Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Formation of buried superconducting Mo2N by nitrogen-ion-implantation†
Joonhyuk Lee a,
Jun Kue Parkb,
Joon Woo Leec,
Yunseok Heoa,
Yoon Seok Ohc,
Jae S. Leeb,
Jinhyung Chod and
Hyoungjeen Jeen
a,
Jun Kue Parkb,
Joon Woo Leec,
Yunseok Heoa,
Yoon Seok Ohc,
Jae S. Leeb,
Jinhyung Chod and
Hyoungjeen Jeen *a
*a
aDepartment of Physics, Pusan National University, Busan 46241, Korea. E-mail: hjeen@pusan.ac.kr
bKorea Multi-purpose Accelerator Complex, Korea Atomic Energy Research Institute, Gyeongju 38180, Korea
cDepartment of Physics, Ulsan National Institute of Science and Technology, Ulsan 44919, Korea
dDepartment of Physics Education, Pusan National University, Busan 46241, Korea
First published on 16th December 2020
Abstract
Nitrogen ion implantation is a useful technique to put nitrogen ions into lattices. In this work, nitrogen ion implantation into epitaxial Mo films is performed to create a buried superconducting γ-Mo2N. Atomically flat epitaxial (110) Mo films are grown on (0001) Al2O3. By impinging nitrogen ions, where the beam energy is fixed to 20 keV, we observe (111) γ-Mo2N diffraction and the formation of a γ-Mo2N layer from X-ray reflectivity. Magnetization and transport measurements clearly support a superconducting layer in the implanted film. Our strategy shows that formation of a buried superconducting layer can be achieved through ion implantation and self-annealing.
Introduction
Ion implantation is a versatile technique to incorporate ions into crystalline lattices.1,2 Through ion implantation, electrical, magnetic, and optical properties have been tuned. Fractional boron doping into silicon using ion implantation enabled formation of the desired level of doping in semiconductors.3,4 In addition, for fabricating dilute magnetic semiconductors, magnetic ion implantation was used for room temperature ferromagnetism.2,5–7 Unusual luminescence and photo-activity in the implanted films were also reported.8–11 However, the implantation strategy is not limited to semiconductors. In recent, it was adopted for stabilizing meta-stable phase such as rare-earth-free permanent magnet such as Fe16N2 by implanting nitrogen ions into iron lattices.12 Thus, combining ion implantation technique in crystal synthesis may bring an another degree of freedom for tuning materials properties. In this regard, creation of a metal nitride from nitrogen ion implantation is important, since nitrogen molecules are normally very stable. So, it is not easy to decompose nitrogen molecules, incorporate nitrogen ions into the lattices, and form desired stoichiometry. Ones often used ammonia as a processing agent for nitridation.13–15 Even if ion implantation is rather destructive method to impinge small atomic or molecular nitrogen ions, usually N+ or N2+, into crystal lattices, it is expected to intercalate nitrogen ions effectively and stabilize them in the lattice through post-process: i.e. heat treatment. This leads to stabilize a highly-stabilized resistive surface and/or exotic physical properties like superconductivity, which will be introduced in this work.Nitridation of molybdenum using ion implantation is of considerable interest, since molybdenum nitrides can be mechanically strong and superconducting materials with different critical temperatures depending on nitrogen content.16–21 It has been proven that ion beam implantation with wider nitrogen beam energy (up to 200 keV) and relatively high nitrogen ion dose (1016 ∼ 1017 ions cm−2) can induce the formation of γ-Mo2N, δ-MoN, and B1–MoN. It is generally known that higher ion incorporation could be possible, when lower energy and higher dose used.20 In this work, we observed evidence of buried superconducting-phase formation by ion implantation on (110) epitaxial Mo thin films using relatively low energy. First, we synthesized atomically flat (110) Mo thin films. The films were transferred to ion beam facility for atomic nitrogen ion (N+) beam implantation at low energy to minimize disordering of Mo atoms. The implanted films were tested to find potential formation of superconducting nitrides using X-ray scattering, cross-section transmission electron microscopy, atomic force microscopy (AFM), transport, and magnetization measurements.
