Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In situ high-pressure 13C/1H NMR reaction studies of benzyl alcohol oxidation over a Pd/Al2O3 catalyst†
Carmine
D'Agostino
 *ab,
Mick D.
Mantle
*ab,
Mick D.
Mantle
 a and
Lynn F.
Gladden
a and
Lynn F.
Gladden
 a
a
aDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Philippa Fawcett Dr, Cambridge CB3 0AS, UK. E-mail: cd419@cam.ac.uk; mdm20@cam.ac.uk; lfg1@cam.ac.uk
bDepartment of Chemical Engineering and Analytical Science, The University of Manchester, The Mill, Sackville Street, Manchester, M13 9PL, UK. E-mail: carmine.dagostino@manchester.ac.uk
First published on 9th April 2020
Abstract
We report in situ high-pressure NMR kinetic studies of catalytic oxidations inside heterogeneous catalysts exploiting distortionless enhancement by polarisation transfer (DEPT) 13C NMR. 1H NMR diffusion and relaxation time measurements are then used to elucidate trends in reaction kinetics in different solvents. The work shows the feasibility of non-invasively monitoring intra-particle kinetics, transport and adsorption in porous catalysts using a comprehensive NMR toolkit.
The application of NMR spectroscopy to study chemical reactions in solid catalysts has grown in recent years. The approach offers several advantages as it allows one to carry out reaction studies non-invasively, exploiting the intrinsic ability of NMR to resolve the structure of chemical species formed during reaction and, unlike conventional approaches based on analysis of bulk fluids surrounding the catalyst particles, allows probing directly chemical kinetics and composition within the catalyst particles. Using such an approach it becomes possible to resolve chemical compositions of species inside the pore space, as for example in heterogeneous catalysis. This has recently been demonstrated when monitoring the evolution of conversion and product distribution during the heterogeneous catalytic ethene oligomerisation reaction at 110 °C and 28 barg over a 1 wt% Ni/SiO2–Al2O3 catalyst.1
Several other examples of such studies have been reported in the literature. For example, Koptyug et al.2 have used 1H NMR to produce spatially resolved spectra within a 2D slice along the axial direction of a fixed bed of 1% Pd/Al2O3 catalyst pellets. The reaction considered was that of the hydrogenation of α-methyl styrene to cumene. Although the spectra showed evidence of changes in chemical composition along the length of the bed, no attempt was made to quantify conversion. This was most likely due to problems in deconvolving the broad 1H resonances associated with the reactant and product species. In recent work, Leutzsch et al.3 have used 2H NMR combined with neutron scattering to monitor the kinetics of the catalytic hydrogenation of benzene in 3 wt% Pt/MCM-41. Catalytic hydrogenation reactions over solid catalysts have also been studied using 1H NMR spectroscopy, exploiting parahydrogen-induced polarisation (PHIP) to enhance the NMR signal.4
There are cases in which the use of 1H NMR becomes prohibitive when studying reactions in solid catalysts as the relatively narrow chemical shift range of the 1H nucleus and the line broadening of 1H resonances, due to contrast in magnetic susceptibility, give rise to a large number of overlapping resonances, making the resulting NMR spectrum almost featureless and any further analysis difficult if not impossible. In such cases, 13C NMR spectroscopy is undoubtedly the preferred approach. The principle disadvantage of 13C NMR is the low signal-to-noise ratio, due to both low natural abundance (∼1%) and low sensitivity of the 13C nucleus relative to the 1H nucleus. Polarisation transfer techniques such as the disortionless enhancement by polarisation transfer (DEPT) 13C NMR spectroscopy technique5 overcomes some of these disadvantages, enhancing the 13C signal by up to a factor of four. Moreover, the pulse sequence relies on the 1H spin–lattice relaxation time (T1) rather than 13C spin–lattice relaxation time, which is usually three to five times longer. Therefore, data acquisition can be much faster and this implies a higher signal-to-noise ratio, for a fixed acquisition time. Mantle et al.6 used DEPT 13C NMR spectroscopy to study hydrogenation and isomerisation of pentenes over commercial Pd/Al2O3 catalyst. When trans-2-pentene and hydrogen were both adsorbed onto the support, partial hydrogenation to n-pentane was observed in addition to the presence of both cis-2- and trans-2-pentenes. All pentene isomers hydrogenated over the Pd/Al2O3 catalyst to give predominantly n-pentane and a small amount of the trans-2-pentene isomer. 13C DEPT-MRI was also used by Sederman et al.7 to spatially map the isomerisation and hydrogenation of 1-octene over Pd/Al2O3 occurring within a trickle-bed reactor.
