Open Access Article
Open Access ArticleControllable growth of MoS2 nanosheets on TiO2 burst nanotubes and their photocatalytic activity†
Qiushi Jiang‡
a,
Shang Wang‡a,
Xue Lib,
Zhaolian Hana,
Chunli Zhao *c,
Tingting Did,
Siyuan Liua and
Zhiqiang Cheng
*c,
Tingting Did,
Siyuan Liua and
Zhiqiang Cheng *a
*a
aCollege of Resources and Environment, Jilin Agricultural University, Changchun 130118, People's Republic of China. E-mail: czq5974@163.com
bCollege of Information Technology, Jilin Agricultural University, Changchun 130118, People's Republic of China
cCollege of Horticulture, Jilin Agricultural University, Changchun 130118, People's Republic of China. E-mail: zcl8368@163.com
dNortheast Electric Power Design Institue Co.,LTD. of China Power Engineering Consulting Group, Changchun 130021, People's Republic of China
First published on 10th November 2020
Abstract
MoS2 nanosheets were grown on TiO2 nanotubes by the simple hydrothermal method for the first time. The layer-by-layer growth of MoS2 nanosheets led to a significant increase in the specific surface area of TiO2/MoS2 burst tube composites compared with TiO2 burst tubes, a significantly enhanced ability to separate photo-induced carriers, and synergistic adsorption and visible light catalytic activity of dye molecules. The maximum adsorption (qmax) of MB was 72.46 mg g−1. In addition, 94.1% of MB could be degraded after 30 minutes of visible light irradiation. The microsurface morphology, structure, chemical composition, element valence and band width of TiO2/MoS2 nanocomposites were analyzed by scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDS), X-ray photoelectron spectroscopy (XRD), X-ray photoelectron spectroscopy (XPS), diffuse reflectance spectroscopy (DRS) and photoluminescence spectroscopy (PL). The mechanism of photocatalytic reaction was studied via free radical capture experiments.
1. Introduction
TiO2 has a low price, non-toxicity, no secondary pollution, high photo-sensitivity and excellent ability to oxidize and decompose organic matter, and its processing is relatively mature.1–3 However, TiO2 can only use ultraviolet light, which accounts for 4% of solar energy. Meanwhile, the rapid recombination of photogenerated electrons and holes in the reaction process also limits the photocatalytic efficiency of TiO2, which greatly limits its wide application.4–7 In the previous study, TiO2 burst nanotubes (TiO2NBTs) were prepared by combining electrospinning and dipping calcination. Compared with TiO2 nano-fiber tubes, TiO2NBTs have larger specific surface area and more pore structure.8The band bending at the catalyst interface due to the composite of other nanomaterials provides a driving force for the separation of photogenerated electrons and holes, thus reducing the influence of carrier recombination and improving the photocatalytic activity.9–12 For example, precious metals Pt, Ru, Rh, Au and Ag and their corresponding oxides are common cocatalysts.13–15 However, these materials are expensive and scarce, making it difficult to scale up. Strategies have been proposed to replace precious metals with cheap nanocrystalline materials,16–31 and the semiconductor MoS2 is one of the hot options,32 which has a layered structure similar to graphene, anisotropy, chemical inertia, electron transfer rate, photoresistance33 and specific optical properties,34 and is also a catalyst for dehydrogenation of complex hydrocarbons. Since the conduction band of MoS2 is below the conduction band of anatase TiO2, and the valence band exceeds that of anatase TiO2, TiO2 can transfer photoelectrons and holes to MoS2, and its narrow band gap causes the photocatalyst complex of MoS2 and TiO2 to broaden the wavelength absorption range and reduce the recombination rate of photoexcited electron–hole pairs. A large number of studies have proved that the combination of MoS2 and TiO2 as a transition metal sulfide can improve the activity of photocatalyst.35–39 However, most of them modify MoS2 on the surface of TiO2 nanofiber40–43 and its array44,45 as well as nano–microspheres,46,47 and the composite structure between TiO2 burst nanotubes matrix and MoS2 with special morphology has not been described. As we all know, the performance of photocatalysts largely depends on their morphologies. Therefore, it is of great significance to prepare nanocomposites with multiple catalytic active sites and stable morphology and to further explore their catalytic mechanisms.48–51
In this paper, TiO2/MoS2 burst nanotube composites were easily synthesized on TiO2 burst nanotubes by hydrothermal method. The uniform growth of MoS2 nanosheets on TiO2 burst tubes not only maintains the structural stability, but also achieves rapid electron transfer through close contact between TiO2 and MoS2. The uniform layer by layer growth of MoS2 nanosheet results in a larger exposed surface and more porous structure of TiO2/MoS2 nanotube burst composite, which enhances the photocatalytic activity driven by UV-vis while maintaining the structural stability. During the catalytic degradation of MB, relative to TiO2 burst tubes and hydrothermal MoS2 nanosheets, the ability of TiO2/MoS2 burst nanotube composites to adsorb MB dye molecules and separation of photoinduced carriers is greatly enhanced. Under visible light irradiation, MB solution is completely degraded within 70 min, thus improving the utilization efficiency of solar energy.
2. Experimental
2.1 Materials
Polystyrene (PS) was provided by Shanghai Youngling Electromechanical Technology Co., Ltd (the PS with Mw = 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1). Tetrabutyl titanate (TBOT 97%), N,N-dimethylformamide (DMF), methylene blue (MB), benzoquinone (BQ), and isopropanol (IPA) and triethanolamine (TEA) were purchased from Aladdin Industrial Corporation. Absolute ethanol, oxalic acid (H2C2O4), glucose, sodium molybdate (Na2MoO4·2H2O) and thiourea (CSN2H4, 99%) were bought from Beijing chemical works. All chemicals were of analytic grade and without further purification.
000 g mol−1). Tetrabutyl titanate (TBOT 97%), N,N-dimethylformamide (DMF), methylene blue (MB), benzoquinone (BQ), and isopropanol (IPA) and triethanolamine (TEA) were purchased from Aladdin Industrial Corporation. Absolute ethanol, oxalic acid (H2C2O4), glucose, sodium molybdate (Na2MoO4·2H2O) and thiourea (CSN2H4, 99%) were bought from Beijing chemical works. All chemicals were of analytic grade and without further purification.