Experimental
80 nm-thick epitaxial Mo thin films were grown on (0001) Al2O3 substrates (Crystal bank, Pusan National University) using custom-made DC magnetron sputtering. The detailed growth condition for Mo films are following: 5 mTorr as forming gas pressure (Pforming), 50 W of DC power, and 700 °C of substrate temperature (TS). X-ray diffraction (D8 Discover, Bruker) techniques such as X-ray reflectivity, 2θ–ω scan, φ-scan were employed to characterize structural information of epitaxial Mo films (see Fig. 1). After confirming epitaxial growth of Mo films, N+ beam implantation experiments were performed in Korea Multi-purpose Accelerator Complex. Beam condition was fixed at 20 keV with different ion doses: 1015, 1016, and 5 × 1016 ions cm−2. To predict potential ion distribution, we used the transport of ions in matter (TRIM) results22 to estimate profile of implanted nitrogen ions and recoiled or disordered molybdenum ions. X-ray 2θ–ω scan and X-ray reflectometry (XRR) were specially adopted to see formation of buried molybdenum nitrides and destabilization of molybdenum by ion implantation. To observe microstructure and chemical inhomogeneity due to nitrogen ion implantation, cross-sectional transmission microscope (TALOS F200X, FEI) was used. Z-contrast imaging and energy dispersive X-ray spectroscopy (EDS) are performed on the highest nitrogen dosed sample (5 × 1016 ions cm−2 of N+). X-ray reflectivity fitting was performed with GenX software.22 After confirming the formation of the nitrides, we used SQUID magnetometer (MPMS3, Quantum Design) and Physical Property Measurement System (Quantum design) to observe temperature dependence of magnetization and transport property. For a SQUID measurement, we used 100 Oe of magnetic field. For a transport measurement, we used conventional four probe transport geometry and the data is normalized by resistance value at T = 8 K (normal state).Results and discussion
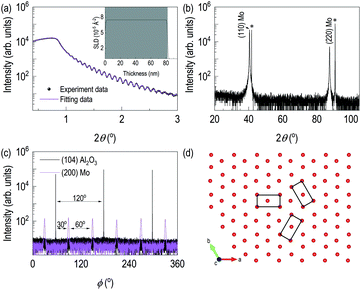
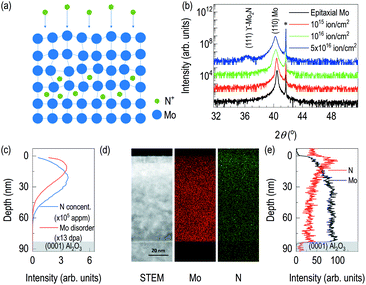
Fig. 1 showed the evidence of epitaxial synthesis of Mo films. Fig. 1(a) showed X-ray reflectivity of Mo films on (0001) Al2O3. Clear Kiessig fringes show well-defined interfaces with about 80 nm in thickness. The obtained electron scattering length density (eSLD) of a Mo layer is 7.54 Å−2, which is very similar to the bulk value of Mo (eSLD = 7.64 Å−2). In addition, from X-ray reflectivity fitting, we also obtained the surface roughness information, which is about 1 nm. The value is similar to the value obtained from atomic force microscopy (see Fig. S1†) Fig. 1(b) showed 2θ–ω scan. It showed (110) Mo is stabilized on (0001) Al2O3. In addition to the determination of out-of-plane information, we performed off-axis φ scan to figure out complete epitaxial relationship. φ scans around (104) Al2O3 and (200) Mo were selected. We observed Al2O3 peaks with 120° apart, while we obtained six peaks from (200) Mo with 60° apart. Also, Mo peaks are 30° apart from the nearest Al2O3 peaks. It indicates potential texture in the plane. In Fig. 1(d), lattice oxygens on (0001) Al2O3 are shown with rectangular (110) Mo lattices. From the figure, it can be easily seen that [001] Mo does not coincide with [100] Al2O3. In addition, [001] Mo is at least 30° apart from [100] Al2O3.After confirming epitaxial synthesis of (110) Mo thin film on (0001) Al2O3, we performed nitrogen-ion implantation with 20 keV and various doses. A schematic diagram in Fig. 2(a) describes how nitrogen ions may be intercalated. Since ion energy is high enough, it creates recoiling of Mo ions from its equilibrium positions. Fig. 2(b) shows XRD results of Mo films with various doses of the implantation. First, there is no shoulder peak near the substrate peak, which is likely to be associated with the effect of lattice distortion or implanted ions. It is surprising that a new diffraction peak was observed in addition to