run in the range of a 2θ from 5 to 80° with a step size of 0.02 and step time of 1 s. The d-spacing was calculated in the diffraction patterns using Bragg's equation (nλ = 2d
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ).
θ).
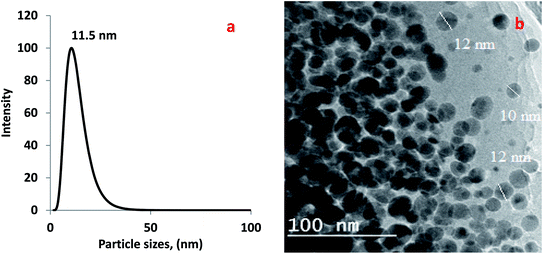
2.2.3.2. Morphological characterization. The morphology of the fabricated bionanocomposites samples was done on surfaces of the prepared bionanocomposite films and examined using scanning electron microscopy (SEM) model JEM-1230, Japan, operated at 120 kV, with a maximum magnification of 600 × 103 and a resolution until 0.2 nm. Also, the morphology of prepared CuO-NPs was evaluated through drying a drop of the solution on a carbon-coated copper grid of the Joel-100S transmission electron microscope, of resolution of 0.3 nm. The SEM microscope was attached to a dispersive energy spectrometer (EDEX).
2.2.3.3. Evaluation of particle size of the prepared CuO-NPs. The particle size of prepared CuO nanoparticles was evaluated via dynamic light scattering (NICOMP 380 ZLS, Dynamic light scattering (DLS) instrument, USA).
2.2.3.4. Barrier properties. Water vapor transmission rate (WVTR) was carried out using GBI W303 (B) Water Vapor Permeability Analyzer (China) using the cup method. The water vapor transmission rate was calculated by way of the amounts of water vapor transferred through a unit area of the film in a unit time below exact conditions of temperature (38 °C) and humidity (4%) as stated by the subsequent standards (ASTM E96). Furthermore, the oxygen transmission rate (O2) was evaluated by N530 Gas Permeability Analyzer (China), using (ISO2556-2001) as international standard.
2.2.3.5. FT-IR spectra. The interactions established in fabricated bionanocomposites were assessed by FT-IR spectra and recorded in the range from 400 to 4000 cm−1 using (Shimadzu 8400S) FT-IR Spectrophotometer.
2.2.3.6. Biodegradation characterization. The biodegradation test of bionanocomposite films carried out in soil conditions by burying bionanocomposites samples in soil. Firstly, the bionanocomposites samples were dried till constant weight at 50 °C then the bionanocomposites samples (3 cm × 3 cm) with 2 mm thickness were buried in the soil (pH = 7.5) and the relative humidity was retained at 50–60% by spraying water and the temperature was 20–25 °C. Finally, the bionanocomposites samples were weighed every week for three times, and washed with distilled water, afterward; the bionanocomposites samples were dried at 50 °C for 24 h and then weighed. Results were repeated three times.
2.2.4.1. Physicochemical analysis of cheese. Cheese samples were analyzed for moisture, fat, ash and total nitrogen (TN) contents total solid (TS) and titratable acidity (TA), as described in ref. 23. The pH values of cheeses were measured using pH meter via glass electrode (Hanna Co., Italy).
2.2.4.2. Textural profile analysis of cheese. The texture profile measurements (TPA) of processed cheese samples were evaluated according to the International Dairy Federation24 using the double compression test (TMS-Pro texture analyzer, Food Technology Co., USA). The values for texture features, i.e., hardness (N), adhesiveness (N s), gumminess (N), cohesiveness, chewiness (N), and springiness were planned from the TPA graphic.
2.2.5.1. Antimicrobial activity of fabricated CMC/PVA/CuO bionanocomposites as coating suspensions. Firstly, activated test bacterial strains in trypton soya broth, incubated at 37 °C for 24 h and activated tested fungi strains in potato dextrose broth, incubated at 25 °C for 72 h. The antimicrobial assay of bionanocomposites was carried out using the diffusion plate method according to Dodiya & Amin.25 0.1 mL of the different tested strains (∼105 cells per mL) was spread on the surface of the plate containing nutrient agar media and the plates were left at 37 °C for 2 h. Using cork borer (0.5 cm) to prepare wells in the agar layer and after that full each well with 50 μL of different bionanocomposites suspension. After incubation at 37 °C/24 h, the diameter of the clear inhibition zone surrounding the wells was measured.
2.2.5.2. Microbiological properties of processed cheese during storage. Ten grams of each processed cheese samples were homogenized individually with 90 mL of sterilized tri-sodium citrate solution (2% w/v). Decimal dilutions were prepared in 9 mL sterile saline solution. Potato dextrose agar medium was used to count yeasts and moulds after incubated at 25 °C for 4 days in aerobic condition.26 Violet red bile agar medium was used to detect coliforms after incubated at 37 °C for 18 h. Also, plate count agar medium was used to counted total bacteria after incubated at 37 °C for 48 h.27 Plate count agar medium was used to determined psychotropic bacteria after incubated at 7 °C for 7 days.26 Also, spore forming counts were enumerated by plate count agar medium after heated the tubes contained samples for 80 °C for 10 min and cold rapidly28 and plates were incubated at 35 °C for 48 h.
