Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Influence of Cu doping on the visible-light-induced photocatalytic activity of InVO4
Natda Wetchakun *a,
Pimonrat Wanwaena,
Sukon Phanichphant
*a,
Pimonrat Wanwaena,
Sukon Phanichphant b and
Khatcharin Wetchakun
b and
Khatcharin Wetchakun c
c
aDepartment of Physics and Materials Science, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand. E-mail: natda_we@yahoo.com
bMaterials Science Research Center, Faculty of Science, Chiang Mai University, Chiang Mai 50200, Thailand
cProgram of Physics, Faculty of Science, Ubon Ratchathani Rajabhat University, Ubon Ratchathani 34000, Thailand
First published on 13th October 2020
Abstract
Correction for ‘Influence of Cu doping on the visible-light-induced photocatalytic activity of InVO4’ by Natda Wetchakun et al., RSC Adv., 2017, 7, 13911–13918, DOI: 10.1039/C6RA27138C.
The authors regret errors in Fig. 4, 7, and 9 in the previously published article. The corrections for the errors in the article are described as follows:
(1) The diffuse reflectance spectra of pure InVO4 and 1.0 mol% Cu-doped InVO4 are shown in Fig. 4. The absorption margin of 1.0 mol% Cu-doped InVO4 was shifted to a longer wavelength, indicating a decrease in the band gap with respect to pure InVO4. The absorption margins of the pure InVO4 and 1.0 mol% Cu-doped InVO4 samples were 505 nm and 510 nm, corresponding to band gaps of 2.51 eV and 2.45 eV, respectively (Fig. 4a and b).
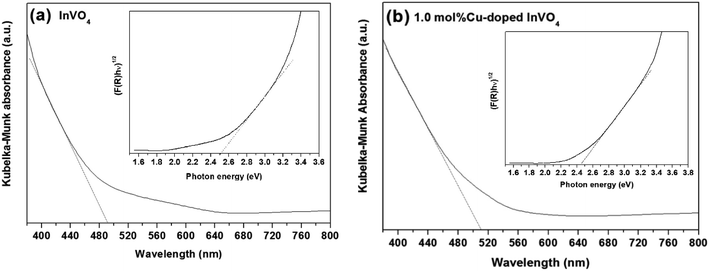 | ||
| Fig. 4 Kubelka–Munk absorbance spectra and band gaps (insets) of the pure InVO4 (a) and 1.0 mol% Cu-doped InVO4 (b) samples. | ||
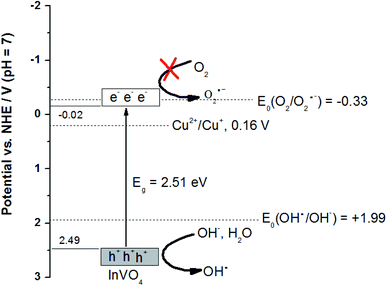
(2) The band edge positions of the conduction band (CB) and the valence band (VB) of InVO4 can be calculated by the following equation: E0CB = χ − EC − 0.5Eg,1 where χ is the electronegativity of the semiconductor, EC is the energy of free electrons on the hydrogen scale of 4.5 eV, Eg is the band gap of InVO4, and the χ value of InVO4 is 5.74 eV.2 The Eg value of InVO4 evaluated from the UV-vis DRS analysis was about 2.51 eV. The valence band energy (EVB) can be calculated by the following equation:3 EVB = ECB + Eg, where ECB is the conduction band energy. Based on the equation above, the calculated CB and VB edge potentials of InVO4 were −0.02 eV and 2.49 eV, respectively. Now, we are in a position to discuss the photocatalytic mechanism of Cu-doped InVO4 for MB degradation (Fig. 7). In the photocatalysis process, when the absorbed photon energy (hν) equals or exceeds the band gap, the Cu-doped InVO4 generates electron–hole (e−/h+) pairs. In that case, the generated electrons from the valence band can be transferred to the conduction band of InVO4. Since the CB edge potential of InVO4 (−0.02 eV) is higher than the standard redox potential, E0(O2/O2˙−) = −0.33 V vs. NHE at pH 7, this suggests that the electrons in the CB of InVO4 cannot reduce O2 to the superoxide radical ion (O2˙−). In addition, the VB of InVO4 (2.49 eV) is higher than the standard redox potential, E0(OH−/OH˙) = 1.99 V vs. NHE at pH 7. This indicates that the photogenerated holes in the valence band of InVO4 can oxidize the hydroxyl ion (OH−) or water (H2O) to form the hydroxyl radical (OH˙).
(3) Due to the contradiction between the scavenging test and the proposed photocatalytic mechanism, Fig. 9 was removed from the original article.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
References
- H. Q. Jiang, H. Endo, H. Natori, M. Nagai and K. Kobayashi, Mater. Res. Bull., 2009, 44, 700–706 CrossRef CAS.
- F. Guo, W. Shi, X. Lin, X. Yan, Y. Guo and G. Che, Sep. Purif. Technol., 2015, 141, 246–255 CrossRef CAS.
- G. Magesh, B. Viswanathan, R. P. Viswanath and T. K. Varadarajan, Indian J. Chem., 2009, 48A, 480–488 CAS.
| This journal is © The Royal Society of Chemistry 2020 |