the broadening of (110) Mo peak. (110) Mo peak are broadened and shifted toward lower 2θ angle as the dose increases. It indicates, by recoiling of Mo atoms, lattice expansion is taken place. Note that the significant lattice expansion was found, when the dose is above 1016 ions cm−2. In addition, when we checked rocking curve of (110) Mo, we observed its full width half maximum (FWHM) changes from 0.08° for as-grown Mo film to 0.14° for N+ implanted Mo film with 5 × 1016 ions cm−2. A new diffraction peak is shown when the dose reached 5 × 1016 ions cm−2. The new peak is located to that of (111) γ-Mo2N.20,23 The ion implantation experiments were performed by cooling the backplate of sample stage using chilled water, and the temperature of backplate is kept to 24 °C. It is likely that the temperature of Mo films during ion implantation is different from that of backplate potentially due to self-annealing during ion implantation,24–26 which will be a potential reason for formation of crystalline molybdenum nitride. From AFM results in Fig. S1,† the surface roughness of the ion-implanted films is about 2 nm, which is higher than the value from an as-grown Mo film. Interestingly, as the dose increases, the grain size increases but the surface roughness decreases. It can be evidence of self-annealing of the surface through ion beam implantation.
After finding the formation new phase in 5 × 1016 ions cm−2 of nitrogen ion dose, to estimate depth information of recoiled Mo and implanted nitrogen, TRIM was used to simulate distribution of nitrogen ions in the Mo film and distribution of recoiled Mo atoms (Fig. 2(c)). It is noted that surface Mo atoms are likely to lose their equilibrium positions during nitrogen implantation. From our TRIM simulation, the distribution of recoiled Mo atoms is limited to the surface. However, nitrogen ion distribution is bit different. Its center position is likely to locate deeper than that of recoiled Mo. In addition, the simulation shows nitrogen ions will reside within the 80 nm-thick Mo films. Thus, it is unlikely that the results of physical properties are originated from the modification of Al2O3 by nitrogen ions. Scanning transmission electron microscopy was also performed on the sample with 5 × 1016 ions cm−2. A Z-contrast imaging in Fig. 2(d) show clear contrasts. The first region is recognized from top surface down to 7 nm below the surface. Second layer is formed in between 7 nm and 20 nm from the top surface. It is likely due to changes in chemical composition and density. So, we additionally performed EDS of Mo and N. For the case of EDS Mo, it is clearly seen that less bright signals near the surface. Fig. 2(e) shows depth profile of relative Mo signals from EDS. The result shows the region up to 4 nm from the surface is low density, which is less than 50% of the signals from the bulk region, found at 32 nm and below from the surface. The depth profile of Mo signal from EDS shows 20 keV of nitrogen ion beam significantly disorders Mo layer near the surface. Also, Fig. 2(d) and (e) include information of implanted nitrogen. It is clearly seen that brighter region is found near the surface. However, at the proximity of the surface, relative nitrogen dose is not the high. It indicates potential formation of the buried Mo2N superconducting layer. From the depth profile of relative N signals from EDS, the highest nitrogen signal is found at 4 nm below the surface. It is seen that sufficient amount of nitrogen ions are found down to 30 nm. From STEM/EDS, it is clearly seen that nitrogen implantation in Mo layer, disordering of Mo layer, and no effect on Al2O3. Fig. S2 (b) and (c)† are FFTs from the lattice image in Fig. S2(a).† The zone axes are determined as [−1 1 −1] of Mo and [0 −1 1] of γ-Mo2N, which are well-matched with the simulation results. In addition, we clearly observed lattice expansion upon nitrogen implantation.