3. Results and discussion
3.1. Evaluation of the prepared CuO-NPs and the bionanocomposites films
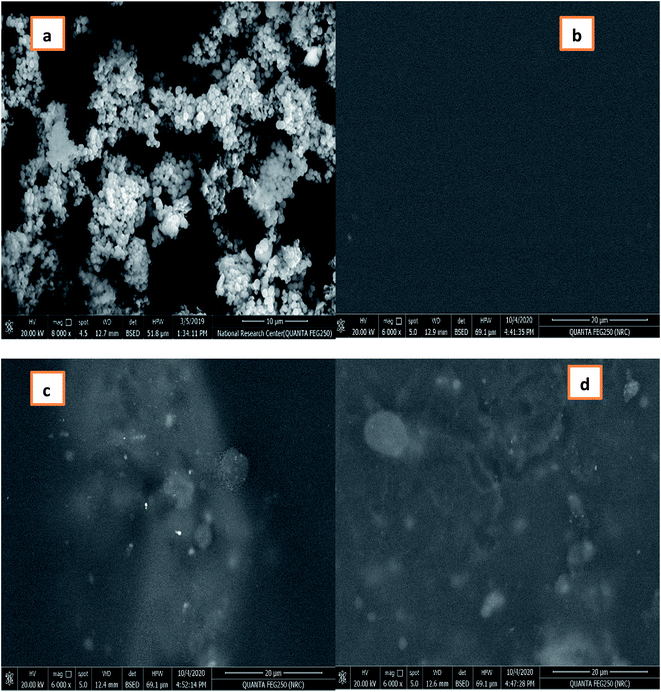 | ||
| Fig. 3 SEM image of (a) CuO-NPs, (b) CMC/PVA blend, (c) and (d) CMC/PVA/CuO bionanocomposites containing different amounts of CuO-NPs (0.3% and 0.9%), respectively. | ||
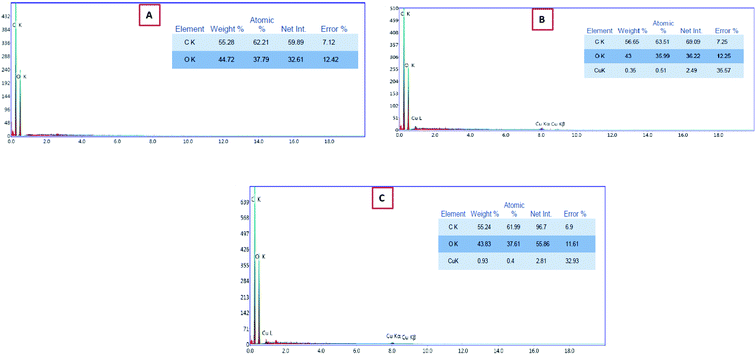
The EDEX analysis of the CMC/PVA blend as well as the prepared CMC/PVA/CuO bionanocomposites film fabricated using different loadings % of CuO-NPs, the existences of CuO nanoparticles into the bionanocomposites matrix was proven using EDEX analysis (Fig. 4) which displays that the presence of CuO-NPs in the EDEX and the percent of CuO-NPs in the matrix about 0.3 and 0.9% that demonstrated by SEM analysis (Fig. 3).
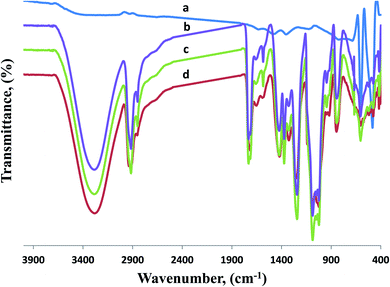 | ||
| Fig. 5 FT-IR spectra of (a) CuO-NPs, (b) CMC/PVA blend as well as the prepared CMC/PVA/CuO bionanocomposites containing different amounts of CuO-NPs, (c) 0.3% and (d) 0.9%. | ||
The vibrational peak detected between 2860 and 3986 cm−1 are attributed to the stretching C–H from alkyl groups also the stretching of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O from acetate group from PVA were detected at peaks around 1740 and 1720 cm−1. Correspondingly, the peaks corresponding to hydroxyl groups shifted to 3312 cm−1 and altered into weaker signs for CMC/PVA/CuO bionanocomposites films likened to CMC/PVA blend. Also, it has clear bands at 528 cm−1 and 580 cm−1 that are correlated to CuO stretching modes. Samples have the absorption bands in range from 1385 to 1642 cm−1 which might be associated to OH bending vibrations joined with copper atoms. Thus, FT-IR data proposes that the presence of CuO-NPs in the CMC/PVA blend.
O and C–O from acetate group from PVA were detected at peaks around 1740 and 1720 cm−1. Correspondingly, the peaks corresponding to hydroxyl groups shifted to 3312 cm−1 and altered into weaker signs for CMC/PVA/CuO bionanocomposites films likened to CMC/PVA blend. Also, it has clear bands at 528 cm−1 and 580 cm−1 that are correlated to CuO stretching modes. Samples have the absorption bands in range from 1385 to 1642 cm−1 which might be associated to OH bending vibrations joined with copper atoms. Thus, FT-IR data proposes that the presence of CuO-NPs in the CMC/PVA blend.
| Samples | CuO-NPs, ratio | OTR, cc per m2 per day | WVTR, g per m2 per day |
|---|---|---|---|
| a All samples are represented as mean of replicates ± standard deviation. | |||
| CMC/PVA | 0.0 | 71.63 ± 0.67 | 730.23 ± 0.57 |
| CMC/PVA/CuO | 0.3% ± 0.88 | 33.07 ± 0.58 | 669.35 ± 0.88 |
| CMC/PVA/CuO | 0.9% ± 0.58 | 25.55 ± 0.33 | 169.13 ± 0.88 |
Thus, the adding of 0.3% and 0.9% CuO-NPs decreased dramatically the WVTR value of the prepared bionanocomposites film to 669.35 and 169.13 g per m2 per day respectively in comparison to 730.23 g per m2 per day for CMC/PVA film. Also, Table 1 demonstrates that resistance for gas permeation of the fabricated bionanocomposites films develops by increasing the percentage of added CuO-NPs in the CMC/PVA blend. Hence the permeability of (O2) decreased from 33.07 to 25.55 cc per m2 per day compared by the CMC/PVA films which display higher GTR compared to the prepared bionanocomposites films. The present results can be explained by the tortuosity effect.33 Incorporation of impermeable nanoparticles within a polymer matrix represents an obstacle to the diffusion of gases and water vapors. For the permeation of diffusing molecules it has to follow a more tortuous pathway. The volume fraction and shape of the added nanoparticles and their dispersion and distribution within the polymer matrix influences the tortuosity effect.
