Open Access Article
Open Access ArticleDesmoschinensisflavones A and B, two rare flavones having a hybrid benzyl benzoate ester-flavone structural framework from Desmos chinensis Lour†
Isaraporn Polbuppha ab,
Virayu Suthiphasilp
ab,
Virayu Suthiphasilp a,
Tharakorn Maneeratac,
Rawiwan Charoensupcd,
Thunwadee Limtharakul
a,
Tharakorn Maneeratac,
Rawiwan Charoensupcd,
Thunwadee Limtharakul e,
Sarot Cheenpracha
e,
Sarot Cheenpracha f,
Stephen G. Pyne
f,
Stephen G. Pyne *b and
Surat Laphookhieo
*b and
Surat Laphookhieo *ac
*ac
aCenter of Chemical Innovation for Sustainability (CIS) and School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand. E-mail: surat.lap@mfu.ac.th
bSchool of Chemistry and Molecular Bioscience, University of Wollongong, Wollongong, New South Wales 2522, Australia. E-mail: spyne@uow.edu.au
cMedicinal Plant Innovation Center of Mae Fah Luang University, Chiang Rai 57100, Thailand
dSchool of Integrative Medicine, Mae Fah Luang University, Chiang Rai 57100, Thailand
eDepartment of Chemistry, Faculty of Science, Research Center on Chemistry for Development of Health Promoting Products from Northern Resources, Chiang Mai University, Chiang Mai 50200, Thailand
fSchool of Science, University of Phayao, Phayao 56000, Thailand
First published on 21st December 2020
Abstract
Two rare flavones having a hybrid benzyl benzoate ester-flavone structural framework, desmoschinensisflavones A and B (1 and 2), together with 12 known compounds (3–14) were isolated from the fruit, leaf, and twig extracts of Desmos chinensis (red flower). The new structures were characterized by UV, IR, NMR, and HRESITOFMS data. Desmoschinensisflavones A and B have a distinctive skeleton of benzoate ester-flavones with a C-4′′ and C-6 and C-8 connection via a methylene group, respectively. Plausible biosynthesis pathways to compounds 1 and 2 are proposed based on an intermolecular nucleophilic 1,4-addition to ortho-quinone intermediates. Compounds 6–8 and 12 showed weakly antioxidant inhibition with IC50 values in the range of 65.4–74.6 μM.
Introduction
The genus Desmos belongs to the Annonaceae family. These plants are a rich source of flavonoids, alkaloids, benzoate esters, chalcones, and oxepinones.1–4 Hybrid-flavonoids have rarely been reported from this genus, such as biflavones from D. chinensis1 and flavan-chalcones from D. cochinchinensis.5 Several species of this genus have been used in Chinese folk medicines.4 For example, the roots of D. cochinchinensis have been used as an antimalarial,6 while the stem and root of D. dumosus have mainly been used to treat muscle pain in Thai traditional medicine.7 Desmos chinensis Lour. is a large climbing shrub, known in the local name (Thailand) as ‘Sai-Yud’, which is distributed widely in Southern and South East Asian countries.1 The root part of D. chinensis has been popularly used as treatment of various conditions in Southern and South East Asia. For example, the decoction of D. chinensis roots has been used for the treatment of malaria.8 In India, Malaysia, and Thailand, the roots of D. chinensis have been used to treat diarrhea, dysentery, fever, parturition vertigo, and postpartum pot herbs.1,9 Previous phytochemical studies on D. chinensis produced several types of compounds, including biflavones,1 benzyl benzoate esters,10 C-benzylated chalcones,9 oxoaporphine alkaloids,10 and flavonoids.11 Some of these compounds showed antimicrobial,12 antifungal,11 antitumor, and acute toxic activities.13Of the three different flowering species of D. chinensis growing at Mae Fah Luang University, the red, yellow, and giant flowering forms, herein, we report the isolation and structure elucidation of two new hybrid benzoate ester-flavones (1 and 2) together with 12 known compounds (3–14) isolated from the fruit, leaf, and twig extracts of the red flowering species of D. chinensis. This is the first report on the isolation of natural products having a hybrid benzyl benzoate ester-flavone skeleton from the Annonaceae family to the best of our knowledge. The antioxidant and α-glucosidase inhibitory properties of some of the compounds are also reported.
