Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
2-Amino-3′-dialkylaminobiphenyl-based fluorescent intracellular probes for nitric oxide surrogate N2O3†
P. Rogelio
Escamilla‡
 c,
Yanming
Shen‡
a,
Quanjuan
Zhang
a,
Derek S.
Hernandez
c,
Cecil J.
Howard
c,
Xuhong
Qian
ab,
Daria Y.
Filonov
d,
Alexander V.
Kinev
d,
Jason B.
Shear
*c,
Eric V.
Anslyn
c,
Yanming
Shen‡
a,
Quanjuan
Zhang
a,
Derek S.
Hernandez
c,
Cecil J.
Howard
c,
Xuhong
Qian
ab,
Daria Y.
Filonov
d,
Alexander V.
Kinev
d,
Jason B.
Shear
*c,
Eric V.
Anslyn
 *c and
Youjun
Yang
*c and
Youjun
Yang
 *ab
*ab
aState Key Laboratory of Bioreactor Engineering, East China University of Science and Technology, Meilong Road 130, Shanghai 200237, China. E-mail: youjunyang@ecust.edu.cn
bShanghai Key Laboratory of Chemical Biology, School of Pharmacy, East China University of Science and Technology, Meilong Road 130, Shanghai 200237, China
cDepartment of Chemistry, University of Texas at Austin, Austin, Texas, USA. E-mail: anslyn@austin.utexas.edu; jshear@cm.utexas.edu
dCreative Scientist, Inc., Durham, NC, USA
First published on 2nd January 2020
Abstract
Fluorescent probes for nitric oxide (NO), or more frequently for its oxidized surrogate dinitrogen trioxide (N2O3), have enabled scientists to study the contributions of this signaling molecule to many physiological processes. Seeking to improve upon limitations of other probes, we have developed a family of fluorescent probes based on a 2-amino-3′-dialkylaminobiphenyl core. This core condenses with N2O3 to form benzo[c]cinnoline structures, incorporating the analyte into the newly formed fluorophore, which results in product fluorescence with virtually no background contribution from the initial probe. We varied the substituents in the core in order to optimize both the reactivity of the probes with N2O3 and their cinnoline products' fluorescence wavelengths and brightness. The top candidates were then applied to cultured cells to verify that they could respond to NO within cellular milieus, and the top performer, NO530, was compared with a “gold standard” commercial probe, DAF-FM, in a macrophage-derived cell line, RAW 264.7, stimulated to produce NO. NO530 demonstrated similar or better sensitivity and higher selectivity for NO than DAF, making it an attractive potential alternative for NO tracking in various applications.
Introduction
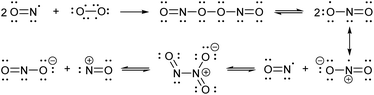
Nitric oxide (NO) is a blood vessel relaxation factor with multiple roles in physiological signaling.1–5 Considering how NO is integrally involved in cardiovascular, nervous, immune, and other human body systems, it is no surprise that abnormal NO levels are implicated in numerous pathological conditions, including cardiovascular diseases, circulatory shock, local inflammation, asthma, cancer, stroke, ischemic reperfusion injury, neurodegenerative disorders, depression and diabetes.6–25 In order to establish NO levels and activity, scientists have employed NO-specific electrodes26–34 and chemical probes that, upon reaction with NO or its surrogates, produce a change in electron paramagnetic resonance (EPR),35–39 UV-vis absorbance,40,41 chemiluminescence,42–45 and/or fluorescence.46–59 For monitoring NO in cells and tissues, fluorescence imaging provides a sensitive and spatially defined signal that can be followed over time, often with minimal interference to cell or tissue processes.Nitric oxide synthases (NOSs) produce the free radical NO when they convert L-arginine to L-citrulline.60 This neutral and slightly polar dissolved gas diffuses through both aqueous and non-polar (lipid membrane) environments, where it has several fates.61–63 Transition metals such as those bound to heme proteins, as well as other radicals, such as superoxide, scavenge NO and therefore contribute to its short lifetime.24 NO binds readily (often under diffusion control) to paramagnetic metals. In addition, high oxidation-state metals can be reduced by NO, with concomitant or swiftly ensuing nitrosation of a proximal nucleophile, such as a thiol.64–66 If present, superoxide will react with NO in a diffusion-controlled rate. It produces the peroxynitrite anion, a potent oxidizer responsible for DNA mutations.67 When triplet oxygen reacts with four equivalents of NO, it forms two equivalents of the potent nitrosating dinitrogen trioxide (N2O3, Scheme 1). The diradical molecular oxygen first reacts with two NO radicals to form the dinitroso peroxide which homolyzes to the nitrogen dioxide (NO2) radical. Reaction with another NO radical produces the asymmetric N2O3, rather than the symmetric nitrous anhydride.
