Direct comparison of safer or sustainable alternative dipolar aprotic solvents for use in carbon–carbon bond formation†
Suwiwat
Sangon
a,
Nontipa
Supanchaiyamat
 a,
James
Sherwood
a,
James
Sherwood
 b,
Con R.
McElroy
b,
Con R.
McElroy
 b and
Andrew J.
Hunt
b and
Andrew J.
Hunt
 *a
*a
aMaterials Chemistry Research Center, Department of Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen, 40002, Thailand. E-mail: andrew@kku.ac.th
bGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, Heslington, York, YO10 5DD, UK
First published on 10th August 2020
Abstract
There is a lot of interest in the development of new, safer and more sustainable polar aprotic solvents due to their importance in industrial applications and significant safety issues with the most commonly used examples. One such area of application is in pharmaceutically relevant C–C coupling reactions, where polar aprotic solvents are commonly used for solubility and to stabilise reaction intermediates. Although there are now a number of excellent alternatives in the literature, to date they have not been compared in a single study. This study demonstrates the effectiveness of the green solvents N-butylpyrrolidinone (NBP), γ-valerolactone (GVL), propylene carbonate (PC) and dihydrolevoglucosenone (Cyrene) in Heck and Baylis–Hillman reactions. Good conversions and initial rates were observed in GVL and NBP in Heck reactions. Cyrene exhibited high initial rates of reaction and high yields in the Baylis–Hillman reaction. This demonstrates Cyrene to be a promising alternative polar aprotic solvent for this reaction.
Introduction
Solvents are essential for chemical synthesis and play a particularly significant role in the pharmaceutical industry. A fundamental process in the production of active pharmaceutical ingredients is carbon–carbon bond formation, typified by the Heck reaction,1 and the Baylis–Hillman reaction.2 Polar solvents are traditionally used to accelerate these reactions and stabilise intermediates during synthesis.3 The Heck cross-coupling reaction is a palladium catalysed reaction of an organohalide with an alkene leading to the formation of a substituted alkene.4–6 The solvent is influential in every step of the reaction, including palladium speciation.3 In the absence of auxiliary ligands, β-hydride elimination is the rate determining step,7,8 and accelerated by dipolar solvents such as DMF.9 Density functional theory (DFT) calculations indicate that coordination of a dipolar aprotic solvent molecule through an associative mechanism enables the reaction to proceed through a low-energy pathway.10The Baylis–Hillman reaction is used to form a C–C bond between a α,β-unsaturated carbonyl and an aldehyde, activated ketone, or other carbon electrophile.11 The reaction is typically catalysed by DABCO. Liu and co-workers have indicated that predicting the exact mechanism of the Baylis–Hillman reaction with various combinations of solvent, substituents, and catalysts is currently challenging.12 The rate of the reaction is thought to be dependent on the ability of the solvent to stabilize the zwitterionic intermediate which is created from nucleophilic attack of the catalyst on the β-carbon of the activated vinyl species.13
Conventional polar aprotic solvents used in C–C bond forming reactions include N-methyl pyrrolidinone (NMP), N,N-dimethylformamide (DMF) and N,N-dimethylacetamide (DMAc). These solvents are reprotoxic and their commercial use is restricted.3 As a result, there is a significant need for reliable and safer substitute solvents. Wherever feasible, sustainable or bio-derived alternative solvents should be considered instead of traditional problematic solvents.
