Open Access Article
Open Access ArticleSynthesis and stereocomplex formation of enantiomeric alternating copolymers with two types of chiral centers, poly(lactic acid-alt-2-hydroxybutanoic acid)s†
Hideto Tsuji *,
Kazuya Nakayama and
Yuki Arakawa
*,
Kazuya Nakayama and
Yuki Arakawa
Department of Applied Chemistry and Life Science, Graduate School of Engineering, Toyohashi University of Technology, Tempaku-cho, Toyohashi, Aichi 441-8580, Japan. E-mail: ht003@edu.tut.ac.jp
First published on 23rd October 2020
Abstract
Stereocomplex (SC) formation was reported for the first time for enantiomeric alternating copolymers consisting of repeating units with two types of chiral centers, poly(lactic acid-alt-2-hydroxybutanoic acid)s [P(LA-alt-2HB)s]. L,L-Configured poly(L-lactic acid-alt-L-2-hydroxybutanoic acid) [P(LLA-alt-L-2HB)] and D,D-configured poly(D-lactic acid-alt-D-2-hydroxybutanoic acid) [P(DLA-alt-D-2HB)] were amorphous. Blends of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) were crystallizable and showed typical SC-type wide-angle X-ray diffraction profiles similar to those reported for stereocomplexed blends of poly(L-lactic acid) and poly(D-lactic acid) homopolymers and of poly(L-2-hydroxybutanoic acid) and poly(D-2-hydroxybutanoic acid) homopolymers, and of L,L-configured poly(L-lactic acid-co-L-2-hydroxybutanoic acid) [P(LLA-co-L-2HB)] and D,D-configured poly(D-lactic acid-co-D-2-hydroxybutanoic acid) [P(DLA-co-D-2HB)] random copolymers. The melting temperature values and melting enthalpy values at 100% crystallinity for stereocomplexed solvent-evaporated and precipitated P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends were correspondingly 187.5 and 187.9 °C, and 98.1 and 91.8 J g−1. Enantiomeric polymer blending of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) can confer crystallizability by stereocomplexation and the biodegradable materials with a wide variety of physical properties and biodegradability are highly expected to be prepared by synthesis of alternating copolymers of various combinations of two types of chiral α-substituted 2-hydroxyalkanoic acid monomers and their SC crystallization.
1. Introduction
Poly(L-lactide) or poly(L-lactic acid) (PLLA), which is produced from renewable plant resources and biodegradable in the environment and the human body, has mechanical properties appropriate for a wide range of applications such as general, biomedical, pharmaceutical, and environmental applications.1–11 To diversify its application, stereocomplex (SC) formation of PLLA with its enantiomer, poly(D-lactide) or poly(D-lactic acid) (PDLA) and D-configured poly(lactide) or poly(lactic acid) (PLA)-like homopolymers and random copolymers has been widely inquired.12–23 Using the method of SC formation between PLLA and PDLA homopolymers and in stereo block copolymers, mechanical properties and resistance to thermal and hydrolytic degradation of PLA-based materials can be achieved.12–23 Furthermore, SC formation of PLA-based block copolymers with water-soluble polymers facilitates the preparation of aqueous biomedical gels.12–23 Various parameters affect SC formation. Such parameters include the types, concentrations and sequence of monomer units,13,14,18,24–29 thermal history,13,18,30,31 external forces,13,18,32–36 the types and concentrations of solvents for casting,18,37–39 the types and concentrations of third polymers and additives.18,40–47Recently, the symmetry between PLLA and PDLA or their alternating packing was found to be retained in the SC crystal lattice,48,49 whereas PDLA and PLLA chains were reported to be contained in the SC crystal lattice with a wide PLLA fractions from 30 to 70%.50,51 The elastic modulus of the PLLA/PDLA SC crystalline region (20 GPa) in the direction parallel to the c-axis was higher than that of PLLA or PDLA homo-crystalline regions (14 GPa),52 which should have been originated from the stronger interaction between PLLA and PDLA chains than that between PLLA chains or PDLA chains, as evidenced by atomic force microscopy.53 Also, utilizing hydrogen-bonding, stereo diblock-like PLAs with high stereocomplexationability were prepared from PLLA and PDLA having 2-ureido-4[1H]-pyrimidinone terminal,54 whereas stereo multiblock PLA was synthesized from DL-lactide at ambient temperature using achiral iron complexes.55 The SC formation of lactic acid (LA) and α-amino acid-based enantiomeric random copoly(esteramides), poly(lactic acid-co-alanine)s was reported.56 Utilizing the stereocomplexed PLA nanofibers, highly transparent self-reinforced PLA composites were prepared.57 PLA stereocomplexed materials having different memory shape effects were prepared by network formation or supramolecular formation and incorporation of elastomeric block.58,59
Similar to SC formation between PLLA and PDLA homopolymers, SC formation took place between the enantiomeric PLA-like homopolymers, poly(α-substituted 2-hydroxyalkanoic acid)s (Fig. 1), such as poly(2-hydroxybutanoic acid) [P(2HB)]18 and poly(2-hydroxy-3-methylbutanoic acid) [P(2H3MB)],18 and poly(mandelic acid).60 Hetero SC formation occurred between PLA and P(2HB), P(2HB) and P(2H3MB) with the different chemical structures and opposite configurations. Ternary SC formation took place in the blends of L- and D-configured P(2HB)s and L- or D-configured PLA,18 L- and D-configured P(2H3MB)s and L- or D-configured P(2HB),61 and D-configured PLA, L-configured P(2HB), and D-configured P(2H3MB) [or L-configured PLA, D-configured P(2HB), and L-configured P(2H3MB)],62 whereas quaternary SC occurred in the blend of L- and D-configured P(2HB)s and L- and D-configured P(2H3MB)s.18
Also, SC formation took place in enantiomeric random copolymer blends of poly(L-lactic acid-co-L-2-hydroxybutanoic acid) [P(LLA-co-L-2HB)] (56/44) and poly(D-lactic acid-co-D-2-hydroxybutanoic acid) [P(DLA-co-D-2HB)] (52/48)63 and of poly(L-lactic acid-co-L-2-hydroxy-3-methylbutanoic acid) [P(LLA-co-L-2H3MB)] (47/53) and poly(D-lactic acid-co-D-2-hydroxy-3-methylbutanoic acid) [P(DLA-co-D-2H3MB)] (47/53),64 and the staggered random copolymers, L-configured P(LLA-co-L-2HB) (50/50) and D-configured poly(D-2-hydroxybutanoic acid-co-D-2-hydroxy-3-methylbutanoic acid) (50/50).65 It should be noted that in these cases, all types of monomer units were confirmed to be packed in the SC crystalline lattice by wide-angle X-ray diffractometry (WAXD). This is in marked contrast with normal crystallization of random copolymers, wherein minor monomer units are excluded from and not included in the crystalline regions.