2.2 Preparation of TiO2 burst nanotubes
The preparation of TiO2 burst tube has been described in our previous work, weighing 1.15 g PS and 2.85 g DMF, and then adding oxalic acid (0.3 wt%) to the mixture. The spinning precursor solution was prepared by stirring the mixture at 60 °C for 6 h. PS fibers are manufactured using electrospinning equipment with a high voltage power supply, rotary nozzles and grounded collectors. When the ambient temperature is 25 °C and the ambient humidity is 30%, transfer the spinning solution to the syringe and connect it to the 14 kV high voltage power supply. The flow rate of the spinning solution was controlled at 1.0 mL h−1, and the distance between the needle and the receiver was 16 cm to obtain electrospun nanofiber membrane. The collected nanofiber membrane was fully immersed in TBOT ethanol solution with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 for 15 min, and then taken out. After the soaking process, the composite membranes were dried in an oven at 60 °C for 1 h. Finally, the dried PS/TBOT fiber membrane was calcined at a heating rate of 3 °C min−1 to 550 °C in a tube furnace, and kept 30 min. Naturally cool after annealing to obtain TiO2 burst nanotubes.8
10 for 15 min, and then taken out. After the soaking process, the composite membranes were dried in an oven at 60 °C for 1 h. Finally, the dried PS/TBOT fiber membrane was calcined at a heating rate of 3 °C min−1 to 550 °C in a tube furnace, and kept 30 min. Naturally cool after annealing to obtain TiO2 burst nanotubes.8
2.3 Preparation of TiO2/MoS2 nanocomposite by hydrothermal method
First, 0.2 g sodium molybdate and 0.4 g thiourea were dissolved in 30 mL glucose solution (0.05 M), and then 100 mg TiO2 burst nanotubes and the above mixed solution were simultaneously transferred to a 50 mL polytetrafluoroethylene lined stainless steel autoclave, which was then placed in an oven at 200 °C for 24 h. After the autoclave was naturally cooled, the black products were collected by centrifugation and washed with ethanol for 3–5 times. The washed composites were dried overnight in the oven at 60 °C to obtain TiO2/MoS2 burst nanotube composites. As a contrast, a single MoS2 nanosheet was prepared, and the amount of material used in the hydrothermal process was exactly the same as that in the preparation of TiO2/MoS2 nanocomposites, except that no TiO2 burst nanotubes were added.2.4 Characterization
The surface morphology of the sample was observed by scanning electron microscope (SSX-550, Shimadzu). The EDS mapped image and microstructure of the samples were observed by transmission electron microscope (TEM, TecnaiF20) of energy dispersive X-ray spectrometer (EDS). X-ray photoelectron spectroscopy (XPS, VGESCALABLKII) is used to determine the chemical composition of the complex. The specific surface area and pore diameter of the samples were analyzed using a specific surface & pore size analysis instrument (BET, 3H-2000PS1, BeishideInstrument). The crystal structure of the sample can be determined by X-ray diffractometer (XRD, XRD-7000, Shimadzu). The absorption characteristics of the samples and the absorptivity of degraded dyes were studied by UV-vis spectrophotometer with integrating sphere (TU-1950, Beijing Purkinje Corporation). The photoluminescence (PL) spectrum excited by 325 nm wavelength was measured at room temperature on a fluorescence spectrophotometer (F-4500, Hitachi, Japan).2.5 Organic adsorption characterization
As a common industrial dye and fungicide, MB has low biodegradability in water. In this work, methylene blue (MB) was used as a simulated organic pollutant to evaluate the adsorption properties of TiO2/MoS2 nanocomposites. The solution of 10 mg L−1, 20 mg L−1, 30 mg L−1, 40 mg L−1, 50 mg L−1 and 60 mg L−1 MB were put into the quartz tubes. 10 mg TiO2 burst nanotubes, MoS2 nanosheets and TiO2/MoS2 nanocomposites were added to each quartz tube. The suspension was magnetically stirred in the dark for 24 hours, and 3 mL solution was extracted from it for centrifugation to measure the absorbance value of the solution.2.6 Photocatalytic test
The photocatalytic activity of TiO2/MoS2 nanocomposites was studied by the degradation of MB solution under visible light. First, the nanocomposites of 10 mg were mixed with the 60 mL (10 mg L−1) MB solution and stirred by magnetic force in dark for 30 minutes to achieve the adsorption–desorption equilibrium. Then the mixed solution was irradiated with the visible band of a mercury lamp (360 W), and the distance between the solution and the light source was 10 cm. What's more, the irradiation power used for photocatalysis was 450 mW cm−2. The UV-vis absorption spectrum can be measured when the 3 mL solution is taken with a filter after each 10 min irradiation of UV-vis, and a blank control test is carried out.2.7 Possible mechanism of photocatalytic activity
In order to explore the charge transfer process of TiO2/MoS2 nanocomposites during photocatalytic degradation, free radical capture experiments of different scavengers were carried out. The effects of superoxide radical (·O2−), hydroxyl radical (·OH) and hole (h+) on the photodegradation of MB were investigated. Benzoquinone (BQ, 1 mM), isopropanol (IPA, 1 mM) and triethanolamine (TEA, 1 mM) were added to the reaction system as scavengers of ·O2−, ·OH and h+, respectively. The remaining steps were the same as those of photocatalysis test.3. Results and discussion
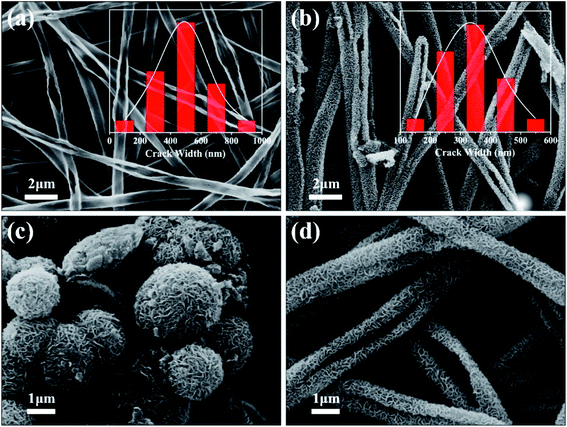
Fig. 1(a) shows the TiO2 burst nanotubes with a diameter of 600–1000 nm. Due to the gradual decomposition of oxalic acid with the increase of temperature in the calcination stage of PS/TBOT fiber membrane, CO2 gas is released. Under the high temperature condition, CO2 gas will leave tiny holes on the surface of TiO2 nanotube when it escapes, and PS will degrade completely as a sacrificial template, causing disintegration of TiO2 nanotubes and forming TiO2 burst nanotubes. As shown in Fig. 1(b), after the MoS2 nanosheets are composited on the TiO2 burst nanotubes by a hydrothermal method, the diameter of the composite has also increased accordingly. However, by comparing Fig. 1(a) and (b), it is found that the cracking degree of the nanocomposite after the composite of MoS2 and TiO2 nanosheets is significantly smaller than that of the TiO2 burst nanotubes. The reason is that MoS2 nanosheets grow layer by layer at the position where the TiO2 burst nanotubes. It is found that compared to the micron-scale irregular spherical agglomerates produced by the aggregation and growth of MoS2 nanosheets without a growth template (Fig. 1(c)), the tiny holes provided by the TiO2 burst nanotubes provide suitable nucleation sites for the growth of MoS2 nanosheets. Moreover, in TiO2/MoS2 material, TiO2 nano burst-tubes as basal hydrothermal preparation of TiO2/MoS2 overall still had a tubular cracking morphology, while MoS2 combination in the form of three-dimensional layered nanometer sheets in both internal and external TiO2 nano burst-tubes, improving the porosity of the composite material in general (Fig. 1(d)).The morphological changes of the TiO2/MoS2 composite nano-burst tube materials were confirmed by TEM observations in Fig. 2(a) and (b). Typical layered structure MoS2 particles were observed on the TiO2/MoS2 composite burst tube, while no layered structure MoS2 were observed in the separate TiO2 nano bursttubes catalyst. By measuring the HRTEM image of the MoS2 nanosheets (Fig. 2(c)), the lattice spacing d is 0.62 nm, which corresponds to the hexagonal MoS2 (002) plane (JCPDS37-1492). In the TiO2/MoS2 composite nanoburst tube material shown in (Fig. 2(d)), not only the hexagonal MoS2 (002) plane can be detected, but also the (101) plane of anatase TiO2 (JCPDS211272), and the lattice spacing d is 0.35 nm. Based on the above analysis, it is concluded that MoS2 nanosheets grow on TiO2 burst nanotubes through a simple hydrothermal reaction, and the two grow closely together. We believe that this composite heterostructure is beneficial to the transfer of photogenic charge carriers, and at the same time can enhance the charge separation ability and photocatalytic activity.