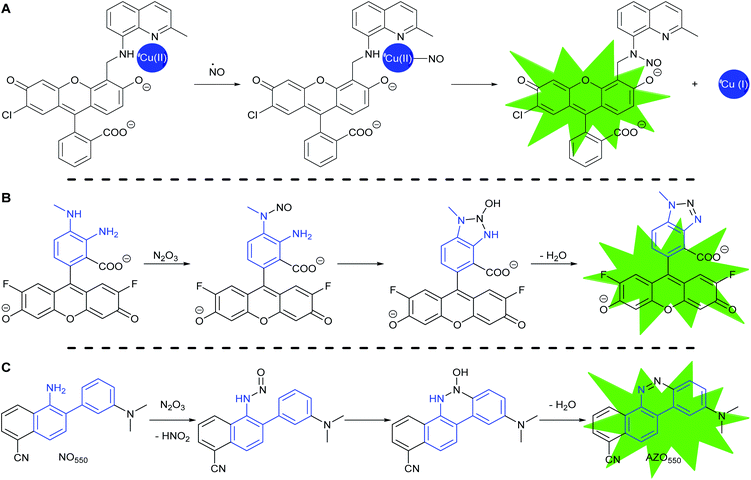
Most fluorescent probes for NO fall into three categories. First are probes inspired by high oxidation state transition metal NO scavengers, in which a fluorescent ligand binds a fluorescence-quenching metal, such as copper(II), as exemplified by Lippard's Cu(FL) probes.68–74 After NO complexation to Cu(II), nucleophilic attack by the ligand amine reduces the metal to Cu(I). Metal reduction and N-nitrosation lower the affinity of the ligand for the metal, to the extent that the metal dissociates and no longer quenches ligand fluorescence (Scheme 2A).
The second category consists of the ortho-diaminobenzene family, which detects N2O3 (Scheme 2B).75–79 The diaminobenzene moiety is attached to a fluorophore to produce photoinduced electron-transfer (PET) quenching of fluorescence. Nitrosation of either of the amines, followed by condensation of the other amine upon the N-nitrosamine, produces a triazole that can no longer efficiently PET quench fluorescence. In this category lies DAF-FM, one of the most cited probes for NO imaging. It has also been suggested that the nitrosation occurs via peroxynitrite, which is formed due to a reaction between superoxide and NO,80 however the probes cannot differentiate the source of nitrosation.
Both sensing strategies produce an enhancement, not a wavelength-shift, of emission as the signal of reaction with NO or its oxidized surrogate. Incomplete quenching of fluorescence in the unreacted probes can result in significant background signal. With no change in wavelength, this background degrades the detection limits of the reaction with NO. Transition metal-based probes are most affected by this incomplete quenching, with relatively small increases (3–40×) in fluorescence due to NO addition when compared to the non-metal-based probes (40–1500×). In addition, the performance of ortho-diaminobenzene-based probes is limited both by chelation to alkali-earth metals and reaction with other bioavailable dielectrophiles, such as dehydroascorbic acid (DHA) and methyl glyoxal, resulting in the attenuation of PET quenching and thus a non-NO-selective signal.81–84
In 2010 Shear, Anslyn, and Yang presented a new fluorescence detection mechanism for NO embodied in the probe NO550 (probe 9, Table 1).85 This probe comprises a 2-amino-3′-dimethylaminobiphenyl core that condenses with N2O3 to produce a benzo[c]cinnoline via nitrosation of the amine, followed by nucleophilic aryl attack on the nitrosamine, with subsequent loss of water – an intramolecular diazotization, creating AZO550 (Scheme 2C).