On the other hand, SC formation took place in enantiomeric alternating copolymer blends of poly(L-lactic acid-alt-6-hydroxycaproic acid) and poly(D-lactic acid-alt-6-hydroxycaproic acid)66 and of poly(L-lactic acid-alt-glycolic acid) [P(LLA-alt-GA)] and poly(D-lactic acid-alt-glycolic acid) [P(DLA-alt-GA)].67 These enantiomeric alternating copolymers which formed SC had the repeating or monomer units of lactic acid-6-hydroxycaproic acid or 6-hydroxycaproic acid–lactic acid and of lactic acid–glycolic acid or glycolic acid–lactic acid. In these repeating units, only one type of chiral center from lactic acid units existed. On the other hand, Tabata and Abe synthesized alternating copolymers composed of repeating or monomer units of two types of chiral centers, D,D-configured poly(D-lactic acid-alt-D-3-hydroxybutanoic acid) and L,D-configured poly(L-lactic acid-alt-D-3-hydroxybutanoic acid) and observed the melting temperature (Tm) difference between the two polymers (233 and 83 °C).68
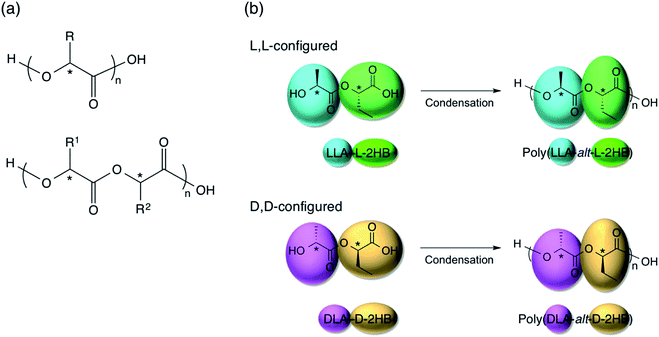
In the present study, we synthesized enantiomeric alternating copolymers of poly(L-lactic acid-alt-L-2-hydroxybutanoic acid) [P(LLA-alt-L-2HB)] and poly(D-lactic acid-alt-D-2-hydroxybutanoic acid) [P(DLA-alt-D-2HB)] (Fig. 1) and report SC formation between alternating copolymer blends of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB). These alternating copolymers are composed of repeating unit of lactic acid-2-hydroxybutanoic acid or 2-hydroxybutanoic acid–lactic acid, which contain two types of chiral centers from lactic acid and 2-hydroxybutanoic acid units (Fig. 1). So, this is the first report for SC formation between enantiomeric alternating copolymers consisting of repeating units with two types of chiral centers. To investigate the physical properties, crystalline species, and crystallization behavior of unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB), and their blends, WAXD, differential scanning calorimetry (DSC), and Fourier transform infrared (FTIR) were carried out.