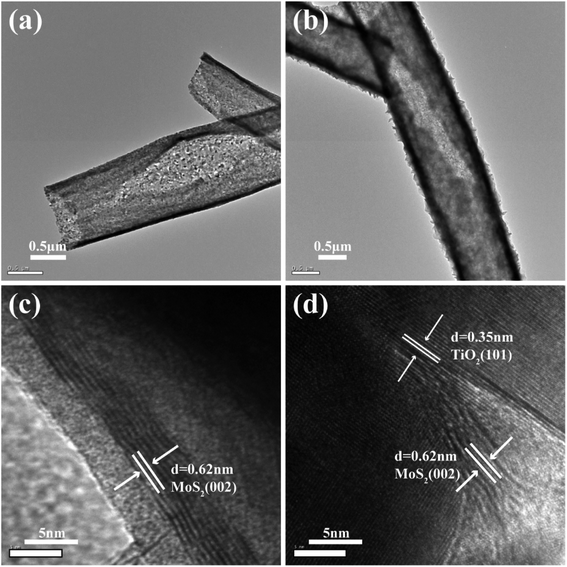 | ||
| Fig. 2 TEM image of the TiO2 burst nanotubes (a) TiO2/MoS2 nanocomposite (b), HRTEM images of several MoS2 nanosheets (c) and TiO2/MoS2 nanocomposite (d). | ||
The elemental mapping analysis of energy dispersion-X-ray spectroscopy (EDX) of the black box for the selected area in Fig. 3(a) visually shows the distribution of Mo, S, Ti and O (Fig. 3(b)–(e)) in the TiO2/MoS2 composite burst tube. In Fig. 3(a), the cracked part can be clearly observed, while in Fig. 3(b) and (c), it can be observed that Mo and S completely cover the TiO2 burst nanotubes. It can be seen that MoS2 nanosheets grow uniformly on the inside and outside of the tube. Fig. 3(f), as the EDX spectrum of the TiO2/MoS2 composite, also detected all peaks representing Mo, S, Ti and O elements. The unlabeled peaks are derived from Cu and C in the carbon-coated copper grid. Besides, the calculated content of MoS2 loaded on TiO2 base was 71.7% (see the ESI†).
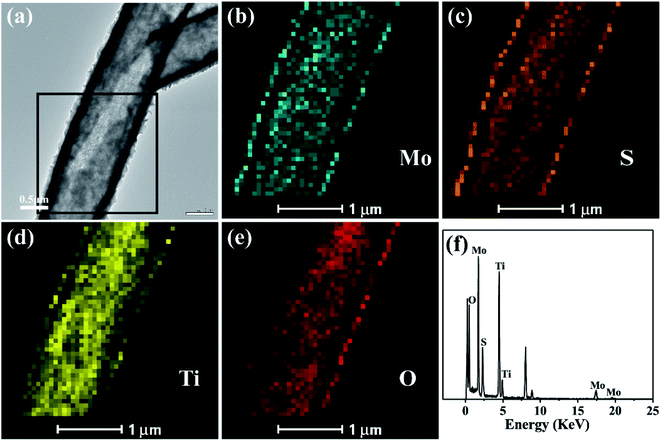 | ||
| Fig. 3 TEM image (a) of the TiO2/MoS2 nanocomposite and TEM-EDS elemental mapping (b–e) of the selected area by black box in (a), EDS spectrum of the TiO2/MoS2 nanocomposite (f). | ||
X-ray photoelectron spectroscopy ((XPS)) analysis is used to determine the chemical composition and bonding configuration of the prepared samples. The complete spectra of TiO2 and TiO2/MoS2 (0–1000 eV) are shown in Fig. 4(a). As shown in Fig. 4(b) and (c), for pure MoS2 nanosheets, the binding energies of Mo 3d3/2, Mo 3d5/2, S 2p1/2 and S 2p3/2 are 231.75 eV, 228.65 eV, 162.65 eV and 161.5 eV, respectively. When MoS2 nanosheets were grown into TiO2 burst nanotubes by hydrothermal synthesis, the binding energies of Mo 3d3/2, Mo 3d5/2, S 2p1/2 and S 2p3/2 peaks were converted to 231.7 eV, 228.6 eV, 162.55 eV and 161.4 eV, respectively, which were significantly lower than those of pure MoS2 nanosheets. This transition means that there is an electronic interaction between TiO2 burst nanotubes and pure MoS2 nanosheets. As shown in Fig. 4(d), the O 1s peak of TiO2/MoS2 complex, in addition to the peak value of 529.9 eV attributed to Ti–O–Ti bond, and the peak of 532.1 eV corresponds to the OH bond of water adsorbed on the surface. The peak of 531.75 eV cannot be observed in TiO2 burst nanotubes, which may be related to the formation of Ti–O–Mo bond between MoS2 nanosheets and TiO2 burst nanotubes. Fig. S1† shows the O 1s peak of TiO2 burst nanotubes, which is significantly different from that of TiO2/MoS2 composite.