3.2. Soil burial degradation test
The biodegradation investigation was used to achieve the ecofriendly compatibility of prepared CMC/PVA/CuO bionanocomposites. The biodegradation of the CMC/PVA blend as well as the fabricated bionanocomposites was achieved through a soil burial degradation technique for four weeks. The CMC/PVA blend demonstrated degradation rate in soil around 75% weight loss after 4 weeks of soil burial degradation as shown in (Fig. 6). Furthermore, the biodegradation examination of CMC/PVA/CuO bionanocomposites containing different ratios of CuO-NPs during 4 weeks, displays the biofilms biodegradation (between 78 to 84%) after four week. It can be realized that the highest weight loss was observed for the bionanocomposite film containing high content of CuO-NPs. Furthermore, the obtained data revealed that with raising the CuO-NPs contents from 0.3 to 0.9 wt%, the biodegradation rate was slightly improved as revealed in Fig. 6. It appears the following cause's for instance large cavity creation by presences of CuO-NPs in the bionanocomposites film surface and rising of agglomeration could improve the biodegradability of the prepared bionanocomposites.34 Moreover, the percentage of weight loss are effected by the composition of bionanocomposites film, and many factors including the type of present microorganisms and its quantity, as well as the degradation conditions (for example; pH, temperature, moisture, and oxygen), impurities amount; and the soil features (specifically the particle size distribution in the soil).35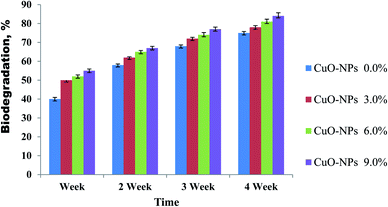 | ||
| Fig. 6 The biodegradation activities of CMC/PVA/CuO bionanocomposites containing different ratios of CuO-NPs during 4 weeks of soil burial. | ||
3.3. Physicochemical properties of processed cheese
Table 2 shows that the moisture content of processed cheese was affected by packaging and storage period. The significant decrease in the moisture content was more pronounced in the surface coated cheese using a film without added CuO-NPs (control) compared to those coated with bionanocomposites containing different percentages of CuO-NPs. At the end of the storage period processed cheese coated with 0.9% CuO-NPs film had the highest moisture content whereas the lowest was in the control. The increased in moisture content in coated cheese during storage can be explained by the improved barrier properties of the used films as evident from the reduced WVTR as revealed in Table 1. Also, there were significant differences in the moisture content of processed cheese from all treatments as affected by the coating materials which can be attributed to differences in their WVTR.| Storage period (month) | CMC/PVA/CuO bionanocomposites | ||||
|---|---|---|---|---|---|
| 0.0 CuO-NPs% | 0.3 CuO-NPs% | 0.6 CuO-NPs% | 0.9 CuO-NPs% | ||
| a Means with same superscript, lower case (effect of coating) and upper case (effect of storage) are not significantly different (P < 0.05). | |||||
| Moisture % | Fresh | 59.76aA | 59.76aA | 59.76aA | 59.76aA |
| 2 | 57.11dB | 57.53cB | 58.32bB | 59.00aB | |
| 4 | 56.16cC | 57.12bC | 57.78aC | 57.96aC | |
| 6 | 55.17bD | 56.89aC | 57.10aD | 57.29aD | |
| TN % | Fresh | 1.92aD | 1.92aD | 1.92aD | 1.92aD |
| 2 | 2.10aC | 2.05bC | 2.02bC | 1.98cC | |
| 4 | 2.18aB | 2.14bB | 2.08cB | 2.06cB | |
| 6 | 2.24aA | 2.21aA | 2.16bA | 2.12cA | |
| Fat % | Fresh | 19.67aC | 19.67aC | 19.67aC | 19.67aC |
| 2 | 20.67aB | 20.67aB | 20.50aB | 20.17bB | |
| 4 | 21.17aA | 21.00aA | 20.67bB | 20.67bA | |
| 6 | 21.17aA | 21.17aA | 21.00aA | 20.83aA | |
| Ash % | Fresh | 4.37aB | 4.37aB | 4.37aC | 4.37aD |
| 2 | 4.66aA | 4.65aA | 4.55bB | 4.47bC | |
| 4 | 4.72aA | 4.70aA | 4.62aB | 4.60aB | |
| 6 | 4.76aA | 4.74aA | 4.74aA | 4.73aA | |
| Acidity % | Fresh | 1.55aD | 1.55aD | 1.55aD | 1.55aD |
| 2 | 1.64bC | 1.65bC | 1.68aC | 1.70aC | |
| 4 | 1.75cB | 1.77cB | 1.82bB | 1.85aB | |
| 6 | 1.79bA | 1.84aA | 1.86aA | 1.88aA | |
| pH | Fresh | 6.03aA | 6.03aA | 6.03aA | 6.03aA |
| 2 | 5.93aB | 5.92aB | 5.89bB | 5.83cB | |
| 4 | 5.86aC | 5.82bC | 5.78cC | 5.73dC | |
| 6 | 5.79aD | 5.74bD | 5.71cD | 5.68dD | |
The fat content of processed cheese increased through storage but without significant differences among treatments (Table 2). The increase in the fat percentage caused mainly from the increase in the total solid (TS) during storage.
Also, Table 2 shows that the total nitrogen (TN) content of coated processed cheese increased slightly at the end of the storage period basically due to loss of moisture. The control cheese had the highest TN content compared to the other coated processed cheese treatments (Table 2), which can be attributed to its low moisture content. There were no significant differences in the content of ash observed between all treatments of the cheese while, with advanced of the storage, ash contents increased significantly.
In all cheese treatments the acidity was significantly raised and pH decreased through storage period (Table 2). Also, slight but significant differences were found in percentage of the acidity of processed cheese from different treatments. These results point to that cheese coating didn't interfere with regular changes in processed cheese during storage.