2. Experimental section
Materials and synthesis (Scheme 1),69–72 sample preparation, physical measurements, and observation62,64,67,73–75 were performed according to the literatures. The details of the experiments are stated in the ESI (Section S1).† The weight-average molecular weight (Mw), number average molecular weight (Mn), polydispersity index, and specific optical rotation in chloroform at 25 °C and 589 nm ([α]25589) of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) synthesized and used in the present study are 3.00 × 103, 3.08 × 103, g mol−1, 1.53 × 103 and 1.54 × 103 g mol−1, 1.96, 2.00, and −122.3, 120.3 deg dm−1 g−1 cm3, respectively.3. Results and discussion
3.1. Synthesis
In order to corroborate the alternating monomer unit sequences of the synthesized copolymers, 1H and 13C NMR measurements were performed (Fig. 2, S1(a), and S2†). All 1H and 13C NMR spectra for P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) are shown in ESI as Fig. S1(a) and S2,† respectively, together with those reported for P(L-2HB), P(LLA-co-L-2HB) random copolymer, and PLLA75 [Fig. S1(b) and S3†]. The quartet and doublet peaks at 5.2 and 1.6 ppm observed for the 1H NMR spectra of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) are attributed to correspondingly methine (b) and methyl (a) protons of lactic acid (LA) units, whereas the quartet, multiplet, and triplet peaks at 5.0, 2.0, and 1.0 ppm are ascribed to correspondingly methine (e), methylene (d), and methyl (c) protons of 2-hydroxybutanoic acid (2HB) units [Fig. S1(a)†], in agreement with the 1H NMR spectrum reported for the random copolymer, P(LLA-co-L-2HB).75,76 However, methine peaks for LA and 2HB units [(b) and (e)] of P(LLA-alt-L-2HB) are sharp due to their regular alternating monomer sequences, in contrast with broad ones of P(LLA-co-L-2HB) random copolymer.The singlet peaks seen at 169.0, 69.0, and 16.8 ppm for 13C NMR spectra of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) are attributed to carbonyl, methine, and methyl carbons of LA units, whereas those at 169.9, 73.6, 24.4, and 9.3 ppm are ascribed to carbonyl, methine, methylene, and methyl carbons of 2HB units [Fig. 2(b) and S2†].75,77 In the case of P(LLA-co-L-2HB) random copolymer, triplet peaks are seen for the methylene carbon of 2HB units (24.2 ppm) and the methine carbon of LA units (16.8 ppm), whereas singlet peaks are observed for P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) [Fig. 2(b)]. Also, in the cases of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB), singlet peaks are observed for the carbonyl carbons of 2HB and LA units, whereas in the case of P(LLA-co-L-2HB) random copolymer, dual peaks are seen for carbonyl carbons of 2HB and LA units [Fig. S2(a) and S3(a)†]. The sharp 1H NMR peaks and singlet 13C NMR peaks of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) indicate the fixed circumstances of LA and 2HB units in alternating sequences but not in the various circumstances in random sequences, confirming the successful synthesis of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) alternating copolymers.
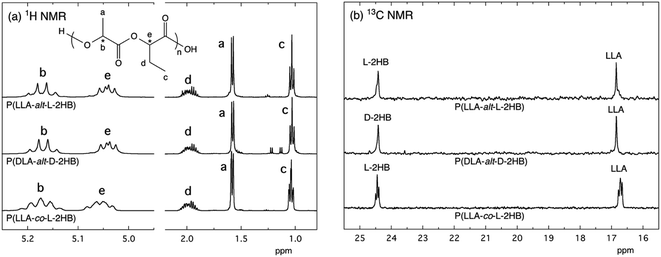 | ||
| Fig. 2 (a) 1H NMR spectra and (b) 13C NMR spectra of methylene carbon of L-2HB units and methyl carbon of LLA units for P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) alternating copolymers and P(LLA-co-L-2HB) random copolymer in CDCl3. The spectra for P(LLA-co-L-2HB) random copolymer were reproduced from ref. 75 with permission from Elsevier. | ||
3.2. Wide-angle X-ray diffractometry
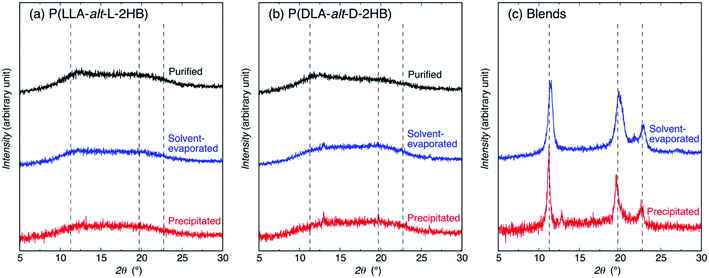
For estimating the crystalline species and crystallinity (Xc) values of the samples, WAXD measurements were carried out [Fig. 3(a–c)]. The very weak crystalline peaks seen at 12.8, 19.6, 22.9, and 25.9° for solvent evaporated and precipitated P(DLA-alt-D-2HB) and precipitated P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blend are ascribed to those of N,N′-diisopropylurea (DIU) formed during polymerization and remaining in the samples (Fig. S4†). The unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) showed broad diffraction profiles, irrespective of sample preparation methods, indicating that the unblended polymer samples were amorphous. This result is in contrast with the results of P(LLA-co-L-2HB) (56/44) and P(DLA-co-D-2HB) (52/48) random copolymers63,75 and P(LLA-alt-GA) and P(DLA-alt-GA) alternating copolymers,67 which were homocrystallizable. The noncrystallizability of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) may be due to their lower Mw values (Mw = 3.0 × 103 and 3.1 × 103, g mol−1, respectively) compared to those of the P(LLA-co-L-2HB) (56/44) and P(DLA-co-D-2HB) (52/48) random copolymers (Mw = 1.4 × 104 and 1.6 × 104, g mol−1, respectively) and P(LLA-alt-GA) and P(DLA-alt-GA) alternating copolymers (Mw = 4.8 × 103 and 5.9 × 103, g mol−1, respectively).On the other hand, both solvent-evaporated and precipitated P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends showed the crystalline peaks at 11.4° (or 11.2°), 19.8° (or 19.5°), 22.9° (or 22.6°). Here, the data outside and inside parentheses are for the solvent-evaporated and precipitated samples, respectively. The diffraction patterns of P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends are similar to those of PLLA/PDLA homopolymer SC,78 poly(L-2-hydroxybutanoic acid) [P(L-2HB)]/poly(D-2-hydroxybutanoic acid) [P(D-2HB)] homopolymer SC,79 and P(LLA-co-L-2HB) (56/44)/P(DLA-co-D-2HB) (52/48) random copolymer SC,63 but completely different from that of P(LLA-alt-GA)/P(DLA-alt-GA) alternating copolymer SC with the crystalline peaks at 17.3°, 18.5°, 21.3° (ref. 67) [Fig. 4]. The observed 2θ values of P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends are similar to those of P(LLA-co-L-2HB) (56/44)/P(DLA-co-D-2HB) (52/48) random copolymer SC [11.5° (or 11.3°), 20.0° (or 19.6° and 20.1°), 22.9° (or 22.7°), the data outside and inside parentheses are for the solvent-evaporated and melt-crystallized samples, respectively],63 but lower and higher than those for the melt-crystallized PLLA/PDLA homopolymer SC (11.9°, 20.7°, 24.0°)78 and P(L-2HB)/P(D-2HB) homopolymer SC (10.7°, 18.6° and 19.3°, 21.5°),79 respectively. The interplanar distance (d) and crystalline diffraction angle (2θ) values of samples were obtained from Fig. 4, except for P(LLA-alt-GA)/P(DLA-alt-GA) alternating copolymer SC are tabulated in Table S1,† together with those for poly(L-2-hydroxy-3-methylbutanoic acid) [P(L-2H3MB)]/poly(D-2-hydroxy-3-methylbutanoic acid) [P(D-2H3MB)] homopolymer SC.80 The diffraction peak patterns indicate SC formation between P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB), whereas the 2θ and d values exhibit their SC crystalline lattice sizes are very similar to those of P(LLA-co-L-2HB) (56/44)/P(DLA-co-D-2HB) (52/48) random copolymer SC and between those of PLLA/PDLA homopolymer SC and P(L-2HB)/P(D-2HB) homopolymer SC. Very interestingly, comparison between the WAXD profiles of P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) SC and P(LLA-alt-GA)/P(DLA-alt-GA) SC [Fig. 4] reveals that their SC crystal lattice types of enantiomeric LA-based alternating copolymer are completely different.