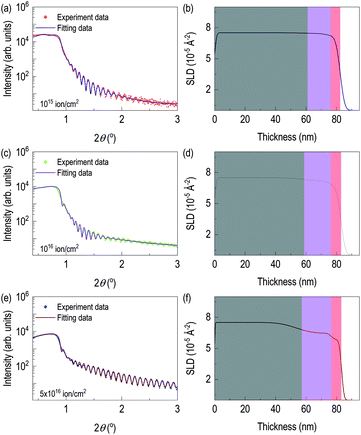
After checking the formation of γ-Mo2N from X-ray diffraction and chemical depth profile of the highly dosed sample, we performed X-ray reflectivity of N+ implanted Mo films. Fig. 3 shows X-ray reflectivity, fitting, and depth profile of electron scattering length density (eSLD). In all cases, we observed clear kiessig fringes. While Fig. 3(a) is less pronounced, Fig. 3(c) and (e) show clear evidence of lattice modulation, since they show non-monotonic decay of X-ray reflectivity. In order to reflect the results of TRIM simulation and STEM/EDS results, we modeled the system with three layers: (i) defective-surface Mo layer possibly due to recoiled Mo atoms, (ii) nitrogen-implanted Mo layer, and (iii) unperturbed Mo layer. XRR fitting was performed on the XRR data from the film with 5 × 1016 ions cm−2, since it is expected to have the highest contrast due to high concentration of recoiled Mo and high dose of N+. After getting thickness parameters, we performed XRR fitting of other two samples, which are chemically less distinct. From XRR fitting of the film with 5 × 1016 ions cm−2, thicknesses of recoiled Mo layer, nitrogen implanted Mo layer, and unperturbed Mo layer are 6.64 nm, 19.31 nm, and 57.16 nm, respectively. The corresponding electron scattering length density (eSLD) of recoiled Mo layer, nitrogen implanted Mo layer, and unperturbed Mo layer are 5.87, 6.48, and 7.54 Å−2. Note that when comparing the eSLD values of the nitrogen-implanted Mo layer, the value is similar to that of γ-Mo2N within 3% of error.27
It confirms ion beam implantation creates three distinct layers (see Table 1) as we saw in Z-contrast imaging. Also, large amount of volume is still from unreacted Mo layer. Note that the eSLD of recoiled Mo layer is significantly lower value, and this may be due to continuous damage at the surface, which is related to disorder of Mo atoms. After getting full information of the highly dosed Mo films, XRR fitting of the remaining samples was performed. There are three major changes on recoiled-Mo layer and nitrogen-implanted Mo layer. Electronic SLD values of the recoiled-Mo layer are progressively decreasing with higher doses: 7.08 Å−2 for the case of 1015 ions cm−2, 6.60 Å−2 for the case of 1016 ions cm−2, and 5.87 Å−2 for the case of 5 × 1016 ions cm−2. It is rather drastic change above 1016 ions cm−2. However, eSLDs of nitrogen-implanted Mo layer are monotonically reduced by increase of dose: 7.45 Å−2 for the case of 1015 ions cm−2, 7.20 for the case of 1016 ions cm−2, and 6.48 for the case of 5 × 1016 ions cm−2. Lastly, we tracked roughness of each layer. Interestingly roughness of both nitrogen-implanted Mo layer and unperturbed Mo layer are high for cases of the lower ion dose, while for the case of 5 × 1016 ions cm−2, interfacial roughness significantly reduced. Note that the surface roughness of the ion-implanted films was significantly reduced. The results from XRR fitting is consistent with those of AFM in Fig. S1.†
| txrr (nm) | eSLD (10−5 Å−2) | rxrr (nm) | txrr (nm) | eSLD (10−5 Å−2) | rxrr (nm) | txrr (nm) | eSLD (10−5 Å−2) | rxrr (nm) | txrr (nm) | eSLD (10−5 Å−2) | rxrr (nm) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Defective surface Mo layer | 6.44 | 7.08 | 2.30 | 6.81 | 6.60 | 2.27 | 6.64 | 5.87 | 1.29 | |||