Dihydrolevoglucosenone (Cyrene) is a bio-based molecule derived from cellulose, which demonstrates significant promise as a safer polar aprotic solvent.14,15 Cyrene was previously reported as an alternative solvent for the synthesis of metal–organic frameworks (MOFs),16 Suzuki–Miyaura cross coupling reactions,17 and the preparation of polyethersulfone and poly(vinylidene fluoride) membranes.18 The lignocellulosic bio-based solvent γ-valerolactone (GVL) is also attracting interest as a polar reaction medium.19,20 Cross couplings, including the Heck, Sonogashira and Hiyama reactions have been used to establish applications of GVL, demonstrating the ability of GVL to produce high yields and reduce palladium contamination in isolated products.19,21–24
N-Butyl pyrrolidinone (or 1-butyl-2-pyrrolidone, NBP) is another new polar aprotic solvent. Although structurally similar to NMP and N-ethyl pyrrolidinone, NBP is classified as a safer and also inherently biodegradable alternative.25 The performance of NBP is equivalent to NMP in various examples of organic synthesis.25
Organic cyclic carbonates such as ethylene carbonate and propylene carbonate (PC) are also classified as alternative greener solvents owning to their low toxicity, use of CO2 as a feedstock and rapid biodegradation.26 Significant work has focused on the use of cyclic carbonate solvents in organic synthesis.27 Cyclic carbonates are found in cosmetics and battery electrolytes, and are also appropriate for Heck reactions,1 the Suzuki–Miyaura reaction28 and Sn(IV)-catalysed hydroxymethylfurfural production from starchy wastes.29
Herein, the aforementioned greener dipolar aprotic solvents have been applied to the Heck and Baylis–Hillman reactions to enable a direct comparison of solvent performance. Polar aprotic solvents are preferentially used to conduct the Heck reaction.30 The Baylis–Hillman reaction was conventionally performed in protic solvents.13 However due to hydrolysis of reactants, the application of protic solvents limits the potential scope of this reaction. There is still a significant need for solvent optimization due to the typically slow kinetics of this reaction,31 and the application of dipolar aprotic solvents can be used to promote coupling of a much broader range of substrates.32,33 To date a number of studies have investigated the use of greener dipolar solvents in synthesis.1,14,19,25,28,34 However, few have made direct comparisons between alternative safer or sustainable solvents. This work also discusses the green credentials of these solvents to aid in selecting alternatives for conventional dipolar aprotic solvents. The solvents used in this study are listed in Fig. 1. In addition, non-polar aprotic solvents have also been selected as a point of comparison.
Experimental
Materials and methods
Cyrene was kindly supplied by Circa group Ltd. All other chemicals and solvents were purchased from Sigma Aldrich and used without further purification.Heck reaction
Into a 100 mL two-neck round bottom flask the following reagents were added: iodobenzene (30 mmol), methyl acrylate or styrene (30 mmol), and triethylamine (30 mmol). The chosen solvent (30 mL) was added, and the reaction heated to 100 °C with agitation at 300 rpm. Once the solution stabilised at the desired temperature, Pd(OAc)2 (0.1 mol%) was added. Samples were taken at designated intervals and monitored by GC-FID using diethyl succinate as an internal standard.Baylis–Hillman reaction
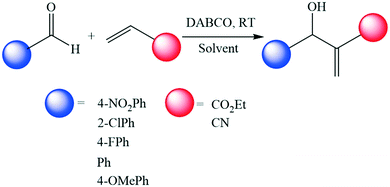
To a solution of 4-nitrobenzaldehyde (2 mmol) in the chosen solvent (2 mL) was added DABCO (1 mmol) and methyl acrylate (6 mmol). The progression of the reaction was monitored by 1H-NMR spectroscopy. The reaction mixture was diluted with water (40 mL) and then was extracted by diethyl ether (3 × 20 mL). The organic layer was collected, washed with brine, dried with Na2SO4 and concentrated under vacuum. PC and Cyrene were selected to demonstrate the performance of the reaction for the scale up process: 10 mmol of 4-nitrobenzaldehyde, DABCO (5 mmol) and 30 mmol acrylonitrile were mixed in 10 mL of the solvents. The yield was collected via the previous method. For the recovery of Cyrene, water (3 × 20 mL) was used to extract Cyrene followed by concentration under vacuum.Results and discussion