| Unreacted probe | Pr. no | Cinnoline product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λ abs (nm) | λ em (nm) | ε × 103 M−1 cm−1 | ϕ | Structure | X or Z | λ abs (nm) | λ em (nm) | ε × 103 M−1 cm−1 | ϕ | Relativee,f brightness | |
a Wavelength of maximum absorbance for the most bathochromic peak in the UV-vis spectrum.
b Wavelength of maximum emission for the largest peak in the emission spectrum when excited at the most bathochromic absorbance maximum.
c Absorptivity coefficient/1000.
d Quantum efficiency.
e Brightness relative to the cinnoline product of NO550 (9), previously reported as AZO550.
f Cinnoline brightness is defined as the product of the absorptivity coefficient and the quantum efficiency.
g NO550 does not fluoresce in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 DMSO/50 mM PBS at pH 7.4. The blue values highlight the best performers' fluorescence properties in 1 4 DMSO/50 mM PBS at pH 7.4. The blue values highlight the best performers' fluorescence properties in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 DMSO/50 mM PBS at pH 7.4, of the probes and their cinnoline, or azo, products (hereafter denoted as “AZO-Probe Number”), produced from reaction with nitrite in acid. 4 DMSO/50 mM PBS at pH 7.4, of the probes and their cinnoline, or azo, products (hereafter denoted as “AZO-Probe Number”), produced from reaction with nitrite in acid.
|
|||||||||||
| 300 | 420 | 10.0 | 0.54 |
|
1 | –OMe | 395 | 488 | 14.0 | 0.09 | 3.0 |
| 300 | 420 | 11.0 | 0.47 | 2 | –OH | 395 | 490 | 13.2 | 0.67 | 20.0 | |
| 310 | 415 | 9.0 | 0.27 | 3 | –NMe2 | 415 | 510 | 14.3 | 0.02 | 0.7 | |
| 300 | 390 | 14.4 | 0.003 |
|
4 | — | 450 | 550 | 16.2 | 0.003 | 0.1 |
| 310 | 415 | 9.9 | 0.24 |
|
5 | — | 425 | 508 | 20.8 | 0.62 | 29.3 |
| 300 | 415 | 9.5 | 0.1 |
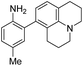
|
6 | — | 447 | 535 | 11.3 | 0.36 | 9.3 |
| 350 | 450 | 9.9 | 0.02 |
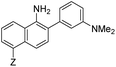
|
7 | –NHAc | 433 | 535 | 13.0 | 0.08 | 2.3 |
| 330 | 445 | 8.0 | 0.04 | 8 | –H | 430 | 530 | 13.9 | 0.06 | 1.9 | |
| 350 | n.d. | 3.5 | n.d.g | 9 | –CN (NO550) | 450 | 550 | 4.0 | 0.11 | 1.0 | |
| 370 | 455 | 9.4 | 0.07 |
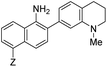
|
10 | –NHAc (NO530) | 445 | 530 | 20.1 | 0.5 | 23.0 |
| 355 | 450 | 10.3 | 0.02 | 11 | –H | 440 | 525 | 24.1 | 0.5 | 27.5 | |
| 345 | 510 | 11.6 | 0.03 | 12 | –CN | 450 | 545 | 7.3 | 0.13 | 2.2 | |
| 420 | 465 | 12.2 | 0.01 |
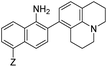
|
13 | –NHAc (NO562) | 470 | 562 | 15.5 | 0.35 | 12.3 |
| 350 | 425 | 11.3 | 0.01 | 14 | H | 466 | 555 | 16.9 | 0.24 | 9.3 | |
| 390 | 515 | 12.4 | 0.02 | 15 | CN | 480 | 580 | 9.0 | 0.11 | 2.3 | |
The excitation and emission wavelengths of AZO550 are sufficiently bathochromically-shifted that excitation of AZO550 produces virtually no excitation of NO550. Such elimination of background signal deriving from NO550 results in a 1500-fold enhancement of emission upon full conversion to AZO550. Moreover, NO550 demonstrates high selectivity for N2O3 in its fluorescence response, showing no response to 100-fold excess of DHA and 1000-fold excess of other biologically relevant analytes. It either fails to react with the competing analytes, or their reactions produce non-fluorescent products. Unfortunately, AZO550's brightness (defined here as the product of molar absorptivity and fluorescence quantum yield: 440 M−1 cm−1) is significantly lower than that for fluorescein-based DAF-FM triazole (from reaction of DAF-FM with N2O3, 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 brightness), due both to AZO550's lower molar absorptivity (4000 M−1 cm−1versus 73