| Nitrogen implanter Mo layer | 15.10 | 7.45 | 4.24 | 17.37 | 7.20 | 3.16 | 19.31 | 6.48 | 1.65 | |||
| Unperturbed Mo layer | 81.07 | 7.54 | 0.72 | 60.90 | 7.54 | 10.32 | 58.81 | 7.54 | 9.97 | 57.16 | 7.54 | 8.28 |
| Epitaxial Mo | 1015 ion cm−2 | 1016 ion cm−2 | 5 × 1016 ion cm−2 | |||||||||
As a buried γ-Mo2N layer is expected to be superconducting, we performed transport measurements and temperature dependent magnetization. First, Fig. 4(a) shows temperature dependent magnetization data. We used 100 Oe of magnetization to observe diamagnetic signal. The sample with 5 × 1016 ions cm−2 shows clear diamagnetic signal below 5 K. However, other lower-dosed samples are not diamagnetic. The superconducting critical temperatures of γ-Mo2N from other groups are listed in Table 2. Note that methods to make γ-Mo2N include solid state reaction,16 ion beam implantation,28 pulsed laser deposition,29 sputtering,30–34 ion beam assisted deposition,34 electron beam evaporation,20,35 plasma immersion ion implantation36,37 and post annealing28,36,37. However, in these papers, there was no information of superconducting critical temperatures, so it was not included in Table 2. In the table, the superconducting critical temperatures are ranged from 2.8 to 7 K, depending on growth method. We would like to emphasize bulk Tc is 5 K.21,23,38–42
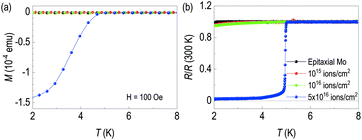 | ||
| Fig. 4 (a) Transport properties and (b) SQUID magnetization data of each sample. In both cases, superconductivity below 5 K is clearly seen from 5 × 1016 ion cm−2 of N+ dose. | ||
This feature of the superconducting zero resistance is also clearly seen in the temperature dependence of resistance in Fig. 4(b). 20 keV beam energy of the 5 × 1016 ions cm−2 shows superconducting transition at around 5 K. It's ascribed to creation of the γ-Mo2N layer through ion implantation. We found small residual resistance of our superconducting sample. The process may not form a perfect defect-free superconducting layer due to the nature of ion implantation. Note that we observed characteristic slope changes from transport results of the low fluence Mo films. This indicates the films are not superconducting at the given temperature ranges, but it is possible to see some difference in superconducting critical temperature at the lower than 1.8 K. Note that Tc of pure Mo is 1 K.43
Conclusions
In conclusion, we observed clear a buried superconducting layer in an epitaxial (110) Mo film grown on (0001) Al2O3 by low energy nitrogen ion implantation. The realization of superconductivity is seen with 5 × 1016 ions cm−2 and 20 keV of atomic nitrogen ion beam. It was checked that structural changes were observed through ion implantation, and the new peak was determined to be (111) γ-Mo2N. We performed the model fitting with three-layer model, and through eSLD and layer tracking, we could trace the γ-Mo2N layer formed on epitaxial Mo.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Research Foundation of Korea [NRF-Korea, NRF-2018M2A2B3A01071859]. JWL and YSO were supported by the Basic Science Support Research Fund [1.200088.01] of Ulsan National Institute of Science & Technology (UNIST). Magnetic characterization was conducted at the core research facility of Pusan national university funded by the Korea Ministry of Education.References
- S. Mantl, Mater. Sci. Rep., 1992, 8, 1 Search PubMed.