Following a previously proposed methodology for solvent development and replacement,35 solvent properties are demonstrated in Table S1 (ESI,† entries 5–14) and also on a solvent polarity map plotting the hydrogen bond accepting ability (β) vs. dipolarity (π*) (Fig. 2) have been used to categorise alternative solvents as dipolar aprotic. Dipolar aprotic solvents share several characteristics beyond a comparatively large permanent dipole moment and being non-protogenic. They typically have high flash points, are water miscible, and (with the exception of acetonitrile, MeCN) boiling points consistently above 150 °C. The high π* values of conventional dipolar aprotic solvents can be matched by bio-derived oxygenated solvents (GVL, PC and Cyrene),14,19,26 but the magnitude of β is sacrificed. Conversely, NBP has a large β value but lower dipolarity.25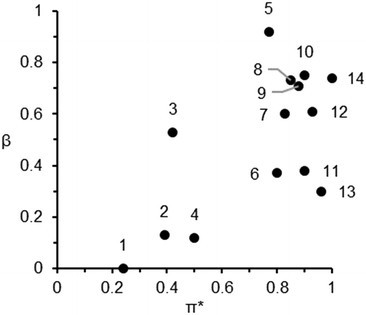 | ||
| Fig. 2 Solvent polarity map of Kamlet–Abboud–Taft solvatochromic parameters. The numbers 1 to 14 correspond to the solvents in Fig. 1. | ||
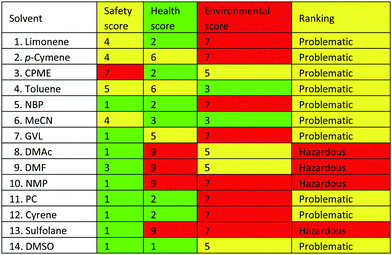
In order to assess the green credentials of the selected solvents, the CHEM21 solvent selection guide has been utilised.36,37 Each category is scored from 1–10, ordered in ascending order of hazard severity (Table 1). All the solvents considered in this work exhibit a problematic or hazardous ranking overall. In a number of instances, the problematic ranking is dictated only by a high boiling point (captured in the environmental score). From the perspective of the pharmaceutical industry, low volatility indicates the need for an energy intensive distillation or removal by aqueous extraction and subsequent treatment. Despite this, Cyrene, PC, NBP and DMSO have low health and safety hazards, thus offering a clear advantage over classical dipolar aprotic solvents. GVL currently has a health score of 5 by default as it is yet to be REACH registered. Reprotoxic solvents receive a score of 9 in the health category. One further consideration is that nitrogen- or sulphur-containing molecules (e.g. MeCN and DMSO) would result in NOx and SOx if incinerated after use.38 Bio-based, oxygenated solvents reduce this particular end-of-life hazard. Additionally, the presence of heteroatoms in solvents reduces their ozone producing capability, while unsaturation and aromaticity increase it.39
| Scores are based on the severity of hazard statements. Key: 1–3, low hazard (green); 4–6, medium hazard (yellow); 7–10, high hazard (red). Original data presented in ref. 38. |
|---|
|
The reaction of iodobenzene and methyl acrylate (Fig. 3a),3,40 was attempted in the solvents specified in Fig. 1. The conversion to methyl cinnamate is presented as a function of time in Fig. S3 (ESI†), with initial rate constants correlated to dipolarity (π*) as a linear solvation energy relationship (LSER) in Fig. 3b. Sulfolane, NBP, DMF and GVL result in rapid reaction rates, while DMAc, NMP and PC exhibited a moderate reaction rate. The general trend is of greater solvent dipolarity further stabilising the activated complex, presumed to be β-hydride elimination (Fig. S1†). The conversions in sulfolane and NBP after 24 hours were 95% and 90% respectively (Fig. 3d). Incomplete conversion in other solvents can be linked to catalyst deactivation because the reaction is irreversible. Firstly, the solvent must assist the reduction of the pre-catalyst. Palladium acetate exists as a trimer, and dissociation into the monomer is thermodynamically preferable in solvents with higher polarity.41 Monomeric palladium acetate is more labile towards reduction to an active Pd(0) species, thought to be Pd(0)OAc− in polar solvents.42 The high conversions in sulfolane and NBP indicate delayed catalyst decomposition to palladium black.