Results and discussion
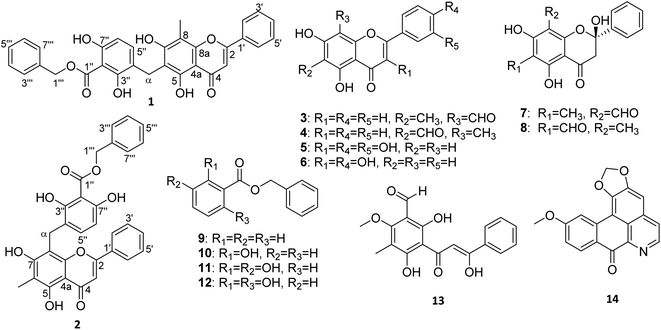
Fourteen compounds, including the hybrid benzyl benzoate ester-flavones 1 and 2, flavonoids (3–8), benzyl benzoate esters (9–12), chalcone (13), and alkaloid (14) were isolated and characterized from the extract of the fruits, leaves, and twigs of D. chinensis. Compounds 1 and 2 are new hybrids of a benzyl benzoate ester and a flavone. The known compounds were identified as isounonal (3),14 unonal (4),14 quercetin (5),15 kaempferol (6),15 desmal (7),8 6-formyl-2,5,7-trihydroxy-8-methylflavanone (8),16 benzyl benzoate (9),17 benzyl-2-hydroxybenzoate (10),17 benzyl-2,3-dihydroxy benzoate (11),18 benzyl-2,6-dihydroxybenzoate (12),17 desmosdumotin D (13),19 and lauterine (14)20 by comparisons with the literature reported spectroscopic data (Fig. 1).Compound 1 was obtained as a yellow powder (mp. 180–183 °C) from the leaf extract. The HR-ESI mass spectrum of 1 showed a negative [M − H]− ion peak at m/z 523.1381 (calcd for 523.1393), corresponding to a molecular formula of C31H24O8 with 20 degrees of unsaturation. The UV absorption maxima at λmax 252, 279, and 331 nm suggested a flavone skeleton,1 while the IR spectrum displayed the characteristics of hydroxy absorption bands at 3442 and 3375 cm−1 and carbonyl absorption bands at 1727 (ester functionality) and 1659 (conjugate ketone functionality) cm−1. The 13C and DEPT 135 NMR spectroscopic data of 1 (Table 1) indicated 31 carbons, including two carbonyls, thirteen methines, two methylenes, one methyl, and thirteen quaternary carbons.
| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δC | δH (mult J in Hz) | HMBC (1H → 13C) | δC | δH (mult J in Hz) | HMBC (1H → 13C) | |
| Flavone skeleton | ||||||
| 2 | 163.4 | 163.3 | ||||
| 3 | 105.1 | 6.65 (s) | 2, 4, 4a, 1′ | 104.1 | 6.70 (s) | 2, 4, 4a |
| 4 | 182.9 | 182.7 | ||||
| 4a | 105.0 | 105.0 | ||||
| 5 | 157.1 | 157.7 | ||||
| 6 | 110.4 | 106.0 | ||||
| 7 | 158.9 | 159.2 | ||||
| 8 | 103.1 | 104.7 | ||||
| 8a | 153.7 | 152.8 | ||||
| α | 21.1 | 3.93 (s) | 5, 6, 7, 4′′, 5′′ | 29.7 | 4.13 (s) | 7, 8, 8a, 3′′, 4′′, 5′′ |
| 1′ | 131.6 | 131.7 | ||||
| 2′ | 126.2 | 7.90 (dd, 7.5, 1.9) | 2, 1′ | 126.2 | 7.91 (dd, 7.4, 2.0) | 2 |
| 3′ | 129.2 | 7.48–7.56 (m) | 2′, 4′ | 129.1 | 7.58–7.59 (m) | 4′ |