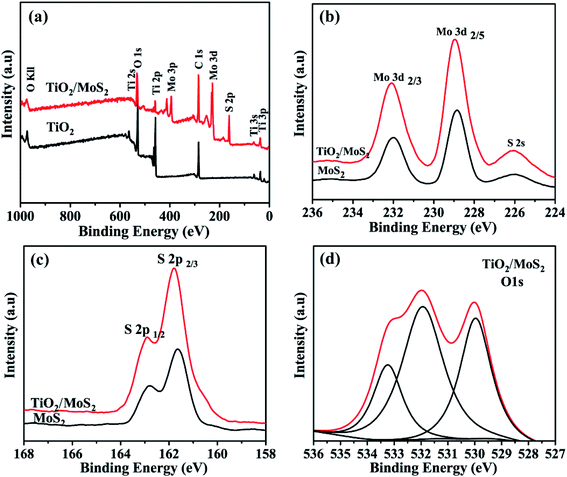 | ||
| Fig. 4 XPS spectrum of the sample: (a) the full survey spectrum of TiO2 and TiO2/MoS2, (b) Mo 3d peaks and (c) S 2p peaks of MoS2 and TiO2/MoS2, respectively. (d) O 1s peaks of TiO2/MoS2. | ||
The nitrogen adsorption–adsorption isotherms of the three samples are shown in Fig. 5(a). The isothermal curves of TiO2 burst nanotubes and TiO2/MoS2 nanocomposites show typical adsorption–desorption behavior, indicating the existence of pores. Among them, the nitrogen absorption isotherm of TiO2/MoS2 nanocomposites shows a typical type IV adsorption isotherm with H3 hysteresis ring, which is the characteristic of mesoporous structure and slit pore. On the contrary, the adsorption–desorption curves of the pure MoS2 nanowires almost coincide, indicating that the pores of pure MoS2 nanosheets are very few. The specific surface areas of TiO2 burst nanotubes, MoS2 nanosheets and TiO2/MoS2 nanocomposites are 41.77, 10.59 and 113.97 m2 g−1, respectively (Table S1† compares the BET surface area and pore volume of the three samples). The existence of pore structure on TiO2 burst nanotubes makes it easier for MoS2 nanosheets to grow. Therefore, with the stacking growth of MoS2 nanosheets, TiO2/MoS2 nanocomposites have more slit-like pores and larger specific surface area. As we all know, the specific surface area is an important property to study the photocatalytic activity of catalysts. In general, the larger specific surface area will lead to higher photocatalytic activity of the catalyst, because a larger surface area can provide more active sites. It can be seen from Fig. 5(b)–(d) that the pore diameter distribution of TiO2 sample is relatively wide, basically 0–120 nm, and the pore size distribution of 0–120 nm TiO2/MoS2 is 0–90 nm. However, the pore diameter of TiO2/MoS2 composites is basically 0–8 nm. Compared with the other two single materials, TiO2/MoS2 composites have smaller pore size and larger specific surface area.
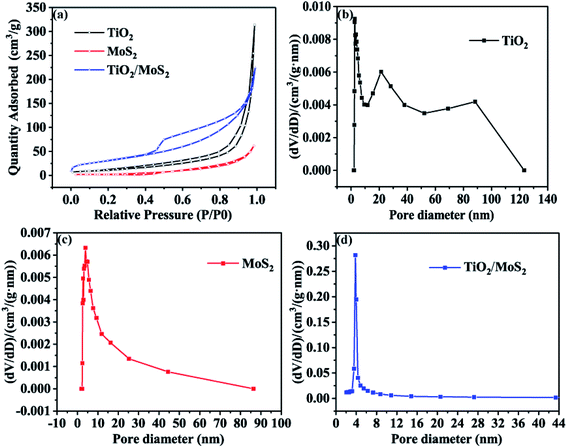 | ||
| Fig. 5 Nitrogen adsorption–desorption isotherms (a) and BJH pore size distribution of three samples (b–d). | ||
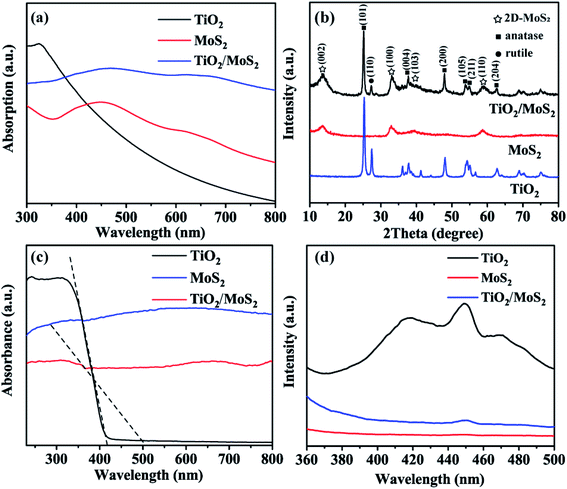
The UV-vis absorption spectra of solid dispersions of TiO2 burst nanotubes, pure MoS2 nanosheets and TiO2/MoS2 nanotube composites are shown in Fig. 6(a), TiO2 burst tubes show significant absorption at less than 400 nm. Both MoS2 nanosheets and TiO2/MoS2 nanotube composites have two absorption peaks in the region of 350–500 and 600–700 nm. However, the absorption in the visible region of TiO2/MoS2 nanoburst tube composites is obviously larger. As shown in Fig. 6(b), the main diffraction peaks of TiO2 at 2θ = 25.28°, 37.80°, 48.05°, 53.89°, 55.06° and 62.69° are in the directions of (101), (004), (200), (105), (211) and (204) respectively, corresponding to the anatase structure of TiO2 (JCPDS 21-1272). While 2θ = 27.45 °corresponds to the direction of (110), which is the characteristic peak of rutile TiO2 (JCPDS 21-1276). The diffraction peaks of pure MoS2 at 2θ = 13.6°, 32.9°, 39.2° and 58.7° correspond to (002), (100), (103) and (110) planes, respectively, which are hexagonal 2D-MoS2 (JCPDS 37-1492). In addition, all the diffraction peaks of TiO2 burst nanotubes and pure MoS2 nanotubes can be found from the curves of TiO2/MoS2 burst nanotube composites, which fully demonstrates the growth of MoS2 nanosheets on TiO2 burst nanotubes, and the crystal structure of TiO2/MoS2 nanocomposites is similar to that of pure MoS2, showing layered structure.