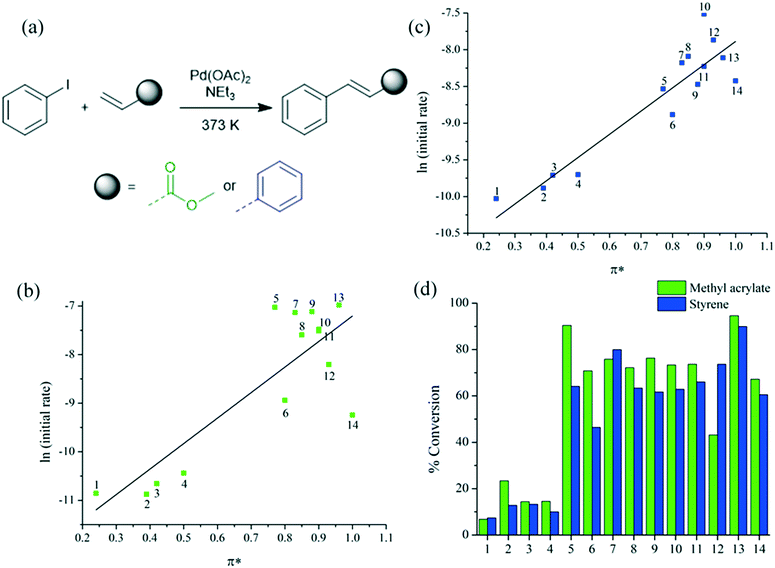 | ||
| Fig. 3 (a) Scheme of a Heck reaction; (b) linear solvation energy relationship of a Heck reaction of methyl acrylate, (c) linear solvation energy relationship of a Heck reaction of styrene and (d) conversion (%) after 24 hours. The numbers 1 to 14 correspond to the solvents in Fig. 1. | ||
A further Heck reaction was studied, namely the reaction between iodobenzene and styrene (Fig. 3). Broadly speaking the solvent effect is unchanged, but there is evidence to suggest the alkene has a role in determining the optimal solvent. Competitive coordination to palladium between reactant and solvent can impede the reaction. The reaction of styrene proceeded at the greatest initial rate in NMP, and Cyrene demonstrated improved performance over the previous case study. GVL and PC produce competitive conversions despite providing slower initial reaction rates.
Regarding the Baylis–Hillman reaction between 4-nitrobenzaldehyde and methylacrylate,43 PC had the highest initial rate of reaction (Fig. 4). Cyrene also had a notably high initial rate of reaction and ultimately led to complete conversion in this solvent. The high dipolarity of Cyrene is expected to stabilise the zwitterionic intermediate formed by the conjugate addition of DABCO to methyl acrylate (Fig. S2 ESI†).13,32 Interestingly, there was no significant difference between the initial rate using NBP, MeCN, GVL, DMAc or NMP as the solvent. Of these, only the reaction in NMP reached completion. Additionally, sulfolane performed with a high initial rate of reaction and provided quantitative conversion in 2 hours, consistent with work of Krishna et al. who demonstrated the utilisation of sulfolane as a solvent for Baylis–Hillman reactions.32 However, NMP and sulfolane are reprotoxic.33 Therefore on balance, Cyrene is an attractive alternative to conventional dipolar aprotic solvents in the Baylis–Hillman reaction.
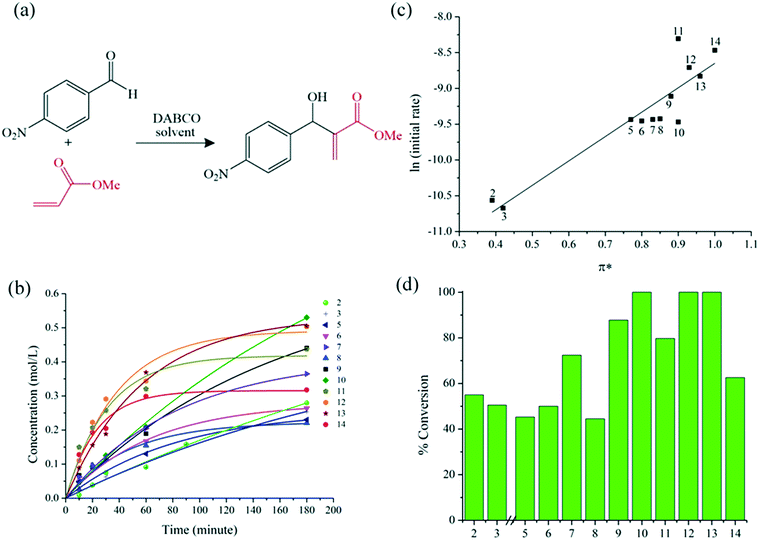 | ||
| Fig. 4 (a) Scheme of the Baylis–Hillman reaction; (b) time (minute) dependence of the product concentration (mol L−1), (c) linear solvation energy relationship and (d) conversion (%) of the Baylis–Hillman reaction (24 hours). The numbers 1 to 14 correspond to the solvents in Fig. 1. | ||
To further investigate which alternative dipolar aprotic solvent is suitable for the Baylis–Hillman reaction the range of substrates has been extended (Scheme 1). A total of 10 reactions were performed with DMF, NMP, PC, Cyrene, sulfolane and DMSO (Table 2). The results of these experiments demonstrate that Cyrene and PC consistently outperform traditional amide solvents and are competitive with sulfolane and DMSO. No solvent promoted any significant conversion with electron rich aldehydes, but the reaction between 4-nitrobenzaldehyde and acrylonitrile repeatedly demonstrated quantitative conversions across all solvent systems. As such this reaction was selected to demonstrate workup by organic aqueous extraction and isolation of product based on a