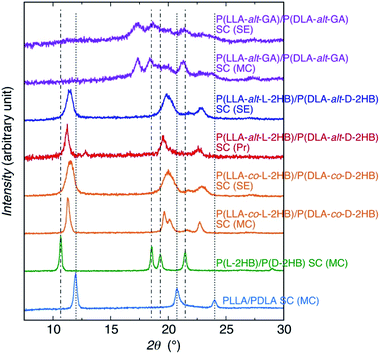 | ||
| Fig. 4 WAXD profiles of solvent-evaporated (SE) and precipitated (Pr) P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) SC, together with solvent-evaporated and melt-crystallized (MC) P(LLA-alt-GA)/P(DLA-alt-GA) alternating copolymer SC,67 solvent-evaporated and melt-crystallized P(LLA-co-L-2HB)/P(DLA-co-D-2HB) random copolymer SC,63 melt-crystalized P(L-2HB)/P(D-2HB) homopolymer SC,79 and melt-crystalized PLLA/PDLA homopolymer SC.78 The main crystalline diffraction angles of SC crystallites for P(L-2HB)/P(D-2HB) homopolymer SC and PLLA/PDLA homopolymer SC are shown with dash-dotted lines and dotted lines, respectively. The data for P(LLA-alt-GA)/P(DLA-alt-GA) alternating copolymer SC, P(LLA-co-L-2HB)/P(DLA-co-D-2HB) random copolymer SC, P(L-2HB)/P(D-2HB) homopolymer SC, and PLLA/PDLA homopolymer SC were adapted from ref. 67, 63, 79, and 78 with permission from American Chemical Society, The Royal Society of Chemistry, American Chemical Society, and Wiley-VCH, respectively. | ||
The crystallinity (Xc) values of the samples were obtained from the WAXD profiles in Fig. 3(a–c) and summarized in Table S2.† The Xc values of unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) (both nil) are much lower than the purified and melt-crystallized unblended P(LLA-co-L-2HB) random copolymer (56/44) (43.7 and 36.5% for samples, respectively)75 and purified and solvent-evaporated unblended P(LLA-alt-GA) and P(DLA-alt-GA) alternating copolymers [8.1% and nil for P(LLA-alt-GA) and 7.2 and 4.8% for P(DLA-alt-GA)]. On the other hand, the solvent-evaporated and precipitated P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends are 66.7 and 60.8%, respectively, which are lower than those reported for the solvent-evaporated and melt-crystallized P(LLA-co-L-2HB) (56/44)/P(DLA-co-D-2HB) (52/48) random copolymer blends (82.5 and 77.7%, respectively),63 but much higher than those reported for solvent-evaporated and melt-crystallized P(LLA-alt-GA)/P(DLA-alt-GA) alternating copolymer blends (20.8 and 29.2%, respectively). The lower Xc values of unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) compared to those of unblended P(LLA-co-L-2HB) random copolymer (56/44) and unblended P(LLA-alt-GA) and P(DLA-alt-GA) alternating copolymers and the lower Xc values of P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends compared to those of P(LLA-co-L-2HB) (56/44)/P(DLA-co-D-2HB) (52/48) random copolymer blends can be ascribed to the lower molecular weights of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB). Interestingly, despite the lower Xc values of unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) compared to those of unblended P(LLA-alt-GA) and P(DLA-alt-GA) alternating copolymers, the higher Xc values of P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends compared to those of P(LLA-alt-GA)/P(DLA-alt-GA) alternating copolymer blends exhibit that dual chiral centers per a repeating unit in P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) enhanced the crystallizability of the enantiomeric polymers by stereocomplexation compared to one chiral center per a repeating unit in P(LLA-alt-GA) and P(DLA-alt-GA).