Whilst hydrogenation reactions over solid catalysts have so far been benefitting from in situ NMR spectroscopy, according to our knowledge there has not been any report on catalytic oxidations occurring in porous catalysts. The methodology, if successfully validated, would represent an invaluable tool to study such reactions. Indeed, in recent years there has been growing attention to catalytic oxidations that make use of atmospheric oxygen as the oxidant. This type of reaction is of particular interest as it represents a more sustainable and alternative route to conventional processes using hazardous inorganic oxidizing agents (e.g., H2Cr2O7, KMnO4), which tend to produce large amounts of hazardous waste.
In this work, we studied the aerobic catalytic oxidation of benzyl alcohol over a Pd/Al2O3 catalyst (supplied by Johnson Matthey, 0.5% Pd by weight, 2.5 mm trilobes, surface area of approximately 100 m2 g−1) at room temperature (20 °C) using air at high pressure (21 bar) as source of oxygen. The reaction was carried out in a home-built batch reactor, made with NMR-compatible materials, and exploiting in situ DEPT-45 13C NMR spectroscopy using 13C natural-abundance species. Oxidation of pure benzyl alcohol as well as benzyl alcohol dissolved in primary aliphatic alcohol solvents (methanol, ethanol and 1-propanol) was chosen as the model system to validate the proposed methodology. The NMR batch reactor was built using polyether ether ketone (PEEK), a thermoplastic polymer with excellent mechanical and chemical resistance properties. The reactor was designed to withstand pressures up to 40 bar and was machined in a cylindrical shape with an external diameter of 20 mm (see Fig. S1 in ESI†). The Pd/Al2O3 catalyst trilobes were first soaked in the reaction mixture, previously purged with nitrogen, for at least 20 hours to make sure the catalyst pores were saturated. The trilobes were then dried on a pre-soaked filter paper to remove any excess liquid on the external surface and transferred into the NMR batch reactor; the pre-soaking of the filter paper prevented liquid from being drawn out of the pores. The cell was then closed, pressurised to 21 bar with air, placed into the magnet and the NMR acquisition started. DEPT-45 13C NMR spectra during reaction were acquired over a period of 15 h. Typical acquisition parameters for the DEPT-45 13C NMR experiments were: 13C and 1H 90° pulses of 20 μs and 25 μs, respectively; 1024 scans; recycle delay of 4 s; evolution delay time, 1/2JCH, equal to 3.4 ms; the value of the echo time, τ, was chosen to be 1/2JCH, where JCH is the spin–spin coupling constant for a 13C–1H group and is approximately 145 Hz. All the 13C NMR spectra were acquired relative to the 13C resonance of tetramethylsilane (TMS).
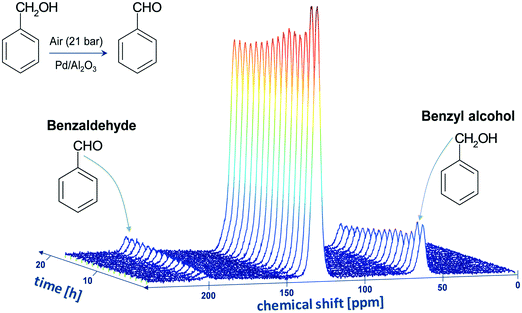
Fig. 1 shows the evolution of the 13C DEPT-45 spectrum as a function of time. The large peak at approximately 130 ppm arise from the overlapping NMR signals coming from the aromatic rings of both benzyl alcohol (reactant) and benzaldehyde (product), hence such peak cannot be used to monitor unambiguously the reaction. However, the unambiguous and clearly distinguishable signals of the –CH2– group of benzyl alcohol, at approximately 60 ppm, and that of the –CHO of the benzaldehyde, at approximately 200 ppm, can be used to monitor both kinetics, conversion and selectivity of the reaction. The formation of benzaldehyde, the main oxidation product, is evident by the rising peak at 200 ppm, whereas the benzyl alcohol consumption can be observed by the decreasing signal at approximately 60 ppm. Spectra of similar quality were observed for benzyl alcohol dissolved in aliphatic alcohols. Again, for all solvents studied, the resonances of the –CH2– group of benzyl alcohol and that of the –CHO of the benzaldehyde were clearly distinguishable. No oxidation of the alcohol solvent was observed at the operating conditions chosen for the reaction. It is noted that Pd/Al2O3 catalysts have been reported to be highly selective to convert benzyl alcohol to benzaldehyde with almost 100% selectivity8 and indeed in our case benzaldehyde was the only oxidation product.