3.4. Texture profile analysis (TPA) of processed cheese
The TPA parameters of processed cheese from different treatments are presented in Table 3. The hardness of processed cheese was affected by coating and storage period. With the progress of the storage period, hardness increased in all treatments compared to hardness of the fresh cheeses. This may be attributed mainly to the moisture losses and increases in the total solids contents of cheese. A close but negative relation was found between hardness and moisture content of cheese with r2 value of 0.82. Also, significant (P > 0.05) differences were found in the hardness of cheese coated with bionanocomposites films containing different levels of CuO-NPs whereas cheese coated with bionanocomposites containing 0.9% CuO-NPs had the lowest hardness contrary the control treatment.| Storage period (month) | CMC/PVA/CuO bionanocomposites | |||
|---|---|---|---|---|
| 0.0 CuO-NPs% | 0.3 CuO-NPs% | 0.6 CuO-NPs% | 0.9 CuO-NPs% | |
| a Means with same superscript, lower case (effect of coating) and upper case (effect of storage) are not significantly different (P < 0.05). | ||||
| Hardness (N) | ||||
| Fresh | 2.30aD | 2.30aD | 2.30aD | 2.30aD |
| 2 | 5.54aC | 5.47aC | 5.30bC | 5.20cC |
| 4 | 7.22aB | 7.00bB | 6.88cB | 6.53dB |
| 9.41aA | 9.16bA | 8.76cA | 8.48dA | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Cohesiveness (area B/A) | ||||
| Fresh | 0.642aA | 0.642aA | 0.642aA | 0.642aB |
| 2 | 0.589aA | 0.571bB | 0.631bA | 0.808aA |
| 4 | 0.530cA | 0.541cB | 0.661bA | 0.847aA |
| 6 | 0.637bA | 0.652bA | 0.685bA | 0.881aA |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Springiness | ||||
| Fresh | 0.119aC | 0.119aC | 0.119aC | 0.119aC |
| 2 | 0.705cA | 0.689cA | 0.758bA | 0.907aA |
| 4 | 0.633cB | 0.577dB | 0.738bA | 0.883aA |
| 6 | 0.691cA | 0.672cA | 0.780Ba | 0.825aB |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Gumminess | ||||
| Fresh | 1.477aD | 1.477aD | 1.477aD | 1.477aD |
| 2 | 3.263cC | 3.123dC | 3.344bC | 4.202aC |
| 4 | 3.826cB | 3.787dB | 4.547bB | 5.531aB |
| 6 | 5.994bA | 5.972bA | 6.000bA | 7.471aA |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Chewiness | ||||
| Fresh | 0.914aD | 0.914aC | 0.914aD | 0.914aD |
| 2 | 2.300cC | 2.152dB | 2.530bC | 3.811aC |
| 4 | 2.422cB | 2.185dB | 3.356bB | 4.884aB |
| 6 | 4.142cA | 4.013dA | 4.680bA | 6.164aA |
Correspondingly, the cheese hardness reduced with the rise in the percentage of the CuO-NPs in the coat film. This may be owed to the low water vapor permeability of the bionanocomposites used.33 Both the storage period and coating had significant effects (P < 0.05) on the gumminess and chewiness of processed cheese. Gumminess and chewiness were increased with storage progress and these increases were more obvious in cheese coated with films containing 0.9% CuO-NPs. From the above results it can be established that the most pronounced changes in the textural properties of processed cheese as affected by storage period and coating happened in hardness, gumminess and chewiness in cheese coated with films containing 0.9% CuO-NPs. Nevertheless, coating and storage time had no important (P > 0.05) effect on springiness of processed cheese.
3.5. Sensory properties
The changes in the sensory scores of processed cheese during storage as affected by coating were presented in Table 4. Fresh coated processed cheeses ranked high scores for colour, odour, taste, oiling off and consistency. During storage the colour scores were reduced for all treatments up to the end of storage period. Colour scores in cheese coated with 0.9% CuO-NPs were constant. Control and coated cheeses gained the similar scores for the consistency when fresh and after then significantly decreased until the end of storage period. Oiling off scores in 0.0% loading of CuO-NPs coated processed cheese were lower than other all treatments.| Storage period (month) | CMC/PVA/CuO bionanocomposites | |||
|---|---|---|---|---|
| 0.0 CuO-NPs% | 0.3 CuO-NPs% | 0.6 CuO-NPs% | 0.9 CuO-NPs% | |
| a Means with same superscript, lower case (effect of coating) and upper case (effect of storage) are not significantly different (P < 0.05). | ||||
| Colour | ||||
| Fresh | 8.0aA | 8.0aA | 8.0aA | 8.0aA |
| 2 | 7.3aA | 7.6aA | 8.0aA | 8.0aA |
| 4 | 5.0cB | 7.0bA | 8.0aA | 8.0aA |
| 6 | 4.0dC | 6.0cB | 7.0bB | 8.0aA |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Odour | ||||
| Fresh | 10.0aA | 10.0aA | 10.0aA | 10.0aA |
| 2 | 10.0aA | 10.0aA | 10.0aA | 10.0aA |
| 4 | 8.0aB | 8.6aB | 8.6aB | 9.0aB |
| 6 | 7.0bC | 8.0aB | 9.0aB | 9.0aB |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Taste | ||||
| Fresh | 10.0aA | 10.0aA | 10.0aA | 10.0aA |
| 2 | 7.7bB | 7.7bB | 9.7aA | 9.7aA |
| 4 | 7.3bB | 7.7bB | 8.7aB | 9.0aB |
| 6 | 6.3bC | 6.7bC | 8.7aB | 9.0aB |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Oiling off | ||||
| Fresh | 10.0aA | 10.0aA | 10.0aA | 10.0aA |
| 2 | 8.3bB | 9.7aA | 10aA | 9.7aA |
| 4 | 8.3bB | 8.7bB | 9.7aA | 9.7aA |
| 6 | 7.3bC | 7.7bC | 8.7aB | 9.0aB |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Consistency | ||||
| Fresh | 10aA | 10aA | 10aA | 10aA |
| 2 | 9.3aB | 9.0aB | 9.7aA | 9.7aA |
| 4 | 7.7cC | 8.0cC | 9.0bB | 9.7aA |
| 6 | 6.0cD | 8.0bC | 8.0bC | 8.7aB |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Total scores | ||||
| Fresh | 48aA | 48aA | 48aA | 48aA |
| 2 | 42.6cB | 44bB | 47.4aA | 47.1aB |
| 4 | 36.3cC | 40.1bC | 44bB | 45.4aC |
| 6 | 30.6dD | 36.4cD | 41.4bC | 43.7Ad |
The maximum scores of consistency, odour and taste were given to cheese coated with film containing 0.9% CuO-NPs. Cheese coated with film contain 0.3% and 0.6% CuO-NPs ranked lower scores than cheese coated with film contain 0.9% CuO-NPs for all parameters, while 0.0% CuO-NPs coated cheese take the minimum scores when fresh and during storage period. The total scores of the cheese showed that the cheese coated with film contain 0.9% CuO-NPs was better acceptable than control and other coated processed cheese treatments.