literature established method.32 Isolated yields for DMF (56% ± 0.8%), NMP (55% ± 3.1%), sulfolane (80% ± 13.1%) and DMSO (31% ± 6.8%) were significantly lower than anticipated due to the formation of emulsions in the workup process. The reaction of 4-nitrobenzaldehyde with acrylonitrile in PC resulted in a greater than 100% crude yield due to residual traces of the solvent. Removal of high boiling point solvents is challenging and a key factor to overcome in the use of bio-based dipolar aprotic solvents for such reactions. After successive washing with water, the sample produced in Cyrene was isolated in 97% yield. The significantly higher yield compared to other dipolar aprotic solvents is presumed to arise due to the ability for Cyrene to form a geminal diol in water.44 Furthermore, over 80% of the Cyrene was recovered through evaporation of water. In the case of PC, expensive and laborious column chromatography techniques were required for solvent recovery and purification, leading to greater losses of the solvent. Interestingly, this reaction was attempted on a gram scale with Cyrene as the solvent, which also resulted in quantitative conversion, comparable crude yields to the lab scale process, and demonstrated excellent solvent recovery. Importantly, Cyrene is a bio-based, sustainable, and low toxicity alternative to traditional dipolar aprotic solvents which demonstrates promise for further scale up and use in the Baylis–Hillman reactions. Future work will introduce DFT calculations to prove the formation of solvent stabilised intermediates and further clarify the mechanisms of these C–C bond forming reactions with bio-based solvents such as Cyrene.
| Reaction | Aldehyde | Activated alkene | Time (h) | % conversion | |||||
|---|---|---|---|---|---|---|---|---|---|
| DMF | NMP | PC | Cyrene | Sulfolane | DMSO | ||||
| a Reaction temperature of 50 °C. b Reaction was attempted 3 times and error presented. | |||||||||
| 1 | 4-Nitrobenzaldehyde | Ethyl acrylate | 3 | 76 | 59 | 90 | 93 | 93 | 94 |
| 2 | 2-Chlorobenzaldehyde | Ethyl acrylate | 5 | 59 | 54 | 75 | 87 | 88 | 87 |
| 3 | 4-Fluorobenzaldehyde | Ethyl acrylate | 5 | 7 | 8 | 10 | 31 | 15 | 13 |
| 4a | Benzaldehyde | Ethyl acrylate | 24 | 14 | 26 | 28 | 69 | 41 | 18 |
| 5a | 4-Methoxy benzaldehyde | Ethyl acrylate | 24 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6b | 4-Nitrobenzaldehyde | Acrylonitrile | 20 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| 7 | 2-Chlorobenzaldehyde | Acrylonitrile | 20 | 100 | 100 | 100 | 100 | 100 | 100 |
| 8 | 4-Fluorobenzaldehyde | Acrylonitrile | 20 | 72 | 73 | 82 | 74 | 93 | 98 |
| 9 | Benzaldehyde | Acrylonitrile | 20 | 50 | 40 | 67 | 67 | 88 | 92 |
| 10a | 4-Methoxy benzaldehyde | Acrylonitrile | 20 | 9 | 7 | 13 | 28 | 29 | 27 |
Conclusions