The optical properties of the samples were studied according to the UV-vis absorption spectrum in Fig. 6(c), As the absorption band of TiO2 burst nanotubes slightly shifts out of the UV light region, which is consistent with the mixed structure of anatase and a small amount of rutile crystal form. According to the formula:
| Eg = 1239.6/λg | (1) |
It can be calculated that the band gaps of TiO2 burst nanotubes and TiO2/MoS2 nanocomposite are 2.97 eV and 2.45 eV respectively. It shows that the excitation energy required by TiO2/MoS2 burst nanotube composite was lower than that of TiO2 burst nanotube. Therefore, the growth of MoS2 nanosheets on TiO2 burst nanotubes is conducive to improve the degradation efficiency and visible light capture capability.
To evaluate the efficiency of charge trapping and photo-induced electron–hole pair recombination in semiconductor composites, Fig. 6(d) shows the photoluminescence (PL) spectra of TiO2 burst nanotubes, MoS2 nanosheets and TiO2/MoS2 nanocomposites. TiO2 burst nanotubes have three main emission peaks. Among them, the peak at 417 nm (2.97 eV) corresponds to its near-forbidden band emission, while the other two peaks, 449 nm (2.76 eV) and 471 nm (2.63 eV), may be related to the defects related to the oxygen vacancy formed in the synthesis process. However, MoS2 nanowires have no significant emission peak. Under the same test conditions, there is only one main luminescence peak in TiO2/MoS2 composites at 450 nm (2.75 eV). In general, PL emission is caused by the recombination of charged carriers, and the lower the emission intensity, the less the charge recombination.52 Obviously, TiO2 has the highest fluorescence intensity, which means the highest photogenerated electron–hole recombination. The fluorescence intensity of TiO2/MoS2 composite is lower than that of TiO2 burst tube, indicating that the recombination of photogenerated electrons and holes is effectively suppressed. Therefore, TiO2/MoS2 can effectively promote the separation of photogenic carriers and prolong the separation time of photogenerated electron–hole pairs, thus showing excellent photocatalytic activity.
The adsorption performance is expressed by adsorption percentage (%) and adsorption capacity qe (mg g−1), which is calculated by eqn (2) and (3):
| Adsorption = (C0 − Ce)/C0 × 100% | (2) |
| qe = (C0 − Ce) × V/W | (3) |
| Ce/qe = 1/KL × qm + Ce/qm | (4) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + log KF + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce/n Ce/n
| (5) |
Fig. 7 shows the adsorption isotherm of the sample. The data were analyzed by Langmuir (Fig. 7(a)) and Freundlich (Fig. 7(b)) equations. The results show that when the concentration of MB solution is different, the correlation coefficients (R2) of TiO2 burst nanotubes, MoS2 nanosheets and TiO2/MoS2 nanocomposites are 0.993, 0.996 and 0.997, respectively. Langmuir model can fit the experimental data well. However, the Freundlich curve fitting effect of the three samples is poor, which is 0.734, 0.658 and 0.784 respectively. (Table S2:† adsorption parameters of THE Langmuir and Freundlich model for MB adsorption by TiO2/MoS2 nanocomposite.)
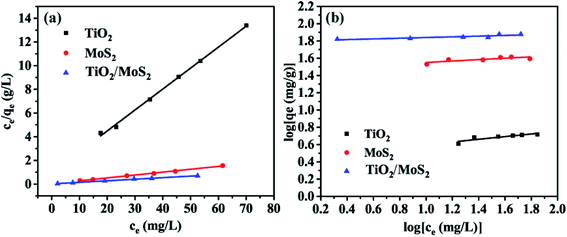 | ||
| Fig. 7 Adsorption Langmuir (a) and Freundlich isotherms (b) of MB by different adsorbents: TiO2 burst nanotubes, MoS2 nanosheets and TiO2/MoS2 nanocomposite. | ||
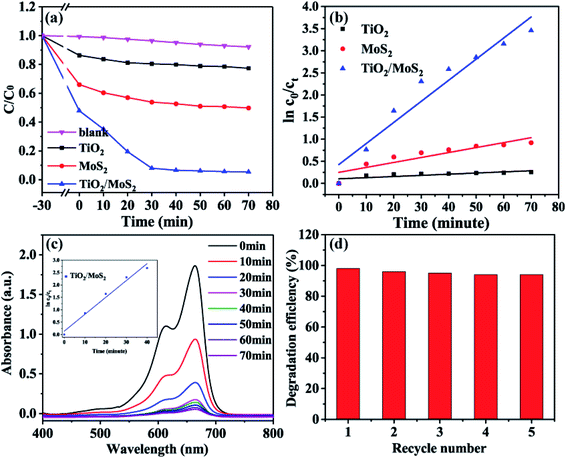
In the photocatalysis experiment, the degradation ability of TiO2/MoS2 nanocomposites was evaluated by degradation of MB solution. In Fig. 8(a) MB solution as a blank control degraded only a very small part under visible light irradiation. Before visible light irradiation, the dark adsorption experiment of 30 min was carried out to ensure that the sample reached the adsorption–desorption equilibrium. In the subsequent photocatalysis experiment, the photocatalytic efficiency of the samples was compared by showing the curve of relative concentration (C/C0) as ordinate and irradiation time (t) as abscissa. Where C0 is the initial concentration of MB solution and C is the concentration of MB solution at t. The dark adsorption results show that TiO2/MoS2 nanocomposites > pure MoS2 nanosheets > TiO2 burst nanotubes, which corresponds to the results of pore diameter distribution and specific surface area analysis. Under visible light irradiation, the dye removal rate of TiO2 burst nanotube within 70 min was only 22.6%, this is because its absorption and utilization efficiency of visible light is not high, corresponding to the calculated band gap 2.97 eV. The pure MoS2 nanometer sheet showed lower catalytic performance, with adsorption and degradation effect of about 50%. Due to the simultaneous crack, layered structure and the synergistic effect of anatase-rutile phase and hexagonal 2D-MoS2, TiO2/MoS2 nanocomposites have the highest degradation efficiency of 96.7% in the reaction of 70 min because of their large specific surface area, low band gap and visible light absorption capacity.
It can be seen from Fig. 8(b) that the degradation process of MB accords with the first-order kinetic reaction of Langmuir–HinShelwood, and the kinetic equation can be expressed as follows:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0/Ct = k × t C0/Ct = k × t
| (6) |
The results showed that TiO2/MoS2 nanocomposite had the highest constant degradation rate of MB. By dividing BET surface area (SBET) by kMB obtained by Langmuir–Hinshelwood first-order kinetic equation fitting, normalized rate constant of MB photodegradation (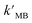 ) by pure TiO2, pure MoS2 and TiO2/MoS2 material was 7.18 × 10−5, 1.04 × 10−3 and 4.21 × 10−4 min−1 m−2, respectively.53 It can be seen that the normalized rate constant of TiO2/MoS2 material was higher than that of pure TiO2 material (see the ESI†).