In order to quantify chemical composition using the spectra reported in Fig. 1 several important points need to be considered. The DEPT 13C NMR signal is influenced by a number of factors that, if not accounted for, will make the acquired signal intensity non-quantitative. These influences are: (a) non-uniform longitudinal relaxation (characterised by T1); (b) non-uniform transverse relaxation (characterised by T2); (c) effects arising from the pulse-sequence itself that leads to differential enhancement of different CHn resonances. Non-uniform longitudinal relaxation may be avoided by ensuring a long recycle delay whereas effects arising from differential enhancement of CHn resonances can be corrected by considering that the intensity of the peaks of a 13C DEPT NMR spectrum is described by:9
I(CHn) = n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cosn−1 cosn−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ |
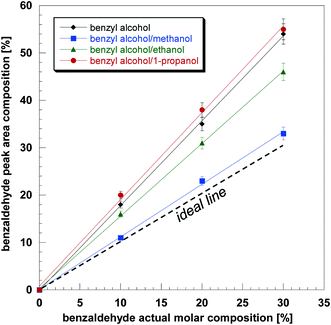
In the present work, quantification of DEPT 13C NMR spectra, in terms of benzaldehyde composition on a solvent-free basis, was carried out using a calibration procedure. Samples of known benzaldehyde composition in benzyl alcohol imbibed in Pd/Al2O3 catalyst pellets, were prepared and DEPT-45 13C NMR spectra acquired, keeping the same parameters used to probe reactions. Spectra were acquired at atmospheric pressure and a check to verify that no reaction occurred during the acquisition time of each spectrum was also carried out. No reaction occurred at ambient conditions during the acquisition time. The actual molar composition was compared with the molar composition calculated from NMR spectra by considering the areas of the –CH2– peak of benzyl alcohol at 60 ppm and that of the –CHO peak of benzaldehyde at 200 ppm.
Results of the calibration are shown in Fig. 2. It can be clearly seen that the calibration plots are in some cases significantly different from the ideal plot (dotted line). To further investigate the trend in Fig. 2 we carried out T21H NMR relaxation measurements of benzyl alcohol and benzaldehyde in the absence and presence of solvents with the Pd/Al2O3 catalyst in order to assess any possible effect of non-uniform T2 relaxation on the DEPT 13C NMR spectra. Here, it is noted that DEPT 13C NMR relies on T2 relaxation of 1H nuclei and not of 13C nuclei, which is due to the nature of the pulse sequence (i.e., 1H–13C polarisation transfer). We found that whilst the T2 of benzaldehyde was approximately the same in all cases (∼45 ms), that of benzyl alcohol was significantly shorter (<25 ms) and especially non-uniform across the various solvents. In particular, we observed that the shorter the T2 of benzyl alcohol the further the calibration curve deviates from the ideal plot. This is because shorter T2 values of the benzyl alcohol peak lead to a larger drop of its NMR signal, hence making the benzaldehyde composition appearing larger than the actual composition. This implies that there is a significant effect on the spectra due to non-uniform T2 relaxation; hence quantification through a calibration procedure is needed to correctly quantify intra-particle composition during reaction.
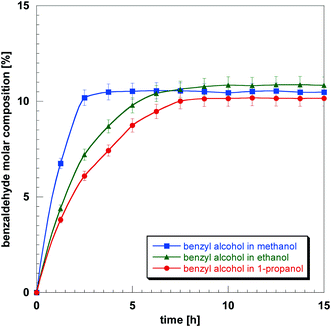
The composition of benzaldehyde within the Pd/Al2O3 catalyst during reaction in different solvents was obtained using the calibration plots of Fig. 2; this was achieved by using the benzaldehyde composition calculated from the NMR peak area measured in a specific solvent (or in solvent-free conditions), hence the vertical axis values of Fig. 2, which were then converted into actual composition, horizontal axis of Fig. 2, using the calibration plot for the solvent (or solvent-free) under investigation. The results are reported in Fig. 3. It can be noted as the benzaldehyde production increases more rapidly in the first 5 h of reaction and then reaches a plateau. Further investigation revealed that such a plateau was due to oxygen depletion. Since air (approximately 20% oxygen and 80% nitrogen) is used as the oxidant gas, the oxygen consumed is being replaced by air, which eventually leads to accumulation of inert nitrogen and consequent oxygen depletion, stopping the reaction. Indeed, after reaching the plateau, when the reactor was depressurised (purged of used air) and pressurised again with fresh air, the conversion started increasing again, confirming our hypothesis.