3.3. Differential scanning calorimetry
For estimating crystallization and thermal properties of the samples, DSC measurements were conducted (Fig. 5). The glass transition temperature (Tg), Tm, and melting enthalpy (ΔHm) were estimated from the DSC thermograms in Fig. 5 and are summarized in Table S2.† The unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) show only glass transition peak at around 15.5–40.4 °C without melting peak, regardless of preparation methods, confirming the WAXD result that unblended P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) were amorphous. On the other hand, the solvent-evaporated and precipitated P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends showed glass transition and melting peaks correspondingly at 33.7, 36.3 °C and 187.5, 187.9 °C. The Tm values for P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) (Mw = 3.0 × 103 and 3.1 × 103, g mol−1, respectively) blends are higher than those for solvent-evaporated and melt-crystallized (Tc = 70 °C) P(L-2HB)/P(D-2HB) (Mw = 1.8 × 103 and 3.3 × 103, g mol−1, respectively) homopolymer blends (Tm = 173.0 and 172.1 °C, respectively)81 but lower than those for melt-crystallized (Tc = 130 °C) PLLA/PDLA (Mw = 4.0 × 103 and 5.4 × 103, g mol−1, respectively) homopolymer blends (Tm = 197.5 °C),78 and solvent-evaporated and melt-crystallized (Tc = 160 °C) P(LLA-co-L-2HB) (56/44)/P(DLA-co-D-2HB) (52/48) (Mw = 1.4 × 104 and 1.6 × 104, g mol−1, respectively) random copolymer blends (203.6 and 198.4 °C),63 and solvent evaporated and melt-crystallized (Tc = 100 °C) P(LLA-alt-GA)/P(DLA-alt-GA) (Mw = 4.8 × 103 and 5.9 × 103, g mol−1, respectively) blends (187.8 and 187.6 °C).67 The lower Tm values of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) can be attributable to the low molecular weights compared to those of PLLA and PDLA, P(LLA-co-L-2HB) (56/44) and P(DLA-co-D-2HB) (52/48), and P(LLA-alt-GA) and P(DLA-alt-GA).Using the ΔHm and Xc of solvent-evaporated (65.4 J g−1 and 66.7%) and precipitated (55.8 J g−1 and 60.8%) P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends, ΔHm values at Xc = 100%, i.e., ΔH0m values were estimated to be 98.1 and 91.8 J g−1, respectively. Using the ΔHm and Xc of solvent-evaporated (83.7 J g−1 and 82.5%) and precipitated (74.3 J g−1 and 77.7%) P(LLA-co-L-2HB)/P(DLA-co-D-2HB) random copolymer blends,63 ΔH0m values were evaluated to be 101.5 and 95.6 J g−1, respectively. Also, using the ΔHm and Xc of solvent-evaporated (53.4 J g−1 and 20.8%) and melt-crystallized (67.7 J g−1 and 29.2%) P(LLA-alt-GA)/P(DLA-alt-GA) blends, ΔH0m values were estimated to be 256.7 and 231.8 J g−1,67 respectively, which are much higher than ΔH0m value of poly(glycolic acid) (206 J g−1)82 and PLLA/PDLA homopolymer SC (142 (ref. 83) and 146 (ref. 77) J g−1). Surprisingly, the ΔH0m values of P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends are similar to those of P(LLA-co-L-2HB)/P(DLA-co-D-2HB) random copolymer blends, despite the fact that P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) have higher sequential regularity compared to that of P(LLA-co-L-2HB) and P(DLA-co-D-2HB). This finding can also be explained by the fact that the molecular weights of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) are lower than those of P(LLA-co-L-2HB) and P(DLA-co-D-2HB) random copolymers. The lower molecular weights or increased density of chain terminals of the former should have increased the defects in the crystalline regions, resulting in lower ΔHm and ΔH0m values.
Finally, the present study reveals that even though alternating copolymers with two types of chiral centers per a repeating unit is amorphous, their enantiomeric polymer blending can impose crystallizability by stereocomplexation and it is strongly expected that the biodegradable materials with a wide range of physical properties and biodegradability can be prepared by various combinations of two types of chiral α-substituted 2-hydroxyalkanoic acid monomers. For this purpose, accumulating the information of physical properties and biodegradability of SCs from enantiomeric alternating α-substituted hydroxyalkanoic acid-based polymers with various combinations of chiral monomers is required.