| 4′ | 131.8 | 7.48–7.56 (m) | 2′, 3′, 5′ | 131.8 | 7.58–7.59 (m) | |
| 5′ | 129.2 | 7.48–7.56 (m) | 2′, 4′ | 129.1 | 7.58–7.59 (m) | 4′ |
| 6′ | 126.2 | 7.90 (dd, 7.5, 1.9) | 2, 1′ | 126.2 | 7.91 (dd, 7.4, 2.0) | 2 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Benzyl benzoate-ester skeleton | ||||||
| 1′′ | 169.7 | 169.6 | ||||
| 2′′ | 99.5 | 99.8 | ||||
| 3′′ | 156.1 | 154.5 | ||||
| 4′′ | 118.8 | 118.2 | ||||
| 5′′ | 139.5 | 7.77 (d, 8.6) | α, 3′′, 7′′ | 138.0 | 7.43–7.45 (m) | |
| 6′′ | 109.7 | 6.51 (d, 8.6) | 2′′, 7′′ | 109.7 | 6.39 (d, 8.6) | 2′′, 4′′ |
| 7′′ | 159.0 | 158.1 | ||||
| 1′′′ | 68.6 | 5.52 (s) | 1′′, 2′′′, 3′′′ | 68.8 | 5.54 (s) | 1′′, 2′′′, 3′′′ |
| 2′′′ | 133.6 | 133.3 | ||||
| 3′′′ | 128.8 | 7.40–7.48 (m) | 2′′′ | 128.9 | 7.43–7.45 (m) | |
| 4′′′ | 129.1 | 7.40–7.48 (m) | 129.3 | 7.43–7.45 (m) | ||
| 5′′′ | 129.4 | 7.40–7.48 (m) | 3′′′, 7′′′ | 129.5 | 7.43–7.45 (m) | |
| 6′′′ | 129.1 | 7.40–7.48 (m) | 129.3 | 7.43–7.45 (m) | ||
| 7′′′ | 128.8 | 7.40–7.48 (m) | 2′′′ | 128.9 | 7.43–7.45 (m) | |
| OH-5 | 13.10 (s) | 4a, 5, 6 | 12.94 (s) | |||
| CH3-6 | 7.7 | 2.15 (s) | 5, 6, 7 | |||
| OH-7 | 8.39 (s) | 7, 8 | 8.19 (s) | 8 | ||
| CH3-8 | 8.1 | 2.32 (s) | 7, 8, 8a | |||
Analysis of the 1H and 13C NMR spectroscopic data of 1 (Table 1) suggested that this compound had a hybrid structure of a benzyl benzoate ester and a flavone. 1H and 13C NMR spectroscopic data of the flavone unit (Table 1) displayed resonances for hydroxy protons at δH 8.39 (1H, s, OH-7) and 13.10 (1H, s, OH-5), a monosubstituted aromatic ring at δH 7.90 (2H, dd, J = 7.5, 1.9 Hz, H-2′,6′)/δC 126.2 and 7.48–7.56 (3H, m, H-3′,5′/H-4′)/δC 129.2/131.8, isolated aliphatic protons at δH 3.93 (2H, s, H-α)/δC 21.1, an olefinic proton at δH 6.65 (2H, s, H-2)/δC 105.1, and a methyl group at δH 2.32 (3H, s, CH3-8)/δC 8.1.
The 1H and 13C NMR spectroscopic data of the benzyl benzoate ester unit (Table 1) displayed resonances for a monosubstituted aromatic ring at δH 7.40–7.48 (5H, m, H-3′′′,7′′′/H-4′′′,6′′′/H-5′′′)/δC 128.8/129.1/129.4, ortho-coupled aromatic protons at δH 6.51 (1H, d, J = 8.6 Hz, H-6′′)/δC 109.7 and 7.77 (1H, d, J = 8.6 Hz, H-5′′)/δC 139.5 and a set of isolated aliphatic protons at δH 5.52 (2H, s, H-1′′′)/δC 68.6.