) by pure TiO2, pure MoS2 and TiO2/MoS2 material was 7.18 × 10−5, 1.04 × 10−3 and 4.21 × 10−4 min−1 m−2, respectively.53 It can be seen that the normalized rate constant of TiO2/MoS2 material was higher than that of pure TiO2 material (see the ESI†).
According to Fig. 8(c), the absorption spectrum of the reaction solution decreases rapidly with the irradiation of visible light, and the degradation process of TiO2/MoS2 nanocomposites in the pre-40 min also consists with the first-order kinetic reaction of Langmuir–HinShelwood, with the degradation rate constant (k, min−1) of 0.0681. 94.1% of MB dyes were degraded in the pre-30 min, which proved that the TiO2/MoS2 nanocomposites had excellent catalytic performance under visible light irradiation. In addition, in order to prove that the absorption spectrum decline in the solution under the action of TiO2/MoS2 nanocomposite was not caused by the increase in adsorption performance due to the changing visible light irradiation conditions. After the catalytic degradation experiment, the samples were recovered and desorbed with ethanol. The results showed that there was no methylene blue in the ethanol solution. Therefore, MB is not adsorbed by TiO2/MoS2 nanocomposites, but degraded under visible light. The cyclic degradation performance of TiO2/MoS2 nanocomposites to MB under visible light was studied, as shown in Fig. 8(d) (Fig. S1† shows the repeated utilization rate of TiO2/MoS2 nanocomposites). Moreover, with the increase of recycling times, the degradation efficiency of TiO2/MoS2 nanocomposites decreased slightly. After 5 times of reuse, the degradation rate of the samples was still 94% (Table 1).
| Photocatalyst catalyst-dosage | Light source | MB dosage | Adsorption (%) | Degradation (%) | Time taken for degradation (min) | k | Ref. |
|---|---|---|---|---|---|---|---|
| 50 mg TiO2/MoS2 | 400 W xenon lamp | 100 mL (50 ppm) | Not mentioned | 99 | 12 | 0.004 min−1 | 50 |
| 50 mg TiO2/MoS2 | 30 W day lamp | 100 mL (10 ppm) | Not mentioned | 99 | 30 | Not mentioned | 54 |
| 20 mg TiO2/MoS2 | 250 W high-pressure mercury lamp | 100 mL (4 × 10−5 M) | Not mentioned | 94 | 60 | 0.048 min−1 | 49 |
| 20 mg MoS2/P25 | 500 W high-pressure mercury lamp | 40 mL (10 ppm) | Not mentioned | 80 | 5 | 0.253 min−1 | 51 |
| 10 mg TiO2/MoS2 | Sunlight | x mL (15 ppm) | 63 | 96 | 25 | Not mentioned | 37 |
| 10 mg TiO2/MoS2 | 360 W high-pressure mercury lamp | 60 mL (10 ppm) | 72 | 94 | 30 | 0.068 min−1 | This work |
Fig. 9 shows the comparison of the performance of TiO2/MoS2 nanocomposites for the degradation of MB dyes after adding different free radical scavengers, in which triethanolamine (TEA), benzoquinone (BQ) and isopropanol (IPA) have quenching effects on hole (h+), superoxide anion radical (·O2−) and hydroxyl radical (·OH), respectively. The adsorption-analysis experiments in the dark showed that the addition of different traps had little effect on the adsorption properties of the composites. After visible light irradiation, the degradation rate of the samples without any free radical scavengers was the highest, reaching 94.2%. After adding BQ, the photodegradation rate decreased slightly to 90.6%. After adding IPA, the photodegradation rate of MB decreased obviously, and the degradation rate was reduced to 86.7%. In addition, after adding TEA into the reaction system, the catalytic activity of TiO2/MoS2 nanocomposites for the degradation of MB solution under visible light was significantly affected. Therefore, ·OH and h+ are the main active species in the visible light degradation of MB by TiO2/MoS2 nanocomposites, while ·O2− has little effect on the degradation of MB.
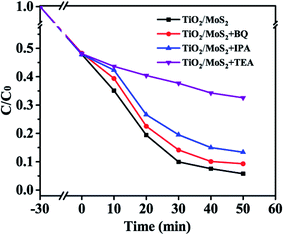 | ||
| Fig. 9 Photocatalytic degradation of MB over the TiO2/MoS2 nanocomposite photocatalyst with the addition of scavengers BQ, IPA and TEA. | ||
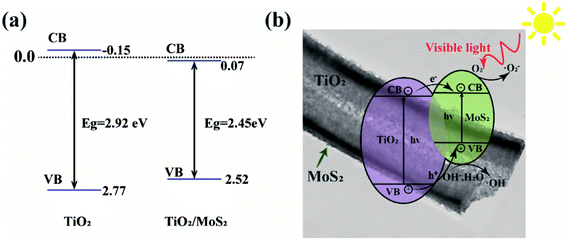
Fig. 10(a) shows the sideband (VB, CB) positions of TiO2 and TiO2/MoS2 nanocomposites, based on the band gap energy obtained in Fig. 6(c), band gaps can be obtained, using the empirical formulas,
| ECB = X − Ee − Eg/2 | (7) |
| EVB = ECB + Eg | (8) |
Based on the above analysis, Fig. 10(b) presents the possible catalytic mechanism of TiO2/MoS2 nanocomposites under visible light irradiation. Because the band gap of MoS2 nanowires is 2.40 eV,48 it can absorb visible light whose wavelength is less than 653 nm. When the appropriate wavelength of light irradiates TiO2/MoS2 nanocomposites, the electrons (e−) in the valence band absorb enough energy to transition to the conduction band and form holes (h+) in the valence band. As the conduction band of MoS2 is close to TiO2, which accelerates the transfer of photoelectrons produced in TiO2 to MoS2, thus promoting the separation of carriers and reducing the recombination of photogenerated electrons and holes.37 Since the CB potential (−0.38 V) of MoS2 is more negative than the reduction potential of O2/·O2− (−0.33 V vs. NHE), e− can effectively reduce the dissolved O2 in the solution to ·O2− radical. Since the VB potential of TiO2 (+2.77 V vs. NHE) is higher than the oxidation potential of H2O/·OH (2.40 V vs. NHE), ·OH will be thermodynamically formed on TiO2.16 On the special surface of TiO2/MoS2 burst tube nanocomposites, photogenerated electrons are quickly captured by oxygen molecules (O2) in dye solution to form superoxide anions (·O2−). At the same time, holes (h+) attack H2O and OH− in organic dye solution to form hydroxyl radical (·OH), resulting in strong oxidation, which can degrade organic dye molecules into environment-friendly H2O, CO2 and other compounds. The photocatalytic mechanism under visible light irradiation is as follows:
| TiO2/MoS2 → e− + h+(TiO2/MoS2) | (9) |
| O2 + e− → ·O2− | (10) |
| OH− + h+ → ·OH | (11) |
| H2O + h+ → ·OH + H+ | (12) |
| ·O2− + H+ + e− → ·OH + OH− | (13) |
| MB + ·OH → CO2 + H2O + NO3− + NH4+ + Cl− | (14) |
4. Conclusions
To sum up, TiO2/MoS2 nanocomposites show multi-functional synergistic properties of MB adsorption and visible light catalysis through low-cost hydrothermal preparation on the matrix of TiO2 burst nanotubes. By characterization, the MoS2 nanosheets grown on TiO2 burst tubes had a layered composite structure, leading to a significant improvement in specific surface area (113.97 m2 g−1) compared with pure TiO2 burst tubes (67.55 m2 g−1), and improved the pollutant adsorption capacity of the sample. The adsorption results of TiO2/MoS2 nanocomposite samples for MB were fitted with the Langmuir model, and the degradation process of MB under visible light accords with the first-order kinetic reaction of Langmuir–Hinshelwood. At the same time, the samples obtained high light capture ability and rapid electron transfer ability. The results of DRS detection calculation showed that the light absorption range of TiO2/MoS2 shifted to visible light, and the forbidden band width decreased. Photocatalytic tests confirmed that the visible light catalytic performance of TiO2/MoS2 nanocomposites got significantly improved compared with single MoS2 nanosheets and TiO2 burst nanotubes. Moreover, free radical trapping experiments confirmed that when TiO2/MoS2 nanocomposites degraded MB, h+ was the main reaction species, while ·O2−and ·OH were secondary reaction species.The improvement of performance can be attributed to the larger specific surface area and more pore structure of TiO2 burst nanotubes compared with TiO2 nanotubes, which limits the aggregation of MoS2 and retains exposed active sites. In addition, the existence of MoS2 nanosheets increases the range of light absorption. Compared with single TiO2 burst nanotubes and MoS2 nanosheets, the heterojunction of TiO2/MoS2 burst nanocomposites is conducive to the effective transfer of carriers, thus improving the visible light catalytic activity.
This work confirmed the feasibility of the application of TiO2/MoS2 burst nanotube composites in the degradation of MB, and provided theoretical guidance and experimental basis for the synthesis of other burst nanotube semiconductor composites. We will devote ourselves to developing more application scenarios and exploring the scheme for large-scale preparation of the composite material.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by Jilin Province Innovation Capacity Building Funds Project (2019lC0050-9), major special science and technology project of pollution control and ecological restoration in Liaohe River Basin of Jilin Province (20200503002SF) and Jilin Province Science and Technology Development Project (20200708066YY and 20200403166SF).References
- S. Zhu, J. Jiang, X. Zhang, Y. Liang, Z. Cui and X. Yang, Mater. Des., 2017, 116, 238–245 CrossRef CAS.
- F. Lu, J. Wang, X. Sun and Z. Chang, Mater. Des., 2020, 189, 108503 CrossRef CAS.
- R. Fagan, D. E. McCormack, S. Hinder and S. C. Pillai, Mater. Des., 2016, 96, 44–53 CrossRef CAS.
- V. Likodimos, Appl. Catal., B, 2018, 230, 269–303 CrossRef CAS.
- J. Low, B. Cheng and J. Yu, Appl. Surf. Sci., 2017, 392, 658–686 CrossRef CAS.
- R. Qian, H. Zong, J. Schneider, G. Zhou, T. Zhao, Y. Li, J. Yang, D. W. Bahnemann and J. H. Pan, Catal. Today, 2019, 335, 78–90 CrossRef CAS.
- M. Sachs, E. Pastor, A. Kafizas and J. R. Durrant, J. Phys. Chem. Lett., 2016, 7, 3742–3746 CrossRef CAS.
- S. Wang, Z. Han, T. Di, R. Li, S. Liu and Z. Cheng, R. Soc. Open Sci., 2019, 6(9) DOI:10.1098/rsos.191019.
- L. Zhang and M. Jaroniec, Appl. Surf. Sci., 2018, 430, 2–17 CrossRef CAS.
- B. Weng, M. Y. Qi, C. Han, Z. R. Tang and Y. J. Xu, ACS Catal., 2019, 9, 4642–4687 CrossRef CAS.
- T. S. Natarajan, K. R. Thampi and R. J. Tayade, Appl. Catal., B, 2018, 227, 296–311 CrossRef CAS.
- J. Yi, X. Yuan, H. Wang, H. Yu and F. Peng, Mater. Des., 2015, 86, 152–155 CrossRef CAS.
- M. Diak, E. Grabowska and A. Zaleska, Appl. Surf. Sci., 2015, 347, 275–285 CrossRef CAS.
- M. Liu, J. Zhao, C. Xiao, Q. Quan and X. Li, Mater. Des., 2016, 104, 428–435 CrossRef CAS.
- S. Li, J. Cai, X. Wu and F. Zheng, Appl. Surf. Sci., 2018, 443, 603–612 CrossRef CAS.
- Y. H. Chiu, T. F. M. Chang, C. Y. Chen, M. Sone and Y. J. Hsu, Catalysts, 2019, 9(5) DOI:10.3390/catal9050430.
- T. H. Do, C. Nguyen Van, K. A. Tsai, L. T. Quynh, J. W. Chen, Y. C. Lin, Y. C. Chen, W. C. Chou, C. L. Wu, Y. J. Hsu and Y. H. Chu, Nano Energy, 2016, 23, 153–160 CrossRef CAS.
- P. Y. Hsieh, Y. H. Chiu, T. H. Lai, M. J. Fang, Y. T. Wang and Y. J. Hsu, ACS Appl. Mater. Interfaces, 2019, 11, 3006–3015 CrossRef CAS.
- Y. S. Chang, M. Choi, M. Baek, P. Y. Hsieh, K. Yong and Y. J. Hsu, Appl. Catal., B, 2018, 225, 379–385 CrossRef CAS.
- J. M. Li, C. W. Tsao, M. J. Fang, C. C. Chen, C. W. Liu and Y. J. Hsu, ACS Appl. Nano Mater., 2018, 1, 6843–6853 CrossRef CAS.