3.6. Antimicrobial activity of CMC/PVA/CuO nanocomposites
Table 5 shows that copper nanoparticles exhibited significant antimicrobial action against different Gram-positive, Gram-negative bacteria and fungi. It also shows that the antimicrobial effect increased significantly (P > 0.05) with increasing the mass of fabricated in the tested bionanocomposites suspensions but these increases were nonlinear. It is showed from the inhibition zone that fabricated CuO-NPs possess effective bactericidal action. An insufficient mechanisms contributing to the antimicrobial belongings of the CuO-NPs have been suggested. CuO-NPs might adhere to the bacterial cell walls, because of their positive charge. CuO-NPs interrelate with amine and carboxyl groups existing on the surfaces of microbial cells. Thus, bacteria with higher density of these ionic groups on their cell surfaces have greater affinity for and are highly subject to CuO-NPs. In living microorganisms, copper is a structural constituent for numerous enzymes. Therefore, a comparatively high concentration of Cu2+ is necessary to produce toxic effects contrary to microbial pathogens. When managed in high dose, Cu2+ ions aid in the creation of ROS, which thereby interact with DNA and intercalate nucleic acid strands. The release of Cu2+ could also disturb the amino acid synthesis in many microbes.36| Strains | CMC/PVA/CuO bionanocomposites | |||
|---|---|---|---|---|
| 0.0, CuO-NPs% | 0.3, CuO-NPs% | 0.6, CuO-NPs% | 0.9, CuO-NPs% | |
| a Means with same superscript, lower case (effect of coating) in the same row are not significantly different (P < 0.05). | ||||
| Bacillus cereus | 9c | 17d | 20b | 23a |
| Staphylococcus aureus | 11d | 19c | 23b | 30a |
| Listeria monocytogenes | 12d | 21c | 26b | 32a |
| Escherichia coli | 13d | 16c | 20b | 25a |
| Yersinia enterocolitica | 14c | 23b | 25b | 31a |
| Salmonella typhimurium | 10c | 14b | 20a | 22a |
| Aspergillus niger | 10d | 14c | 23b | 30a |
| Aspergillus flavus | 14d | 20c | 26b | 35a |
It is clear that the main factor verbalizing antibacterial mechanism is credited to the presence of ROS creation via the CuO-NPs instead of soluble copper ions. Conversely, we cannot exclude certain degree of bacterial ability to solubilize the devoted CuO and so to excerpt harmful Cu2+ ions. Therefore, we suggest that collective actions of the strong adherence of the CuO nanoparticles to the cell membrane of bacterial, along with ROS generation on the surface of CuO nanoparticles, cause rise in cell permeability, leading to an unrestrained transport of CuO nanoparticles over the cytoplasmic membrane and ultimately to cells death. This approach of operation mainly rises in the case of small nanometric level of CuO-NPs as a result of their higher surface-to-volume ratio, resultant in the establishment of more ROS per unit weight, and to their higher probability to pass the cell membrane.
The fabricated CuO-NPs exhibited an average diameter of inhibition zone against pathogenic strains around 23–35 mm indicating biocidal action. The higher effect was observed against A. flavus, L. monocytogenes and S. aureus compared with the CuO-NPs free solution. The soluble ions released from the nanoparticles caused cytotoxicity by interacting either directly with the cellular membrane or intracellularly.37,38 Also, polymer/copper metal nanocomposites were developed using polyvinyl methyl ketone, poly-(vinyl chloride), and polyvinylidene fluoride as the polymer matrices. These composites were able to display bacteriostatic and antifungal activities depending on the polymeric matrix properties.39 Also, Harikumar & Aravind9 found that copper nanoparticles display antibacterial action against E. coil and gained zone was 12 mm.