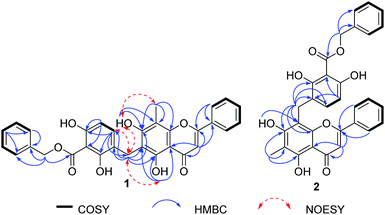
The HMBC correlations (Fig. 2) of the flavone unit indicated that the methyl group was located at C-8 from the HMBC correlations of the methyl proton CH3-8 (δH 2.32) to δC 158.9 (C-7), 103.1 (C-8), and 153.7 (C-8a). The hydroxy group was located at C-7 from the HMBC correlations of the hydroxy proton OH-7 (δH 8.39) to δC 158.9 (C-7) and 103.1 (C-8). The hydrogen-bonded hydroxy proton was located at C-5 from the HMBC correlations of OH-5 (δH 13.10) to δC 105.0 (C-4a), 157.1 (C-5) and 110.4 (C-6) (Fig. 2). Furthermore, the ortho-coupled aromatic protons of the benzoate ester unit were assigned by the HMBC correlations of H-5′′ (δH 7.77) to δC 21.1 (C-α), 156.1 (C-3′′), and 159.0 (C-7′′). However, the key HMBC correlation of H-α (δH 3.93) to δC 157.1 (C-5), 110.4 (C-6), 158.9 (C-7) 118.8 (C-4′′), and 139.5 (C-5′′) clearly indicated the linkage between the benzyl benzoate ester and the flavone moieties. The NOESY correlations between OH-5 (δH 13.10), OH-7 (δH 8.39), H-5′′ (δH 7.77) to methylene protons (H-α, δH 3.93) further confirmed the C-α linkage between these two structural moieties. Therefore, compound 1 was named as desmoschinensisflavone A.
Compound 2 was obtained as a yellow powder (mp. 179–181 °C) from the leaf extract and had the molecular ion peak at m/z 523.1402 [M − H]− (calcd m/z for C31H23O8, 523.1393) based on the HRESITOFMS and 13C NMR spectroscopic data. The 1H and 13C NMR spectroscopic data of 2 (Table 1) were similar to those of 1, except for the replacement of the methyl group at C-8 by a benzyl benzoate ester unit, and attachment of the methyl group at C-6. The HMBC correlations from CH3-6 at δH 2.15 (3H, s) to δC 157.7 (C-5), 106.0 (C-6), and 159.2 (C-7), indicated that the methyl group was attached to the C-6 position. Moreover, the location of benzyl benzoate ester unit was determined to be at the C-8 position based on the HMBC correlations between H-α at δH 4.13 (2H, s) to δC 159.2 (C-7), 104.7 (C-8), 152.8 (C-8a), 154.5 (C-3′′), 118.2 (C-4′′), and 138.0 (C-5′′) (Fig. 2). Thus, structure 2 was identified as a desmoschinensisflavone B.
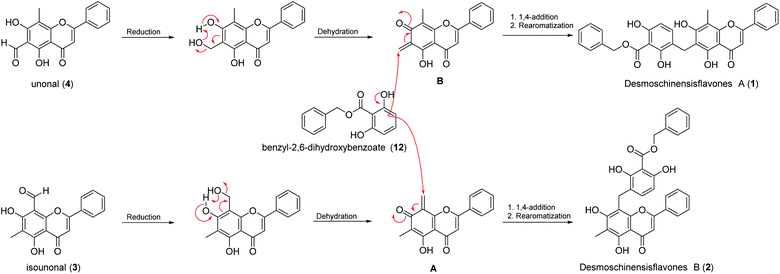
Putative biogenetic pathways to compounds 1 and 2 are shown in Scheme 1. The reduction of the isolated compounds 3 and 4 followed by dehydration would produce the ortho-quinone methide intermediates A and B, respectively. Desmoschinensisflavone A (1) could be derived from intermediate B through coupling with benzyl benzoate 12 via a 1,4-addition reaction followed by rearomatization, whereas desmoschinensisflavone B (2) could be obtained from intermediate A by coupling with benzyl benzoate 12. The isolation of compounds 3, 4, and 12 in this study would support the proposed putative biogenetic pathways of compounds 1 and 2.