4. Conclusions
SC formation was reported for the first time for enantiomeric alternating copolymers with two types of chiral centers per a repeating unit, P(LA-alt-2HB). L,L-Configured P(LLA-alt-L-2HB) and D,D-configured P(DLA-alt-D-2HB) were amorphous polymers. P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends were crystallizable and showed typical SC-type wide-angle X-ray diffraction profiles similar to those reported for PLLA/PDLA homopolymer SC, P(L-2HB)/P(D-2HB) homopolymer SC, and P(LLA-co-L-2HB)/P(DLA-co-D-2HB) random copolymer SC. The Tm and ΔH0m values for stereocomplexed solvent-evaporated and precipitated P(LLA-alt-L-2HB)/P(DLA-alt-D-2HB) blends were correspondingly 187.5 and 187.9 °C, and 98.1 and 91.8 J g−1. Enantiomeric polymer blending of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) can grant crystallizability by stereocomplexation and the biodegradable materials with a wide variety of physical properties and biodegradability are highly expected to be prepared by synthesis of alternating copolymers of various combinations of two types of chiral α-substituted 2-hydroxyalkanoic acid monomers and their SC crystallization.Abbreviations
| 2HB | 2-Hydroxybutanoic acid |
| ΔHm | Melting enthalpy |
| ΔH0m | ΔHm at Xc = 100% |
| DIU | N,N′-Diisopropylurea |
| DSC | Differential scanning calorimetry |
| Mn | Number average molecular weight |
| MC | Melt-crystallized |
| Mw | Weight-average molecular weight |
| LA | Lactic acid |
| P(2H3MB) | Poly(2-hydroxy-3-methylbutanoic acid) |
| P(2HB) | Poly(2-hydroxybutanoic acid) |
| P(D-2HB) | Poly(D-2-hydroxybutanoic acid) |
| P(D-2H3MB) | Poly(D-2-hydroxy-3-methylbutanoic acid) |
| PDLA | Poly(D-lactide) or poly(D-lactic acid) |
| P(DLA-alt-D-2HB) | Poly(D-lactic acid-alt-D-2-hydroxybutanoic acid) |
| P(DLA-alt-GA) | Poly(D-lactic acid-alt-glycolic acid) |
| P(DLA-co-D-2H3MB) | Poly(D-lactic acid-co-D-2-hydroxy-3-methylbutanoic acid) |
| P(DLA-co-D-2HB) | Poly(D-lactic acid-co-D-2-hydroxybutanoic acid) |
| P(L-2HB) | Poly(L-2-hydroxybutanoic acid) |
| P(L-2H3MB) | Poly(L-2-hydroxy-3-methylbutanoic acid) |
| PLA | Poly(lactide) or poly(lactic acid) |
| P(LA-alt-2HB) | Poly(lactic acid-alt-2-hydroxybutanoic acid) |
| PLLA | Poly(L-lactide) or poly(L-lactic acid) |
| P(LLA-alt-GA) | Poly(L-lactic acid-alt-glycolic acid) |
| P(LLA-alt-L-2HB) | Poly(L-lactic acid-alt-L-2-hydroxybutanoic acid) |
| P(LLA-co-L-2H3MB) | Poly(L-lactic acid-co-L-2-hydroxy-3-methylbutanoic acid) |
| P(LLA-co-L-2HB) | Poly(L-lactic acid-co-L-2-hydroxybutanoic acid) |
| Pr | Precipitated |
| SC | Stereocomplex |
| SE | Solvent-evaporated |
| [α] | Specific optical rotation |
| Tg | Glass transition temperature |
| Tm | Melting temperature |
| Xc | Crystallinity |
| WAXD | Wide-angle X-ray diffractometry |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the research grants from The Hibi Science Foundation and JSPS (KAKENHI, Grant Number 16K05912).References
- M. Vert, J. Feijen, A.-C. Albertsson, G. Scott and E. Chiellini, Biodegradable Polymers and Plastics, Royal Society of Chemistry, Cambridge, 1992 Search PubMed.
- D. P. Mobley, Plastics from Microbes, Hanser Publishers, New York, 1994 Search PubMed.
- M. Vert, G. Schwarch and J. Coudane, J. Macromol. Sci., Part A: Pure Appl. Chem., 1995, 32, 787–796 CrossRef.
- A. J. Domb, J. Kost and D. M. Wieseman, Handbook of Biodegradable Polymers (Drug Targeting and Delivery), Harwood Academic Publishers, Amsterdam (The Netherlands), 1997, vol. 7 Search PubMed.
- D. L. Kaplan, Biopolymers from Renewable Resources, Springer, Berlin (Germany), 1998 Search PubMed.
- D. A. Garlotta, J. Polym. Environ., 2001, 9, 63–84 CrossRef CAS.
- A. Södergård and M. Stolt, Prog. Polym. Sci., 2002, 27, 1123–1163 CrossRef.
- A.-C. Albertsson, Degradable Aliphatic Polyesters (Advances in Polymer Science), Springer, Berlin (Germany), 2002, vol. 157 Search PubMed.
- Y. Doi and A. Steinbüchel, Polyesters I, II, III (Biopolymers, Vol. 3a, 3b, 4), Wiley-VCH, Weinheim (Germany), 2002 Search PubMed.
- R. Auras, L.-T. Lim, S. E. M. Selke and H. Tsuji, Poly(lactic acid): synthesis, structures, properties, processing, and applications, (Wiley Series on Polymer Engineering and Technology), John Wiley & Sons, Inc., NJ, 2010 Search PubMed.
- H. Tsuji, in Poly(Lactic Acid). Bio-Based Plastics: Materials and Applications, ed. S. Kabasi, Wiley & Sons, Ltd, Chichester (UK), 2014, ch. 8, pp. 171–239 Search PubMed.
- J. Slager and A. J. Domb, Adv. Drug Delivery Rev., 2003, 55, 549–583 CrossRef CAS.
- H. Tsuji, Macromol. Biosci., 2005, 5, 569–597 CrossRef CAS.
- K. Fukushima and Y. Kimura, Polym. Int., 2006, 55, 626–642 CrossRef CAS.
- P. Pan and Y. Inoue, Prog. Polym. Sci., 2009, 34, 605–640 CrossRef CAS.
- M. Saravanan and A. J. Domb, Eur. J. Nanomed., 2013, 5, 81–86 CAS.
- Y. Jing, C. Quan, Q. Jiang, C. Zhang and Z. Chao, Polym. Rev., 2016, 56, 262–286 CrossRef CAS.
- H. Tsuji, Adv. Drug Delivery Rev., 2016, 107, 97–135 CrossRef CAS.
- B. H. Tan, J. K. Muiruri, Z. Li and C. He, ACS Sustainable Chem. Eng., 2016, 4, 5370–5391 CrossRef CAS.
- Z. Li, B. H. Tan, T. Lin and C. He, Prog. Polym. Sci., 2016, 62, 22–72 CrossRef CAS.
- H. Bai, S. Deng, D. Bai, Q. Zhang and Q. Fu, Macromol. Rapid Commun., 2017, 38, 1700454 CrossRef.