A direct comparison of a variety of sustainable or safer alternative solvents has been performed for two examples of the Heck reaction, and for the first time the Baylis–Hillman reaction. The progression of each Heck reaction (initial rate and final conversion) is solvent dependent, and the substrates were found to influence which solvent was most effective. Cyrene was shown to be a productive solvent for Baylis–Hillman reaction with high initial rates of reaction, quantitative conversions, high yield and solvent recovery. This thus demonstrates Cyrene to be a promising alternative polar aprotic solvent for this reaction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Andrew Hunt would like to acknowledge the financial support of Thailand Research Fund (RSA6280031) and Khon Kaen University. Financial support from the Center of Excellence for Innovation in Chemistry (PERCH-CIC), Ministry of Higher Education, Science, Research and Innovation is gratefully acknowledged. Circa Group Ltd. is graciously thanked for supplying Cyrene. Mr. Suwiwat Sangon would like to thank the support of Institute for the Promotion of Teaching Science and Technology for the Development and Promotion of Science and Technology Talents Project scholarship.References
- H. L. Parker, J. Sherwood, A. J. Hunt and J. H. Clark, ACS Sustainable Chem. Eng., 2014, 2, 1739–1742 CrossRef CAS.
- J. N. Tan, M. Ahmar and Y. Queneau, RSC Adv., 2015, 5, 69238–69242 RSC.
- J. Sherwood, J. H. Clark, I. J. S. Fairlamb and J. M. Slattery, Green Chem., 2019, 21, 2164–2213 RSC.
- R. F. Heck, C. Johansson, G. C. Lloyd-Jones, P. O. Norrby, K. F. Heck and J. P. Nolley, Tetrahedron: Asymmetry, 1968, 21, 1585–1592 Search PubMed.
- R. F. Heck and J. P. Nolley, J. Org. Chem., 1972, 37, 2320–2322 CrossRef CAS.
- H. A. Dieck and R. F. Heck, J. Am. Chem. Soc., 1974, 96, 1133–1136 CrossRef CAS.
- A. F. Schmidt and V. V. Smirnov, Kinet. Catal., 2001, 42, 800–804 CrossRef.
- A. F. Schmidt and V. V. Smirnov, Kinet. Catal., 2003, 44, 518–523 CrossRef CAS.
- G. Strappaveccia, E. Ismalaj, C. Petrucci, D. Lanari, A. Marrocchi, M. Drees, A. Facchetti and L. Vaccaro, Green Chem., 2015, 17, 365–372 RSC.
- L. Xu, M. J. Hilton, X. Zhang, P. O. Norrby, Y. D. Wu, M. S. Sigman and O. Wiest, J. Am. Chem. Soc., 2014, 136, 1960–1967 CrossRef CAS PubMed.
- A. Baylis and M. Hillman, Chem. Abstr., 1972, 77, 34174q Search PubMed.
- Z. Liu, C. Patel, J. N. Harvey and R. B. Sunoj, Phys. Chem. Chem. Phys., 2017, 19, 30647–30657 RSC.
- V. K. Aggarwal, D. K. Dean, A. Mereu and R. Williams, J. Org. Chem., 2002, 67, 510–514 CrossRef CAS PubMed.
- J. Sherwood, M. De Bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC.
- J. E. Camp, ChemSusChem, 2018, 11, 3048–3055 CrossRef CAS PubMed.
- J. Zhang, G. B. White, M. D. Ryan, A. J. Hunt and M. J. Katz, ACS Sustainable Chem. Eng., 2016, 4, 7186–7192 CrossRef CAS.
- K. L. Wilson, J. Murray, C. Jamieson and A. J. B. Watson, Synlett, 2018, 29, 650–654 CrossRef CAS.
- T. Marino, F. Galiano, A. Molino and A. Figoli, J. Membr. Sci., 2019, 580, 224–234 CrossRef CAS.
- G. Strappaveccia, L. Luciani, E. Bartollini, A. Marrocchi, F. Pizzo and L. Vaccaro, Green Chem., 2015, 17, 1071–1076 RSC.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584–595 RSC.