From Fig. 3 it is possible to observe differences in reaction rates for different solvents. In particular the initial reaction rate of benzyl alcohol follows the trend:
| methanol (5.4 h−1) > ethanol (3.5 h−1) > 1 − propanol (3.0 h−1) |
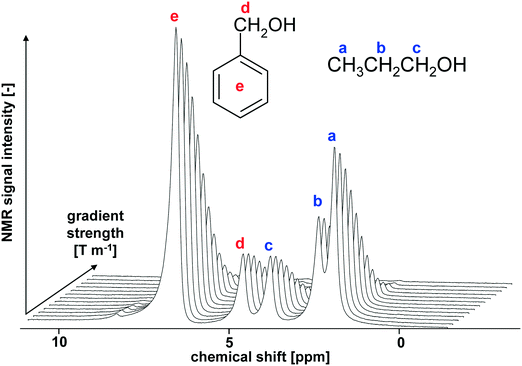 | ||
| Fig. 4 PFG NMR spectra of an equimolar mixture of benzyl alcohol/1-propanol in Pd/Al2O3 catalyst as a function of the gradient strength. | ||
In addition to transport properties, we also estimated solvent affinities with the Pd/Al2O3 catalyst by calculation of the T1/T2 ratio of methanol, ethanol and 1-propanol adsorbed in Pd/Al2O3. Such a ratio has been previously used to assess solvent inhibition in heterogeneous catalysis13 and gives a good indication of surface adsorption strength.10 Solvents with a higher T1/T2 ratio exhibit stronger interaction with the surface,14,15 and hence may inhibit a favourable adsorption of the reactant decreasing reaction rate at the surface.
A summary of initial reaction rates, NMR diffusion coefficients and relaxation time measurements is reported in Table 1. The typical relative error for diffusion and T1/T2 measurements is approximately 2 and 4%, respectively. It is possible to see a trend in diffusion rate of the reactant in different solvents as well as in T1/T2 of the solvent. In particular, solvents with higher reaction rates are associated to a faster diffusion rate of the reactant and to a lower T1/T2 of the solvent, indicating improved transport and a lower degree of solvent inhibition, which leads to favourable adsorption conditions for the reactant over the surface. Such results strongly suggest that both transport and adsorption play an important role in determining catalyst performances. It is interesting to note as a similar trend in T1/T2 for primary alcohols in porous oxide catalysts has been reported by Robinson et al.16 in a study involving both NMR and DFT calculations.
| Solvent | Rate [h−1] | D Reactant [m2 s−1] | T 1/T2 [−] |
|---|---|---|---|
| Methanol | 5.4 ± 0.2 | 1.85 ± 0.04 | 20.6 ± 0.8 |
| Ethanol | 3.5 ± 0.1 | 1.69 ± 0.03 | 29.8 ± 1.2 |
| 1-Propanol | 3.0 ± 0.1 | 1.52 ± 0.03 | 35.0 ± 1.4 |
In summary, we have reported the first in situ1H and 13C NMR measurements to study aerobic, high-pressure oxidation reactions occurring inside heterogeneous catalysts. The subsequent analysis of the NMR data shows that it is possible to monitor, unambiguously, the evolution of intra-particle composition during the oxidation of benzyl alcohol to benzaldehyde inside Pd/Al2O3 catalyst pellets using air as the oxidant. Moreover, the combination of 13C NMR measurements, (to extract kinetic information) with measurements of NMR diffusion coefficients and relaxation times gives a more detailed understanding of the physicochemical process of the catalytic system under different working conditions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge Innovate UK for funding this work, Grant No.: TP/7/ZEE/6/I/N0262B. We would also like to thank Dr. Andy York (Johnson Matthey) for providing catalytic materials.References
- S. T. Roberts, M. P. Renshaw, M. Lutecki, J. McGregor, A. J. Sederman, M. D. Mantle and L. F. Gladden, Operando magnetic resonance: monitoring the evolution of conversion and product distribution during the heterogeneous catalytic ethene oligomerisation reaction, Chem. Commun., 2013, 49, 10519–10521 RSC.