Only compounds isolated in sufficient amounts and that were stable (compounds 1, 3, 4, 6–8, 12, and 13) were evaluated for their antioxidant activities against DPPH and ABTS and α-glucosidase inhibitory activity. Compounds 6, a mixture of 7 and 8, and 12 in the DPPH radical scavenging assay had modest IC50 values of 74.6, 65.4, and 65.5 μM, respectively. While the mixture 7 and 8 in the ABTS•+ scavenging assay showed weak antioxidant activity with an IC50 value of 259.3 μM, while compounds 6 and 12 were inactive in this assay. All tested compounds showed weaker antioxidant activities than ascorbic acid which was used as the positive control (IC50 = 15.9 (DPPH) and 8.2 (ABTS) μM). None of the compounds 1, 3, 4, 6–8, 12, and 13 showed inhibitory activities against α-glucosidase at 200 μg mL−1.
Conclusions
The Desmos genus (Annonaceae) is well known as a rich source of flavonoids. Some minor compounds such as chalcones, benzyl benzoate esters, and alkaloids have also been identified. In this study, eight flavones (1–8), four benzyl benzoate esters (9–12), one chalcone (13), and one alkaloid (14) were isolated and identified from the red flowering species of D. chinensis. To the best of our knowledge, this is the first discovery of unique hybrids of a benzyl benzoate ester and a flavone (1 and 2) from the Annonaceae family. The putative biogenetic pathways of compounds 1 and 2 is proposed via two ortho-quinone methide intermediates of the precursor 3 or 4 coupling with benzyl benzoate ester 12. Some of the isolated compounds from this study were also evaluated for their antioxidant activities and their α-glucosidase inhibitory activities. Unfortunately, none of them showed significant activities.Materials and methods
General experimental procedures
The IR spectra were recorded using a PerkinElmer FTS FT-IR spectrometer and SHIMADSU spectrophotometer. UV-spectra has been recorded with a PerkinElmer or Varian Cary 5000 UV-vis NIR spectrophotometer. The melting point was determined using a Gallenkamp melting point apparatus. The NMR spectra were recorded using a 400 MHz Bruker AM400 spectrometer and 400 MHz Bruker FTNMR Ultra Shield with tetramethylsilane as the internal standard. HRESIMS mass spectra measured on a Bruker micro TOF mass spectrometer. Quick column chromatography (QCC) and column chromatography (CC) were carried out on silica gel 60 (5–40 μm, SiliCycle® Inc.) and silica gel 100 (63–200 μm, SiliCycle® Inc.), respectively. Sephadex LH-20, when indicated, was also used for CC. Precoated thin-layer chromatography (TLC) was performed using silica gel 60 F254 plates (Merck, USA).Plant material
The fruit, leaves, and twigs of D. chinensis were collected from Mae Fah Luang University Botanical Garden, Chiang Rai, Thailand (N: 20.0397°, E: 99.8938°) in June 2017. Plant authentication was verified by Assoc. Prof. Dr Surat Laphookhieo, and a voucher specimen (No. MFU-NPR0162) was deposited at the Natural Products Research Laboratory, School of Science, Mae Fah Luang University.Extraction and isolation
Air-dried leaves of D. chinensis (1.2 kg) were extracted with EtOAc for 3 days at room temperature and concentrated under reduced pressure to give an EtOAc extract (161.2 g). The leaf extract (161.2 g) was subjected to quick column chromatography (QCC) over silica gel (100% hexanes to 100% EtOAc) to give seven fractions (1LA–1LG). Fraction 1LB (24.7 g) was subjected to CC over Sephadex LH-20 (100% MeOH) to give six subfractions (2LA–2LF). Separation of subfraction 2LC (5.53 g) using CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, acetone–hexanes) gave six subfractions (3LA–3LF). Subfraction 3LA (20.8 mg) was further purified by CC (1