- E. Ismalaj, G. Strappaveccia, E. Ballerini, F. Elisei, O. Piermatti, D. Gelman and L. Vaccaro, ACS Sustainable Chem. Eng., 2014, 2, 2461–2464 CrossRef CAS.
- H. Mahmoudi, F. Valentini, F. Ferlin, L. A. Bivona, I. Anastasiou, L. Fusaro, C. Aprile, A. Marrocchi and L. Vaccaro, Green Chem., 2019, 21, 355–360 RSC.
- F. Valentini, H. Mahmoudi, L. A. Bivona, O. Piermatti, M. Bagherzadeh, L. Fusaro, C. Aprile, A. Marrocchi and L. Vaccaro, ACS Sustainable Chem. Eng., 2019, 7, 6939–6946 CrossRef CAS.
- M. Bagherzadeh, H. Mahmoudi, S. Ataie, M. Bahjati, R. Kia, P. R. Raithby and L. Vaccaro, Mol. Catal., 2019, 474 Search PubMed.
- J. Sherwood, H. L. Parker, K. Moonen, T. J. Farmer and A. J. Hunt, Green Chem., 2016, 18, 3990–3996 RSC.
- B. Schäffner, F. Schäffner, S. P. Verevkin and A. Börner, Chem. Rev., 2010, 110, 4554–4581 CrossRef PubMed.
- J. S. Bello Forero, J. A. Hernández Muñoz, J. Jones Junior and F. M. da Silva, Curr. Org. Synth., 2016, 13, 834–846 CrossRef CAS.
- P. Gautam, R. Gupta and B. M. Bhanage, Eur. J. Org. Chem., 2017, 2017, 3431–3437 CrossRef CAS.
- I. K. M. Yu, D. C. W. Tsang, A. C. K. Yip, A. J. Hunt, J. Sherwood, J. Shang, H. Song, Y. S. Ok and C. S. Poon, Green Chem., 2018, 20, 2064–2074 RSC.
- I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009–3066 CrossRef CAS PubMed.
- D. Basavaiah, K. Venkateswara Rao and R. Jannapu Reddy, Chem. Soc. Rev., 2007, 36, 1581–1588 RSC.
- P. R. Krishna, A. Manjuvani, V. Kannan and G. V. M. Sharma, Tetrahedron Lett., 2004, 45, 1183–1185 CrossRef CAS.
- P. Radha Krishna, A. Manjuvani and E. Raja Sekhar, ARKIVOC, 2004, 2005, 99–109 Search PubMed.
- L. Mistry, K. Mapesa, T. W. Bousfield and J. E. Camp, Green Chem., 2017, 19, 2123–2128 RSC.
- S. Jin, F. Byrne, C. R. McElroy, J. Sherwood, J. H. Clark and A. J. Hunt, Faraday Discuss., 2017, 202, 157–173 RSC.
- C. R. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Green Chem., 2015, 17, 3111–3121 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 1–24 CrossRef.
- R. G. Derwent, M. E. Jenkin and S. M. Saunders, Atmos. Environ., 1996, 30, 181–199 CrossRef CAS.
- F. Zhao, B. M. Bhanage, M. Shirai and M. Arai, J. Mol. Catal. A: Chem., 1999, 142, 383–388 CrossRef CAS.
- L. A. Adrio, B. N. Nguyen, G. Guilera, A. G. Livingston and K. K. Hii, Catal. Sci. Technol., 2012, 2, 316–323 RSC.
- C. Amatore and A. Jutand, J. Organomet. Chem., 1999, 576, 254–278 CrossRef CAS.
- D. Basavaiah and G. Veeraraghavaiah, Chem. Soc. Rev., 2012, 41, 68–78 RSC.
- M. De Bruyn, V. L. Budarin, A. Misefari, S. Shimizu, H. Fish, M. Cockett, A. J. Hunt, H. Hofstetter, B. M. Weckhuysen, J. H. Clark and D. J. Macquarrie, ACS Sustainable Chem. Eng., 2019, 7, 7878–7883 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0re00174k |
| This journal is © The Royal Society of Chemistry 2020 |