- Q. Xie, C. Yu and P. Pan, in Crystallization in Multiphase Polymer Systems, ed. S. Thomas, M. Arif P., E. Bhoje Gowd and N. Kalarikkal, Elsevier Inc., Amsterdam (Netherlands), 2018, ch. 17, pp. 535–573 Search PubMed.
- D. Bandelli, J. Alex, C. Weber and U. S. Schubert, Macromol. Rapid Commun., 2020, 41, 1900560 CrossRef CAS.
- N. Yui, P. J. Dijkstra and J. Feijen, Macromol. Chem. Phys., 1990, 191, 481–488 CrossRef CAS.
- K. Fukushima, Y. Furuhashi, K. Sogo, S. Miura and Y. Kimura, Macromol. Biosci., 2005, 5, 21–29 CrossRef CAS.
- M. H. Rahaman and H. Tsuji, Macromol. React. Eng., 2012, 6, 446–457 CrossRef CAS.
- K. Masutani, C. W. Lee and Y. Kimura, Polym. J., 2013, 45, 427–435 CrossRef CAS.
- L. Han, G. Shan, Y. Bao and P. Pan, J. Phys. Chem. B, 2015, 119, 14270–14279 CrossRef CAS.
- L. Han, Q. Xie, J. Bao, G. Shan, Y. Bao and P. Pan, Polym. Chem., 2017, 8, 1006–1016 RSC.
- J. Zhang, K. Tashiro, H. Tsuji and A. J. Domb, Macromolecules, 2007, 40, 1049–1054 CrossRef CAS.
- H. Tsuji and Y. Ikada, Macromol. Chem. Phys., 1996, 197, 3483–3499 CrossRef CAS.
- H. Tsuji, S.-H. Hyon and Y. Ikada, Macromolecules, 1991, 24, 5657–5662 CrossRef CAS.
- H. Tsuji, M. Nakano, M. Hashimoto, K. Takashima, S. Katsura and A. Mizuno, Biomacromolecules, 2006, 7, 3316–3320 CrossRef CAS.
- K. Hemmi, G. Matsuba, H. Tsuji, T. Kawai, T. Kanaya, K. Toyohara, A. Oda and K. Endou, J. Appl. Crystallogr., 2014, 47, 14–21 CrossRef CAS.
- Y. Duan, J. Liu, H. Sato, J. Zhang, H. Tsuji, Y. Ozaki and S. Yan, Biomacromolecules, 2006, 7, 2728–2735 CrossRef CAS.
- T. Serizawa, H. Yamashita, T. Fujiwara, Y. Kimura and M. Akashi, Macromolecules, 2001, 34, 1996–2001 CrossRef CAS.
- H. Tsuji and S. Yamamoto, Macromol. Mater. Eng., 2011, 296, 583–589 CrossRef CAS.
- Y. Furuhashi and N. Yoshie, Polym. Int., 2012, 61, 301–306 CrossRef CAS.
- K. Fukushima, Y.-H. Chang and Y. Kimura, Macromol. Biosci., 2007, 7, 829–835 CrossRef CAS.
- P. Purnama and S. H. Kim, Macromolecules, 2010, 43, 1137–1142 CrossRef CAS.
- C. Samuel, J. Cayuela, I. Barakat, A. J. Müller, J.-M. Raquez and P. Dubois, ACS Appl. Mater. Interfaces, 2013, 5, 11797–11807 CrossRef CAS.
- R.-Y. Bao, W. Yang, X.-F. Wei, B.-H. Xie and M.-B. Yang, ACS Sustainable Chem. Eng., 2014, 2, 2301–2309 CrossRef CAS.
- J. Zhu, B. Na, R. Lv and C. Li, Polym. Int., 2014, 63, 1101–1104 CrossRef CAS.
- L. Gardella, D. Furfaro, M. Galimberti and O. Monticelli, Green Chem., 2015, 17, 4082–4088 RSC.
- H. Xu, D. Wu, X. Yang, L. Xie and M. Hakkarainen, Macromolecules, 2015, 48, 2127–2137 CrossRef CAS.
- L. Han, P. Pan, G. Shan and Y. Bao, Polymer, 2015, 63, 144–153 CrossRef CAS.
- J. Bao, X. Xue, K. Li, X. Chang and Q. Xie, J. Phys. Chem. B, 2017, 121, 6934–6943 CrossRef CAS.
- F. Zhang, H.-W. Wang, K. Tominaga, M. Hayashi, S. Lee and T. Nishino, J. Phys. Chem. Lett., 2016, 7, 4671–4676 CrossRef CAS.
- W. Zhou, K. Wang, S. Wang, S. Yuan, W. Chen, T. Konishi and T. Miyoshi, ACS Macro Lett., 2018, 7, 667–671 CrossRef CAS.
- K. Tashiro, H. Wang, N. Kouno, J. Koshobu and K. Watanabe, Macromolecules, 2017, 50, 8066–8071 CrossRef CAS.
- K. Tashiro, N. Kouno, H. Wang and H. Tsuji, Macromolecules, 2017, 50, 8048–8065 CrossRef CAS.
- S. Lee, M. Kimoto, M. Tanaka, H. Tsuji and T. Nishino, Polymer, 2018, 138, 124–131 CrossRef CAS.
- S. Fujishiro, D. Minamino, I. Obataya, N. Saitoh, Y. Hosokawa and H. Ajiro, Chem. Lett., 2018, 47, 82–84 CrossRef CAS.
- J. Bao, X. Chang, G. Shan, Y. Bao and P. Pan, Polym. Chem., 2016, 7, 4891–4900 RSC.