- S. J. Pearton, C. R. Abernathy, M. E. Overberg, G. T. Thaler, D. P. Norton, N. Theodoropoulou, A. F. Hebard, Y. D. Park, F. Ren, J. Kim and L. A. Boatner, J. Appl. Phys., 2003, 93, 1 Search PubMed.
- M. V. Rao, P. Griffiths, O. W. Holland, G. Kelner, J. A. Freitas, D. S. Simons, P. H. Chi and M. Ghezzo, J. Appl. Phys., 1995, 77, 2479 Search PubMed.
- S. Basu, B. J. Lee and Z. M. Zhang, J. Heat Transfer, 2010, 132, 023301 Search PubMed.
- N. Theodoropoulou, A. F. Hebard, M. E. Overberg, C. R. Abernathy, S. J. Pearton, S. N. G. Chu and R. G. Wilson, Phys. Rev. Lett., 2002, 89, 107203 Search PubMed.
- M. Bolduc, C. Awo-Affouda, A. Stollenwerk, M. B. Huang, F. G. Ramos, G. Agnello and V. P. LaBella, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 033302 Search PubMed.
- S. Q. Zhou, K. Potzger, J. von Borany, R. Grotzschel, W. Skorupa, M. Helm and J. Fassbender, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 035209 Search PubMed.
- G. Franzo, F. Priolo, S. Coffa, A. Polman and A. Carnera, Appl. Phys. Lett., 1994, 64, 2235 Search PubMed.
- A. Polman, J. Appl. Phys., 1997, 82, 1 Search PubMed.
- O. Diwald, T. L. Thompson, E. G. Goralski, S. D. Walck and J. T. Yates, J. Phys. Chem. B, 2004, 108, 52 Search PubMed.
- M. A. Reshchikov and H. Morkoc, J. Appl. Phys., 2005, 97, 061301 Search PubMed.
- Y. F. Jiang, M. Al Mehedi, E. G. Fu, Y. Q. Wang, L. F. Allard and J. P. Wang, Sci. Rep., 2016, 6, 25436 Search PubMed.
- M. Lyutaya, Sov. Powder Metall Met. Ceram., 1979, 18, 190 Search PubMed.
- D. McKay, J. S. J. Hargreaves, J. L. Rico, J. L. Rivera and X. L. Sun, J. Solid State Chem., 2008, 181, 325 Search PubMed.
- C. X. Quintela, J. P. Podkaminer, M. N. Luckyanova, T. R. Paudel, E. L. Thies, D. A. Hillsberry, D. A. Tenne, E. Y. Tsymbal, G. Chen, C. B. Eom and F. Rivadulla, Adv. Mater., 2015, 27, 3032 Search PubMed.
- K. Inumaru, K. Baba and S. Yamanaka, Chem. Mater., 2005, 17, 5935 Search PubMed.
- Y. Y. Zhang, N. Haberkorn, F. Ronning, H. Y. Wang, N. A. Mara, M. J. Zhuo, L. Chen, J. H. Lee, K. J. Blackmore, E. Bauer, A. K. Burrell, T. M. McCleskey, M. E. Hawley, R. K. Schulze, L. Civale, T. Tajima and Q. X. Jia, J. Am. Chem. Soc., 2011, 133, 20735 Search PubMed.
- I. Jauberteau, A. Bessaudou, R. Mayet, J. Cornette, J. L. Jauberteau, P. Carles and T. Merle-Mejean, Coatings, 2015, 5, 656 Search PubMed.
- Y. Shi, B. Zhao, Y. Zhao, L. Li and J. Liu, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 38, 4488 Search PubMed.
- J. G. Choi, D. Choi and L. T. Thompson, J. Mater. Res., 1992, 7, 374 Search PubMed.
- S. M. Wang, D. Antonio, X. H. Yu, J. Z. Zhang, A. L. Cornelius, D. W. He and Y. S. Zhao, Sci. Rep., 2015, 5, 13733 Search PubMed.