3.7. Microbiological properties of processed cheese coated with fabricated copper bionanocomposites
Changes in microbiological population in different treatment of processed cheese coated with fabricated copper bionanocomposites are shown in Table 6 and Fig. 7. The total bacterial count of processed cheese from different treatments increased significantly (P < 0.05) during storage. However, the rate of the total bacterial growth was retarded with the inclusion of CuO-NPs in the coating film and the most pronounced effect was found with the use of 0.9% CuO-NPs containing film. The retarded bacterial growth cannot be attributed solely to the changes in the moisture content, but the decrease in oxygen permeation may play a role in this respect. No coliforms were detected throughout storage in processed cheese coated with bionanocomposites containing the different levels of CuO-NPs while few numbers were found in control after 2 month of storage.| Storage period (month) | CMC/PVA/CuO bionanocomposites | |||
|---|---|---|---|---|
| 0.0, CuO-NPs% | 0.3, CuO-NPs% | 0.6, CuO-NPs% | 0.9, CuO-NPs% | |
| a Means with same superscript, lower case (effect of coating) and upper case (effect of storage) are not significantly different (P < 0.05). | ||||
| Total bacterial count | ||||
| Fresh | 30.3 × 10aD | 30.3 × 10aD | 30.3 × 10aD | 30.3 × 10aA |
| 2 | 17 × 103aC | 7.6 × 103bC | 7.0 × 103bC | 23 × 10cA |
| 4 | 79.1 × 103aB | 42.6 × 103bB | 27 × 103cC | 87.6 × 10dA |
| 6 | 36 × 104aA | 81 × 103bA | 58.3 ×103cA | 92 × 10dA |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Coliforms | ||||
| Fresh | ND | ND | ND | Nil |
| 2 | 1.1 × 10 | ND | ND | ND |
| 4 | 1.6 × 10 | ND | ND | ND |
| 6 | 1.6 × 10 | ND | ND | ND |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Moulds & yeasts | ||||
| Fresh | ND | ND | ND | ND |
| 2 | 2 × 10 | ND | ND | ND |
| 4 | 5 × 10 | ND | ND | ND |
| 6 | 11 × 10 | 3.3 × 10 | 1.3 × 10 | 0.6 × 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Psychrotropics | ||||
| Fresh | ND | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 4 | 8 × 10 | 2.3 × 10 | 1.6 × 10 | ND |
| 6 | 15 × 10 | 7.6 × 10 | 4.3 × 10 | 2.0 × 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Spore formers | ||||
| Fresh | 2.3 × 10 | 2.3 × 10 | 2.3 × 10 | 2.3 × 10 |
| 2 | 4.6 × 10 | 3.6 × 10 | 3.3 × 10 | ND |
| 4 | 18.3××10 | 7.6 × 10 | 8.1 × 10 | 1.1 × 10 |
| 6 | 7.6 × 10 | 6.6 × 10 | 8.1 × 10 | 4.1 × 10 |
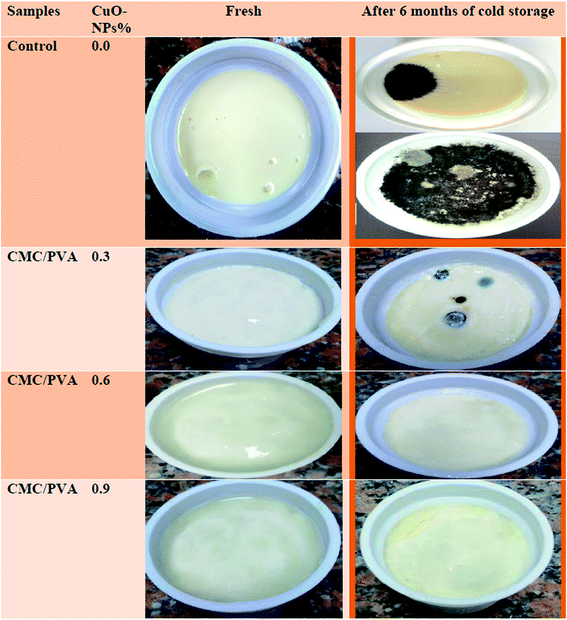 | ||
| Fig. 7 Influence of the prepared CMC/PVA/CuO bionanocomposites coating film on the quality of processed cheese after 6 months of cold storage. | ||
These results confirmed by Delgado, Quijada, Palma & Palza40 who found that the release rate of Cu2+ from polypropylene composites based on copper nanoparticles can kill E. coli bacteria. No moulds and yeasts were detected in processed cheese coated with bionanocomposites containing the different levels of CuO-NPs up to the 4th month of storage and very few numbers were detected after 6 moth of storage. Psychotropics were not detected in control and cheese from different treatments after 2 month of storage and very few were detected thereafter. Small numbers of spore formers were found in control cheese and cheese from the different treatments throughout storage period indicating that the bionanocomposites had no probable effect on the spore formers in processed cheese. The forgoing results indicate coating processed cheese with the bionanocomposites containing CuO-NPs improved the microbiological quality of processed cheese and prevented surface growth of moulds and yeast and the most pronounced effect was obtained with the use of 0.9% CuO-NPs containing bionanocomposites.
3.8. EDEX investigation of cheese after storage period
Simultaneously, EDEX analysis of the processed cheese after storage period (6 months) coated with CMC/PVA/CuO bionanocomposite loaded with different concentrations of CuO-NPs (0.3 and 9%). The cheese was ashing and the resultant powder subject to EDEX investigation to evaluate the migration of CuO-NPs to cheese throughout the storage period. The EDEX data (Fig. 8) pointed out that no migration of CuO-NPs happened from the bionanocomposite film to coat processed cheese. These results demonstrate the safety of utilize CuO-NPs in direct contact with foodstuffs for small storage time (28 days) without paying care to the migration of nanomaterials from the bionanocomposite coating film to the packaged food.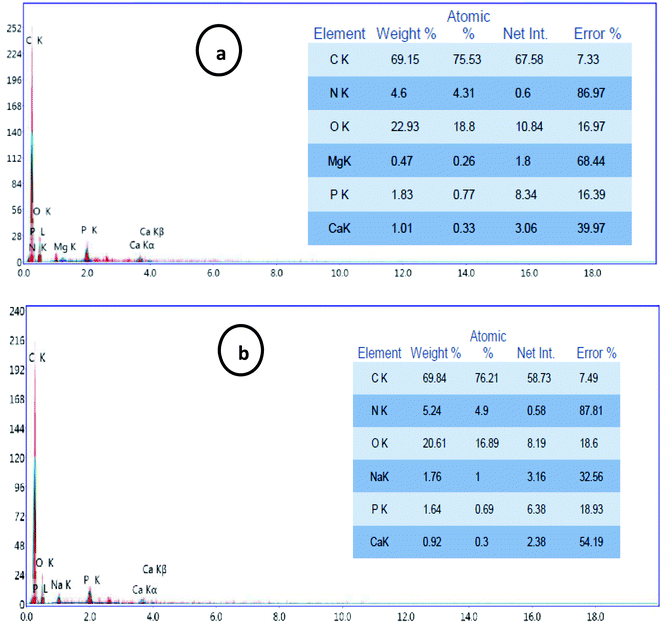 | ||
| Fig. 8 EDEX data of processed cheese coated using CMC/PVA/CuO bionanocomposites containing CuO-NPs with different concentrations, (a) 0.3%, and (b) 0.9% after 6 months of cold storage. | ||
4. Conclusions
In the present work CMC/PVA/CuO bionanocomposite comprising various concentrations of CuO-NPs have been established for processed cheese coating. Inclusion of CuO-NPs enhanced the water vapor permeability, mechanical properties, as well as the antimicrobial properties of the coating material. The coating material containing 0.9% CuO-NPs decreased moisture losses, improved the microbiological quality of cheese and prevented mould growth on the cheese surface during storage. Also, it gained high acceptability for sensory properties. These prepared bionanocomposites reduce growth of microbial contaminants and noticeably extend and enhance the shelf-life of processed cheese.Conflicts of interest
There are no conflicts to declare.References
- N. A. Al-Tayyar, A. M. Youssef and R. R. Al-Hindi, Edible coatings and antimicrobial nanoemulsions for enhancing shelf life and reducing foodborne pathogens of fruits and vegetables: a review, Sustainable Mater. Technol., 2020, 26, e00215 CrossRef.