9 v/v, acetone–hexanes) gave six subfractions (3LA–3LF). Subfraction 3LA (20.8 mg) was further purified by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v, acetone–hexanes) yielding compound 10 (13.3 mg), while subfraction 3LC (127.1 mg) was subjected to CC (1
4 v/v, acetone–hexanes) yielding compound 10 (13.3 mg), while subfraction 3LC (127.1 mg) was subjected to CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, CH2Cl2–hexanes) to give compound 11 (15.2 mg) together with five subfractions (4LA–4LE). Subfraction 4LC (19.1 mg) was further purified by CC (7
1 v/v, CH2Cl2–hexanes) to give compound 11 (15.2 mg) together with five subfractions (4LA–4LE). Subfraction 4LC (19.1 mg) was further purified by CC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, CH2Cl2–hexanes) to provide compound 13 (9.9 mg). Fraction 3LF (781.8 mg) was further separated by CC (100% CH2Cl2) to afford three subfractions (5LA–5LC). Subfraction 5LC (209.1 mg) was further separated by CC (1
3 v/v, CH2Cl2–hexanes) to provide compound 13 (9.9 mg). Fraction 3LF (781.8 mg) was further separated by CC (100% CH2Cl2) to afford three subfractions (5LA–5LC). Subfraction 5LC (209.1 mg) was further separated by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v, acetone–hexanes) to give compound 3 (26.1 mg). Fraction 2LD (7.15 g) was subjected to QCC (1
4 v/v, acetone–hexanes) to give compound 3 (26.1 mg). Fraction 2LD (7.15 g) was subjected to QCC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, EtOAc–hexanes) to give six subfractions (6LA–6LF). Subfraction 6LB (529.8 mg) was chromatographed by CC (1
9 v/v, EtOAc–hexanes) to give six subfractions (6LA–6LF). Subfraction 6LB (529.8 mg) was chromatographed by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 v/v, MeOH–CH2Cl2) to provide compound 4 (5.1 mg) together with five subtractions (7LA–7LE). A mixture of compounds 7 and 8 (23.8 mg) was obtained from 7LE (205.4 mg) by CC (2
99 v/v, MeOH–CH2Cl2) to provide compound 4 (5.1 mg) together with five subtractions (7LA–7LE). A mixture of compounds 7 and 8 (23.8 mg) was obtained from 7LE (205.4 mg) by CC (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, EtOAc–hexanes). Fraction 6LD (116.1 mg) was chromatographed over silica gel CC (3
3 v/v, EtOAc–hexanes). Fraction 6LD (116.1 mg) was chromatographed over silica gel CC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v, CH2Cl2–hexanes) to give compound 1 (4.1 mg) together with five subfractions (8LA–8LE), while compound 2 (1.8 mg) was obtained from 8LB (16.1 mg) by CC (4
2 v/v, CH2Cl2–hexanes) to give compound 1 (4.1 mg) together with five subfractions (8LA–8LE), while compound 2 (1.8 mg) was obtained from 8LB (16.1 mg) by CC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, CH2Cl2–hexanes). Fraction 1LE (1.3 g) was isolated by CC over Sephadex LH-20 to give compound 5 (1.1 mg) together with eight subfractions (9LA–9LH). Subfraction 9LE (80.9 mg) was further separated by CC (1
1 v/v, CH2Cl2–hexanes). Fraction 1LE (1.3 g) was isolated by CC over Sephadex LH-20 to give compound 5 (1.1 mg) together with eight subfractions (9LA–9LH). Subfraction 9LE (80.9 mg) was further separated by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, acetone–hexanes) to give compound 6 (2.7 mg). Subfraction 1LG (5.3 g) was chromatographed by CC (3
1 v/v, acetone–hexanes) to give compound 6 (2.7 mg). Subfraction 1LG (5.3 g) was chromatographed by CC (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v, acetone–hexanes) to afford ten subfractions (10LA–10LJ). After that, compound 14 (1.3 mg) was obtained from 10LB (4.2 mg) by CC (1
7 v/v, acetone–hexanes) to afford ten subfractions (10LA–10LJ). After that, compound 14 (1.3 mg) was obtained from 10LB (4.2 mg) by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, CH2Cl2–hexanes).
9 v/v, CH2Cl2–hexanes).