- M. Björck and G. Andersson, J. Appl. Crystallogr., 2007, 40, 1174 Search PubMed.
- C. L. Bull, T. Kawashima, P. F. McMillan, D. Machon, O. Shebanova, D. Dalsenberger, E. Soignard, E. Takayama-Muromachi and L. C. Chapon, J. Solid State Chem., 2006, 179, 1762 Search PubMed.
- W. X. Li, Y. H. Shen, Y. Q. Zhou, S. Nan, C. H. Chen and R. C. Ewing, Sci. Rep., 2017, 7, 10 Search PubMed.
- A. V. Krasheninnikov and K. Nordlund, J. Appl. Phys., 2010, 107, 70 Search PubMed.
- A. Belattar, G. A. Stephens and P. D. Cardwell, Nucl. Instrum. Methods Phys. Res., Sect. B, 1994, 93, 261 Search PubMed.
- D. Choi and P. N. Kumta, J. Am. Ceram. Soc., 2011, 94, 2371 Search PubMed.
- L. Palmetshofer and P. Rodhammer, Nucl. Instrum. Methods Phys. Res., Sect. B, 1993, 80–1, 340 Search PubMed.
- J. D. Wu, C. Z. Wu, X. X. Zhong, Z. M. Song and F. M. Li, Surf. Coat. Technol., 1997, 96, 330 Search PubMed.
- Y. M. Wang and R. Y. Lin, Mater. Sci. Eng. B Solid State Mater. Adv. Technol., 2004, 112, 42 Search PubMed.
- L. Stober, J. P. Konrath, S. Krivec, F. Patocka, S. Schwarz, A. Bittner, M. Schneider and U. Schmid, J. Micromech. Microeng., 2015, 25, 11 Search PubMed.
- V. P. Anitha, S. Major, D. Chandrashekharam and M. Bhatnagar, Surf. Coat. Technol., 1996, 79, 50 Search PubMed.
- P. Hones, N. Martin, M. Regula and F. Levy, J. Phys. D: Appl. Phys., 2003, 36, 1023 Search PubMed.
- X. D. Zhu, D. Yue, C. Shang, M. T. Fan and B. Hou, Surf. Coat. Technol., 2013, 228, S184 Search PubMed.
- E. P. Donovan, G. K. Hubler, M. S. Mudholkar and L. T. Thompson, Surf. Coat. Technol., 1994, 66, 499 Search PubMed.
- S. Mandl, D. Manova, J. W. Gerlach, W. Assmann, H. Neumann and B. Rauschenbach, Surf. Coat. Technol., 2004, 180, 362 Search PubMed.
- D. Manova, Y. Bohne, J. W. Gerlach, S. Mandl, H. Neumann and B. Rauschenbach, Nucl. Instrum. Methods Phys. Res., Sect. B, 2005, 240, 208 Search PubMed.
- K. Inumaru, T. Nishikawa, K. Nakamura and S. Yamanaka, Chem. Mater., 2008, 20, 4756 Search PubMed.
- K. Inumaru, K. Baba and S. Yamanaka, Appl. Surf. Sci., 2006, 253, 2863 Search PubMed.
- H. M. Luo, G. F. Zou, H. Y. Wang, J. H. Lee, Y. Lin, H. S. Peng, Q. L. Lin, S. G. Deng, E. Bauer, T. M. McCleskey, A. K. Burrell and Q. X. Jia, J. Phys. Chem. C, 2011, 115, 17880 Search PubMed.
- H. Ihara, Y. Kimura, K. Senzaki, H. Kezuka and M. Hirabayashi, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 31, 3177 Search PubMed.
- K. Saito and Y. Asada, J. Phys. F: Met. Phys., 1987, 17, 2273 Search PubMed.
- T. Geballe, B. Matthias, E. Corenzwit and G. Hull Jr, Phys. Rev. Lett., 1962, 8, 313 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08533b |
| This journal is © The Royal Society of Chemistry 2020 |