- S. M. El-Sayed, H. S. El-Sayed, O. A. Ibrahim and A. M. Youssef, Rational design of chitosan/guar gum/zinc oxide bionanocomposites based on Roselle calyx extract for Ras cheese coating, Carbohydr. Polym., 2020, 116234, DOI:10.1016/j.carbpol.2020.116234.
- A. M. Youssef, S. M. El-Sayed, H. S. El-Sayed, H. H. Salama and A. Dufresne, Enhancement of Egyptian soft white cheese shelf life using a novel chitosan/carboxymethyl cellulose/zinc oxide bionanocomposite film, Carbohydr. Polym., 2016, 151, 9–19, DOI:10.1016/j.carbpol.2016.05.023.
- A. M. El Sayed, S. El-Gamal, W. M. Morsi and G. Mohammed, Effect of PVA and copper oxide nanoparticles on the structural, optical, and electrical properties of carboxymethyl cellulose films, J. Mater. Sci., 2015, 50, 4717–4728, DOI:10.1007/s10853-015-9023-z.
- A. M. Youssef and S. M. El-Sayed, Bionanocomposites materials for food packaging applications: Concepts and future outlook, Carbohydr. Polym., 2018, 193, 19–27, DOI:10.1016/j.carbpol.2018.03.088.
- A. M. Youssef, F. M. Assem, M. E. Abdel-Aziz, M. Elaaser, O. A. Ibrahim, M. Mahmoud and M. H. Abd El-Salam, Development of bionanocomposite materials and its use in coating of Ras cheese, Food Chem., 2019, 270, 467–475, DOI:10.1016/j.foodchem.2018.07.114.
- F. Ijaz, S. Shahid, S. A. Khan, W. Ahmad and S. Zaman, Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: Antimicrobial, antioxidant and photocatalytic dye degradation activities, Trop. J. Pharm. Res., 2017, 16(4), 743–753, DOI:10.4314/tjpr.v16i4.2.
- S. Shahzadi, N. Zafar and R. Sharif, Antibacterial activity of metallic nanoparticles, Bact. Pathog. Antibact. Control, 2018, 51 DOI:10.5772/intechopen.72526.
- P. S. Harikumar and A. Aravind, Antibacterial activity of copper nanoparticles and copper nanocomposites against Escherichia coli bacteria, Int. J. Sci., 2016, 5(2), 83–90, DOI:10.1016/j.tox.2013.07.012.
- G. Gautam and P. Mishra, Development and characterization of copper nanocomposite containing bilayer film for coconut oil packaging, J. Food Process. Preserv., 2017, 41(6), e13243, DOI:10.1111/jfpp.13243.
- H. Almasi, P. Jafarzadeh and L. Mehryar, Fabrication of novel nanohybrids by impregnation of CuO nanoparticles into bacterial cellulose and chitosan nanofibers: Characterization, antimicrobial and release properties, Carbohydr. Polym., 2018, 186, 273–281, DOI:10.1016/j.carbpol.2018.01.067.
- Y. Huang, L. Mei, X. Chen and Q. Wang, Recent developments in food packaging based on nanomaterials, Nanomaterials, 2018, 8(10), 830, DOI:10.3390/nano8100830.
- N. A. Al-Tayyar, A. M. Youssef and R. Al-hindi, Antimicrobial food packaging based on sustainable bio-based materials for reducing foodborne pathogens: A review, Food Chem., 2020, 310, 125915 CrossRef CAS.
- H. F. Youssef, M. E. El-Naggar, F. K. Fouda and A. M. Youssef, Antimicrobial packaging film based on biodegradable CMC/PVA-zeolite doped with noble metal cations, Food Packag. Shelf Life, 2019, 22, 100378 CrossRef.
- F. Beigmohammadi, S. H. Peighambardoust, J. Hesari, S. Azadmard-Damirchi, S. J. Peighambardoust and N. K. Khosrowshahi, Antibacterial properties of LDPE nanocomposite films in packaging of UF cheese, LWT–Food Sci. Technol., 2016, 65, 106–111, DOI:10.1016/j.lwt.2015.07.059.
- A. M. Youssef and F. M. Malhat, Selective removal of heavy metals from drinking water using titanium dioxide nanowire, Macromol. Symp., 2014, 337(1), 96–101 CrossRef CAS.