Air-dried fruit of D. chinensis (97.6 kg) were extracted with CH2Cl2 over a period of 3 days at room temperature and concentrated under reduced pressure to give an CH2Cl2 extract (18.0 g). The fruit extract (18.0 g) was subjected to CC over silica gel (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v, acetone–hexanes) to give seven subfractions (1FA–1FG). Fraction 1FC (1.5 g) was purified by CC over Sephadex LH-20 (100% MeOH) to afford compound 11 (3.4 mg), compound 12 (9.2 mg) together with five subfractions (2FA–2FE). While compound 7 (1.3 mg) was obtained from 2FC (14.8 mg) by HPLC RP C18 (2
7 v/v, acetone–hexanes) to give seven subfractions (1FA–1FG). Fraction 1FC (1.5 g) was purified by CC over Sephadex LH-20 (100% MeOH) to afford compound 11 (3.4 mg), compound 12 (9.2 mg) together with five subfractions (2FA–2FE). While compound 7 (1.3 mg) was obtained from 2FC (14.8 mg) by HPLC RP C18 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, ACN–H2O with 0.05% TFA, 2 mL min−1). Subfraction 1FE (1.5 g) was separated by CC (1
3 v/v, ACN–H2O with 0.05% TFA, 2 mL min−1). Subfraction 1FE (1.5 g) was separated by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, acetone–hexanes) to give eight subfractions (3FA–3FH). Compounds 3 (3.0 mg) and 4 (4.1 mg), were obtained from 3FF (439.2 mg) by CC (1
9 v/v, acetone–hexanes) to give eight subfractions (3FA–3FH). Compounds 3 (3.0 mg) and 4 (4.1 mg), were obtained from 3FF (439.2 mg) by CC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v, acetone–hexanes).
9 v/v, acetone–hexanes).
Air-dried twigs of D. chinensis (1.39 kg) were extracted with EtOAc for 3 days at room temperature and concentrated under reduced pressure to give an EtOAc extract (29.9 g). The twig extract (29.9 g) was subjected to QCC over silica gel (100% hexanes to 100% acetone) to afford six subfractions (1TA–1TF). Fraction 1 TB (2.6 g) was subjected to CC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, CH2Cl2–hexanes) to give six subfractions (2TA–2TF). Fraction 2 TB (198.1 mg) was purified by CC (7
3 v/v, CH2Cl2–hexanes) to give six subfractions (2TA–2TF). Fraction 2 TB (198.1 mg) was purified by CC (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v, CH2Cl2–hexanes) to afford compound 11 (3.9 mg) together with four subfractions (3TA–3TD). Purification of subfraction 3 TC (37.1 mg) yielded compound 4 (2.1 mg) by CC (4
3 v/v, CH2Cl2–hexanes) to afford compound 11 (3.9 mg) together with four subfractions (3TA–3TD). Purification of subfraction 3 TC (37.1 mg) yielded compound 4 (2.1 mg) by CC (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, CH2Cl2–hexanes), while subfraction 3TD (170.9 mg) was separated by CC (100% CH2Cl2) to provide compound 3 (2.7 mg).
1 v/v, CH2Cl2–hexanes), while subfraction 3TD (170.9 mg) was separated by CC (100% CH2Cl2) to provide compound 3 (2.7 mg).
Desmoschinensisflavone A (1) yellow powder; mp. 180–183 °C; UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 252 (3.10), 280 (3.06), and 331 (2.96) nm; IR (neat) vmax 3442, 3375, 2923, 2852, 1779, 1727, 1659, and 1129 cm−1; 1H and 13C NMR spectroscopic data, see Table 1; HRESITOFMS m/z 523.1381 [M − H]− (calcd m/z for C31H23O8, 523.1393).
ε) 252 (3.10), 280 (3.06), and 331 (2.96) nm; IR (neat) vmax 3442, 3375, 2923, 2852, 1779, 1727, 1659, and 1129 cm−1; 1H and 13C NMR spectroscopic data, see Table 1; HRESITOFMS m/z 523.1381 [M − H]− (calcd m/z for C31H23O8, 523.1393).
Desmoschinensisflavone B (2) yellow powder; mp. 179–181 °C; UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 255 (3.00), 277 (3.01), and 329 (2.92) nm; IR (neat) vmax 3442, 2955, 2923, 2852, 1787, 1728, 1660, and 1112 cm−1; 1H and 13C NMR spectroscopic data, see Table 1; HRESITOFMS m/z 523.1402 [M − H]− (calcd m/z for C31H23O8, 523.1393).