- P. Marin, M. J.-L. Tschan, F. Isnard, C. Robert, P. Haquette, X. Trivelli, L.-M. Chamoreau, V. Guérineau, I. del Rosal, M. Laurent, V. Venditto and C. M. Thomas, Angew. Chem., Int. Ed., 2019, 58, 12585–12589 CrossRef CAS.
- H. Tsuji, S. Sato, N. Masaki, Y. Arakawa, A. Kuzuya and Y. Ohya, Polym. Chem., 2018, 9, 565–575 RSC.
- N. Kurokawa and A. Hotta, Polymer, 2018, 153, 214–222 CrossRef CAS.
- J. Zhou, H. Cao, R. Chang, G. Shan, Y. Bao and P. Pan, ACS Macro Lett., 2018, 7, 233–7238 CrossRef CAS.
- R. Chang, G. Shan, Y. Bao and P. Pan, Macromolecules, 2015, 48, 7872–7881 CrossRef CAS.
- M. Li, Y. Tao, J. Tang, Y. Wang, X. Zhang, Y. Tao and X. Wang, J. Am. Chem. Soc., 2019, 141, 281–289 CrossRef CAS.
- H. Tsuji, N. Masaki, Y. Arakawa, K. Iguchi and T. Sobue, Cryst. Growth Des., 2018, 18, 521–530 CrossRef CAS.
- H. Tsuji, S. Noda, T. Kimura, T. Sobue and Y. Arakawa, Sci. Rep., 2017, 7, 45170 CrossRef.
- H. Tsuji and T. Sobue, RSC Adv., 2015, 5, 83331–83342 RSC.
- H. Tsuji, K. Osanai and Y. Arakawa, Cryst. Growth Des., 2020, 20, 1047–1057 CrossRef CAS.
- H. Tsuji, K. Osanai and Y. Arakawa, Cryst. Growth Des., 2018, 18, 6009–6019 CrossRef CAS.
- F.-R. Zeng, J.-M. Ma, L.-H. Sun, Z. Zeng, H. Jiang and Z.-L. Li, Macromol. Chem. Phys., 2018, 219, 1800031 CrossRef.
- H. Tsuji, M. Yamasaki and Y. Arakawa, ACS Appl. Polym. Mater., 2019, 1, 1476–1484 CrossRef CAS.
- Y. Tabata and H. Abe, Macromolecules, 2014, 47, 7354–7361 CrossRef CAS.
- J. S. Moore and S. I. Stupp, Macromolecules, 1990, 23, 65–70 CrossRef CAS.
- R. M. Stayshich and T. Y. Meyer, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 4704–4711 CrossRef CAS.
- R. M. Stayshich and T. Y. Meyer, J. Am. Chem. Soc., 2010, 132, 10920–10934 CrossRef CAS.
- H. Tsuji and Y. Arakawa, Polym. Chem., 2018, 9, 2446–2457 RSC.
- Y. Sakamoto and H. Tsuji, Polymer, 2013, 54, 2422–2434 CrossRef CAS.
- H. Tsuji, Y. Arakawa and N. Matsumura, Polym. Bull., 2019, 76, 1199–1216 CrossRef CAS.
- H. Tsuji and T. Sobue, Polymer, 2015, 72, 202–211 CrossRef CAS.
- K. Matsumoto, S. Terai, A. Ishiyama, J. Sun, T. Kabe, Y. Song, J. M. Nduko, T. Iwata and S. Taguchi, Biomacromolecules, 2013, 14, 1913–1918 CrossRef CAS.
- H. Tsuji, F. Horri, S.-H. Hyon and Y. Ikada, Macromolecules, 1992, 25, 4114–4118 CrossRef CAS.
- L. Bouapao and H. Tsuji, Macromol. Chem. Phys., 2009, 210, 993–1003 CrossRef CAS.
- H. Tsuji and A. Okumura, Macromolecules, 2009, 42, 7263–7266 CrossRef CAS.
- H. Tsuji and T. Sobue, Polymer, 2015, 69, 186–192 CrossRef CAS.
- H. Tsuji and S. Shimizu, Polymer, 2012, 53, 5385–5392 CrossRef CAS.
- L. I. Ramdhanie, S. R. Aubuchon, E. D. Boland, D. C. Knapp, C. P. Barnes, D. G. Simpson, G. E. Wnek and G. L. Bowlin, Polym. J., 2006, 38, 1137–1145 CrossRef CAS.
- G. L. Loomis, J. R. Murdoch and K. H. Gardner, Polym. Prepr. (Am. Chem. Soc., Div. Polym. Chem.), 1990, 31, 55 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: S1. Experimental section, S1.1. Materials and synthesis, S1.2. Sample preparation, S1.3. Physical measurements and observation, S2. 1H NMR spectra of P(LLA-alt-L-2HB), P(DLA-alt-D-2HB), P(L-2HB), P(LLA-co-L-2HB), and PLLA (Fig. S1), S3. 13C NMR spectra of P(LLA-alt-L-2HB) and P(DLA-alt-D-2HB) (Fig. S2), S4. 13C NMR spectra of P(L-2HB), P(LLA-co-L-2HB), and PLLA (Fig. S3), S5. WAXD profile of N,N′-diisopropylurea (DIU) (Fig. S4), S6. Interplanar distance and crystalline diffraction angle values of samples (Table S1), S7. Crystallinity and thermal properties of samples (Table S2), S8. FTIR spectroscopy (Fig. S5 and Table S3). See DOI: 10.1039/d0ra08351h |
| This journal is © The Royal Society of Chemistry 2020 |