- Y. H. Chiu and Y. J. Hsu, Nano Energy, 2017, 31, 286–295 CrossRef CAS.
- C. N. Van, T. H. Do, J. W. Chen, W. Y. Tzeng, K. A. Tsai, H. Song, H. J. Liu, Y. C. Lin, Y. C. Chen, C. L. Wu, C. W. Luo, W. C. Chou, R. Huang, Y. J. Hsu and Y. H. Chu, NPG Asia Mater., 2017, 9, e357 CrossRef.
- X. Wang, S. Zhang, B. Peng, H. Wang, H. Yu and F. Peng, Mater. Lett., 2016, 165, 37–40 CrossRef CAS.
- K. A. Tsai and Y. J. Hsu, Appl. Catal., B, 2015, 164, 271–278 CrossRef CAS.
- M. Y. Chen and Y. J. Hsu, Nanoscale, 2013, 5, 363–368 RSC.
- Y. F. Lin and Y. J. Hsu, Appl. Catal., B, 2013, 130–131, 93–98 CrossRef CAS.
- Y. C. Chen, Y. C. Pu and Y. J. Hsu, J. Phys. Chem. C, 2012, 116, 2967–2975 CrossRef CAS.
- Y. H. Chiu, T. H. Lai, M. Y. Kuo, P. Y. Hsieh and Y. J. Hsu, APL Mater., 2019, 7, 080901 CrossRef.
- C.-W. T. Y.-J. H. Mei-jing Fang, J. Phys. D: Appl. Phys., 2018, 51, 404001 CrossRef.
- P. Y. Hsieh, J. Y. Wu, T. F. M. Chang, C. Y. Chen, M. Sone and Y. J. Hsu, Arabian J. Chem., 2020, 13(11), 8372–8387 CrossRef.
- M. Wang, Y. S. Chang, C. W. Tsao, M. J. Fang, Y. J. Hsu and K. L. Choy, Chem. Commun., 2019, 55, 2465–2468 RSC.
- S. Harish, Prachi, J. Archana, M. Navaneethan, M. Shimomura, H. Ikeda and Y. Hayakawa, Appl. Surf. Sci., 2019, 488, 36–45 CrossRef CAS.
- Z. He, W. Que, Y. Xing and X. Liu, J. Alloys Compd., 2016, 672, 481–488 CrossRef CAS.
- S. Gu, M. Qin, H. Zhang, J. Ma, H. Wu and X. Qu, CrystEngComm, 2017, 19, 6516–6526 RSC.
- X. Chen, J. Zhang, X. Jiang, H. Wang, Z. Kong, J. Xi and Z. Ji, Mater. Lett., 2018, 229, 277–280 CrossRef CAS.
- X. Liu, X. Huo, P. Liu, Y. Tang, J. Xu, X. Liu and Y. Zhou, Electrochim. Acta, 2017, 242, 327–336 CrossRef CAS.
- C. Wang, Y. Zhan and Z. Wang, ChemistrySelect, 2018, 3, 1713–1718 CrossRef CAS.
- J. Zhang, L. Huang, Z. Lu, Z. Jin, X. Wang, G. Xu, E. Zhang, H. Wang, Z. Kong, J. Xi and Z. Ji, J. Alloys Compd., 2016, 688, 840–848 CrossRef CAS.
- A. Hamdi, L. Boussekey, P. Roussel, A. Addad, H. Ezzaouia, R. Boukherroub and Y. Coffinier, Mater. Des., 2016, 109, 634–643 CrossRef CAS.
- S. He, Y. Zhang, J. Ren, B. Wang, Z. Zhang and M. Zhang, Colloids Surf., A, 2020, 600, 124900 CrossRef CAS.
- W. Zhou, Z. Yin, Y. Du, X. Huang, Z. Zeng, Z. Fan, H. Liu, J. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS.
- R. Wei, X. Tian, Z. Hu, H. Zhang, T. Qiao, X. He, Q. Chen, Z. Chen and J. Qiu, Opt. Express, 2016, 24, 25337 CrossRef CAS.
- Y. Li, Z. Wang, H. Zhao, X. Huang and M. Yang, J. Colloid Interface Sci., 2019, 557, 709–721 CrossRef CAS.
- J. Dong, J. Huang, A. Wang, G. V. Biesold-McGee, X. Zhang, S. Gao, S. Wang, Y. Lai and Z. Lin, Nano Energy, 2020, 71, 104579 CrossRef CAS.
- L. Zheng, S. Han, H. Liu, P. Yu and X. Fang, Small, 2016, 12, 1527–1536 CrossRef CAS.
- A. Balati, A. Matta, K. Nash and H. J. Shipley, Composites, Part B, 2020, 185, 107785 CrossRef CAS.
- J. Su, S. Yu, M. Xu, Y. Guo, X. Sun, Y. Fan, Z. Zhang, J. Yan and W. Zhao, Mater. Res. Bull., 2020, 130, 110936 CrossRef CAS.
- W. Teng, Y. Wang, H. H. Huang, X. Li and Y. Tang, Enhanced photoelectrochemical performance of MoS2 nanobelts-loaded TiO2 nanotube arrays by photo-assisted electrodeposition, Elsevier B.V., 2017, vol. 425 Search PubMed.
- J. Zhang, L. Zhang, W. Yu, F. Jiang, E. Zhang, H. Wang, Z. Kong, J. Xi and Z. Ji, J. Am. Ceram. Soc., 2017, 100, 5274–5285 CrossRef CAS.
- M. Sabarinathan, S. Harish, J. Archana, M. Navaneethan, H. Ikeda and Y. Hayakawa, RSC Adv., 2017, 7, 24754–24763 RSC.
- D. Wang, Y. Xu, F. Sun, Q. Zhang, P. Wang and X. Wang, Appl. Surf. Sci., 2016, 377, 221–227 CrossRef CAS.
- C. Liu, L. Wang, Y. Tang, S. Luo, Y. Liu, S. Zhang, Y. Zeng and Y. Xu, Appl. Catal., B, 2015, 164, 1–9 CrossRef CAS.
- K. H. Chen, Y. C. Pu, K. Der Chang, Y. F. Liang, C. M. Liu, J. W. Yeh, H. C. Shih and Y. J. Hsu, J. Phys. Chem. C, 2012, 116, 19039–19045 CrossRef CAS.
- K. H. Hu, Y. K. Cai and S. Li, Adv. Mater. Res., 2011, 197–198, 996–999 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08421b |
| ‡ Qiushi Jiang and Shang Wang contribute to the work equally. |
| This journal is © The Royal Society of Chemistry 2020 |