ε) 255 (3.00), 277 (3.01), and 329 (2.92) nm; IR (neat) vmax 3442, 2955, 2923, 2852, 1787, 1728, 1660, and 1112 cm−1; 1H and 13C NMR spectroscopic data, see Table 1; HRESITOFMS m/z 523.1402 [M − H]− (calcd m/z for C31H23O8, 523.1393).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thailand Science Research and Innovation supported this work through the Direct Basic Research Grant (DBG6280007 and DBG6180029) and a full Ph. D. scholarship from the Thailand Research Fund through the Royal Golden Jubilee Ph. D. program (PHD/0098/2560). TL thanks Chiang Mai University for partially supports. Mae Fah Luang University and the University of Wollongong are also acknowledged for laboratory facilities.References
- T. Rittiwong, T. Mutarapat, C. Ponglimanont, W. Mahabusarakam and S. Chakthong, Tetrahedron, 2011, 67, 5444–5449 Search PubMed.
- P. Meesakul, C. Richardson, S. G. Pyne and S. Laphookhieo, J. Nat. Prod., 2019, 82, 741–747 Search PubMed.
- M. Sulaiman, M. T. Martin, M. Pais, A. H. A. Hadi and K. Awang, Phytochemistry, 1998, 49, 2191–2192 Search PubMed.
- J. Wu, X. Wang, Y. Yi and K. Lee, Bioorg. Med. Chem. Lett., 2003, 13, 1813–1815 Search PubMed.
- S. P. Bajgai, V. Prachyawarakorn, C. Mahidol and S. Ruchirawat, Phytochemistry, 2011, 72, 2062–2067 Search PubMed.
- T. Wu, Y. Cheng, F. Cheng, Y. Hsu, T. T. Dinh, F. Chang and Y. Wu, Helv. Chim. Acta, 2014, 97, 1714–1718 Search PubMed.
- W. Chuakul, Thai Pharm. Health Sci. J, 2010, 5, 1–13 Search PubMed.
- H. Kakeya, M. Imoto, Y. Tabata, J. Iwami, H. Matsumoto, K. Nakamura, T. Koyano, K. Tadano and K. Umezawa, FEBS Lett., 1993, 320, 169–172 Search PubMed.
- M. M. Rahman, N. Qais and M. A. Rashid, Fitoterapia, 2003, 74, 511–514 Search PubMed.
- P. V. Kiem, C. V. Minh, H. T. Huong, J. J. Lee, I. M. Lee and Y. H. Kim, Arch. Pharmacal Res., 2005, 28, 1345–1349 Search PubMed.
- M. Tuntipaleepun, S. Chakthong, C. Ponglimanont, P. Plodpai and S. P. Voravuthikunchai, Chin. Chem. Lett., 2012, 23, 587–590 Search PubMed.
- S. Kummee and N. Intaraksa, Songklanakarin J. Sci. Technol., 2008, 30, 635–639 Search PubMed.
- K. Nakanishi, S. Sasaki, A. K. Kiang, J. Goh, H. Kakisawa, M. Ohashi, M. Goto, J. Watanabe, H. Yokotani, C. Matsumura and M. Togashi, Chem. Pharm. Bull., 1965, 13, 882–890 Search PubMed.
- J. Wang, M. Ji, H. Shu, G. Chen, X. Song and J. Wang, J. Nat. Med., 2012, 10, 303–306 Search PubMed.
- Y. Chang, F. Chang and Y. Wu, J. Chin. Chem. Soc., 2000, 47, 373–380 Search PubMed.
- J. Chopin, M. Hauteville, B. S. Joshi and D. H. Gwad, Phytochemistry, 1978, 17, 332–334 Search PubMed.
- M. Kodpinid, C. Sadavongvivad, C. Thebtaranonth and Y. Thebtaranonth, Phytochemistry, 1984, 23, 199–200 Search PubMed.
- B. Rivero-Cruz, I. Rivero-Cruz, R. Rodríguez-Sotres and R. Mata, Phytochemistry, 2007, 68, 1147–1155 Search PubMed.
- J. Wu, Faming. Zhuanli. Shenqing., CN 200610140637.6, 2006 Search PubMed.
- G. G. Harrigan, A. A. L. Gunatilaka and D. G. I. Kingston, J. Nat. Prod., 1994, 57, 68–73 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed explanation of NMR and HRESITOFMS spectra of 1 and 2 and bioactivities. See DOI: 10.1039/d0ra09985f |
| This journal is © The Royal Society of Chemistry 2020 |