- A. M. Youssef, F. M. Malhat and A. F. A. Abd El-Hakim, Preparation and Utilization of Polystyrene Nanocomposites Based on TiO2 Nanowires, Polym.–Plast. Technol. Eng., 2013, 52(3), 228–235 CrossRef CAS.
- A. Z. Noah, M. A. El Semary, A. M. Youssef and M. A. El-Safty, Enhancement of yield point at high pressure high temperature wells by using polymer nanocomposites based on ZnO & CaCO3 nanoparticles, Egypt. J. Petrol., 2017, 26(1), 33–40 CrossRef.
- A. M. Youssef, F. M. Malhat, A. A. Abdel Hakim and I. Dekany, Synthesis and utilization of poly(methylmethacrylate) nanocomposites based on modified montmorillonite, Arabian J. Chem., 2017, 10(5), 631–642 CrossRef CAS.
- G. B. Lomate, B. Dandi and S. Mishra, Development of antimicrobial LDPE/Cu nanocomposite food packaging film for extended shelf life of peda, Food Packag. Shelf Life, 2018, 16, 211–219, DOI:10.1016/j.fpsl.2018.04.001.
- R. Kapoor and L. E. Metzger, Process cheese: Scientific and technological aspects—A review, Compr. Rev. Food Sci. Food Saf., 2008, 7(2), 194–214, DOI:10.1111/j.1541-4337.2008.00040.x.
- E. El Dakhakhny and N. Dabour, Processed Cheese: Basics and Possibility for the Development of Healthier Products, Alexandria J. Food Sci. Technol., 2016, 13(2), 45–62 CrossRef.
- AOAC, Official Methods of Analysis, Association of the Official Analytical Chemists, Arlington, VA, USA, 25th edn, 2002 Search PubMed.
- IDF, Rheological and fracture properties of cheeses, Bulletin No. 268, International Dairy Federation, Brussels, Belgium, 1991 Search PubMed.
- B. Dodiya, B. Amin, S. Kamlaben and P. Patel, Antibacterial activity and phytochemical screening of different parts of Moringa oleifera against selected gram positive and gram negative bacteria, J. Pharm. Chem. Biol. Sci., 2015, 3, 421–425 CAS.
- APHA, Standard Methods for Examination of Dairy Products, American Public Health Association, Washington, USA, 16th edn, 1994 Search PubMed.
- APHA, Compendium of Methods for the Microbiological Examination of Food, American Public Health Association, Washington D.C., USA, 3rd edn, 1992 Search PubMed.
- FDA, Bacteriological Analytical Manual, AOAC International, Arlington, VA, USA, 9th edn, 2002 Search PubMed.
- R. Lowry, VassartStats: web site for statistical computation http://faculty.vassar.edu/lowry.VassarStats.html, 2009 Search PubMed.
- V. S. de Souza, H. O. da Frota and E. A. Sanches, Polyaniline-CuO hybrid nanocomposite with enhanced electrical conductivity, J. Mol. Struct., 2018, 1153, 20–27, DOI:10.1016/j.molstruc.2017.09.084.
- C. Wolf, H. Angellier-Coussy, N. Gontard, F. Doghieri and V. Guillard, How the shape of fillers affects the barrier properties of polymer/non-porous particles nanocomposites: A review, J. Membr. Sci., 2018, 556, 393–418, DOI:10.1016/j.memsci.2018.03.085.
- G. Ren, D. Hu, E. W. Cheng, M. A. Vargas-Reus, P. Reip and R. P. Allaker, Characterization of copper oxide nanoparticles for antimicrobial applications, Int. J. Antimicrob. Agents, 2009, 33(6), 587–590 CrossRef CAS.
- A. M. Youssef, S. M. El-Sayed, H. S. El-Sayed, H. H. Salama, F. M. Assem and M. H. Abd El-Salam, Novel bionanocomposite materials used for packaging skimmed milk acid coagulated cheese (Karish), Int. J. Biol. Macromol., 2018, 115, 1002–1011, DOI:10.1016/j.ijbiomac.2018.04.165.
- Z. W. Abdullah and Y. Dong, Biodegradable and Water Resistant Poly (vinyl) Alcohol (PVA)/Starch (ST)/Glycerol (GL)/Halloysite Nanotube (HNT) Nanocomposite Films for Sustainable Food Packaging, Front. Mater., 2019, 6, 1–17 CrossRef.
- I. Korbag and S. Mohamed Saleh, Studies on mechanical and biodegradability properties of PVA/lignin blend films, Int. J. Environ. Stud., 2016, 73(1), 18–24 CrossRef CAS.
- A. J. Huh and Y. J. Kwon, “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS.
- C. Gunawan, W. Yang Teoh, C. P. Marquis and R. Amal, Cytotoxic origin of copper (II) oxide nanoparticles: comparative studies with micron-sized particles, leachate, and metal salts, ACS Nano, 2011, 5(9), 7214–7225, DOI:10.1021/nn2020248.
- H. L. Karlsson, Pontus Cronholm, Yolanda Hedberg, Malin Tornberg, Laura De Battice, Sofia Svedhem, and Inger Odnevall Wallinder. "Cell membrane damage and protein interaction induced by copper containing nanoparticles—Importance of the metal release process, Toxicology, 2013, 313(1), 59–69 CrossRef CAS.
- N. Cioffi, L. Torsi, N. Ditaranto, G. Tantillo, L. Ghibelli, L. Sabbatini, T. Bleve-Zacheo, M. D'Alessio, P. Giorgio Zambonin and E. Traversa, Copper nanoparticle/polymer composites with antifungal and bacteriostatic properties, Chem. Mater., 2005, 17(21), 5255–5262, DOI:10.1021/cm0505244.
- K. Delgado, R. Quijada, R. Palma and H. Palza, Polypropylene with embedded copper metal or copper oxide nanoparticles as a novel plastic antimicrobial agent, Lett. Appl. Microbiol., 2011, 53(1), 50–54, DOI:10.1111/j.1472-765X.2011.03069.x.
| This journal is © The Royal Society of Chemistry 2020 |