- I. Koptyug, A. A. Lysova, A. Kulikov, V. A. Kirillov, V. N. Parmon and R. Z. Sagdeev, Functional imaging and NMR spectroscopy of an operating gas-liquid-solid catalytic reactor, Appl. Catal., A, 2004, 267, 143–148 CrossRef CAS.
- M. Leutzsch, M. Falkowska, T.-L. Hughes, A. J. Sederman, L. F. Gladden, M. D. Mantle, T. G. A. Youngs, D. Bowron, H. Manyar and C. Hardacre, An integrated total neutron scattering – NMR approach for the study of heterogeneous catalysis, Chem. Commun., 2018, 54, 10191–10194 RSC.
- K. V. Kovtunov, I. E. Beck, V. I. Bukhtiyarov and I. V. Koptyug, Observation of parahydrogen-induced polarization in heterogeneous hydrogenation on supported metal catalysts, Angew. Chem., Int. Ed., 2008, 47, 1492–1495 CrossRef CAS PubMed.
- D. M. Doddrell, D. T. Pegg and M. R. Bendall, Distortionless enhancement of NMR signal by polarization transfer, J. Magn. Reson., 1982, 48, 323–327 CAS.
- M. D. Mantle, P. Steiner and L. F. Gladden, Polarisation enhanced C-13 magnetic resonance studies of the hydrogenation of pentene over Pd/Al2O3 catalysts, Catal. Today, 2006, 114, 412–417 CrossRef CAS.
- A. J. Sederman, M. D. Mantle, C. P. Dunckley, Z. Y. Huang and L. F. Gladden, In situ MRI study of 1-octene isomerisation and hydrogenation within a trickle-bed reactor, Catal. Lett., 2005, 103, 1–8 CrossRef CAS.
- H. L. Wu, Q. H. Zhang and Y. Wang, Solvent-free aerobic oxidation of alcohols catalyzed by an efficient and recyclable palladium heterogeneous catalyst, Adv. Synth. Catal., 2005, 347, 1356–1360 CrossRef CAS.
- T. D. W. Claridge, High resolution NMR techniques in organic chemistry, Elsevier Ltd, Oxford, UK, 1999 Search PubMed.
- C. D'Agostino, J. Mitchell, M. D. Mantle and L. F. Gladden, Interpretation of NMR relaxation as a tool for characterising the adsorption strength of liquids inside porous materials, Chem. – Eur. J., 2014, 20, 13009–13015 CrossRef PubMed.
- V. Mlynarik, Measurement of scalar relaxation of multiple-quantum coherences by spin-echo techniques, Chem. Phys. Lett., 1989, 160, 25–28 CrossRef CAS.
- C. P. Dunckley, PhD, University of Cambridge, 2008 Search PubMed.
- C. D'Agostino, M. R. Feaviour, G. L. Brett, J. Mitchell, A. P. E. York, G. J. Hutchings, M. D. Mantle and L. F. Gladden, Solvent inhibition in the liquid-phase catalytic oxidation of 1,4-butanediol: understanding the catalyst behaviour from NMR relaxation time measurements, Catal. Sci. Technol., 2016, 6, 7896–7901 RSC.
- D. Weber, J. Mitchell, J. McGregor and L. F. Gladden, Comparing strengths of surface interactions for reactants and solvents in porous catalysts using two-dimensional NMR relaxation correlations, J. Phys. Chem. C, 2009, 113, 6610–6615 CrossRef CAS.
- A. T. Krzyżak and I. Habina, Low field 1H NMR characterization of mesoporous silica MCM-41 and SBA-15 filled with different amount of water, Microporous Mesoporous Mater., 2016, 231, 230–239 CrossRef.
- N. Robinson, C. Robertson, L. F. Gladden, S. J. Jenkins and C. D'Agostino, Direct Correlation between Adsorption Energetics and Nuclear Spin Relaxation in a Liquid-saturated Catalyst Material, ChemPhysChem, 2018, 19, 2472–2479 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9re00489k |
| This journal is © The Royal Society of Chemistry 2020 |