000 M−1 cm−1 brightness), due both to AZO550's lower molar absorptivity (4000 M−1 cm−1versus 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and fluorescence quantum yield (0.11 versus 0.81).76 Ghebremariam and co-workers found that not even at 75 μM loading concentration did NO550 match the intensity of only 1 μM DAF-FM in the same endothelial cells at the same time point.86 Loading with higher concentrations of NO550 was also hampered by poor water solubility and cytotoxicity. Herein, we describe the systematic substitution of the 2-aminobiphenyl scaffold, the effect of this substitution on reactivity and fluorescence, and the response of the best candidates to exogenous and endogenous NO in cells, and compare these results to those obtained for DAF-FM.
000 M−1 cm−1) and fluorescence quantum yield (0.11 versus 0.81).76 Ghebremariam and co-workers found that not even at 75 μM loading concentration did NO550 match the intensity of only 1 μM DAF-FM in the same endothelial cells at the same time point.86 Loading with higher concentrations of NO550 was also hampered by poor water solubility and cytotoxicity. Herein, we describe the systematic substitution of the 2-aminobiphenyl scaffold, the effect of this substitution on reactivity and fluorescence, and the response of the best candidates to exogenous and endogenous NO in cells, and compare these results to those obtained for DAF-FM.
Results and discussion
Spectroscopic properties of probes and their cinnoline products
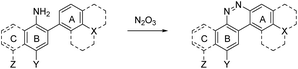
Despite NO550's limitations, the results were sufficiently promising to study the 2-aminobiphenyl core more thoroughly, with the goals of increasing brightness, quantum yield, and red-shifting the fluorescence, all while maintaining the selectivity for NO and the spectral resolution of probe fluorescence versus that of the corresponding cinnoline product. Table 1 shows the permutations synthesized and studied. As N2O3 is dielectrophilic, these probes are dinucleophilic, and positions X and Y on rings A and B, respectively, have the greatest influence on the probes' nucleophilicity. We incorporated ring C to impart bathochromic shifts. Substituent Z on ring C is not only commercially and synthetically convenient but also distant from (and less inductively and sterically interfering with) the N2O3 reaction sites. It is therefore an attractive attachment point for hydrophobic, hydrophilic, cell-compartment directing, or tethering functionalities. We first evaluated the brightness and fluorescence properties (Table 1), in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 DMSO/50 mM PBS at pH 7.4, of the probes and their cinnoline (i.e. azo) products (hereafter denoted as AZO-Probe Number, black column) accessed from the reaction of nitrite with acid (ESI page S9†).
4 DMSO/50 mM PBS at pH 7.4, of the probes and their cinnoline (i.e. azo) products (hereafter denoted as AZO-Probe Number, black column) accessed from the reaction of nitrite with acid (ESI page S9†).
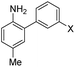
The first set studied consisted of simple variants with rings A and B only (no C annulation). The Y substituent was fixed as a methyl group while varying the X substituent on ring A. Since ring A attacks the trapped N-nitroso group, to X we assigned electron-donating methoxy (OMe, 1), hydroxy (OH, 2), and N,N-dimethylamino (NMe2, 3) groups. By far, the hydroxyl variant surpassed the others in brightness due to AZO-2's much higher quantum yield of 67%. Unfortunately, this brightness showed a dependence upon the protonation state of the phenol around physiological pH. For fluorescein-based probes, the pKa of the phenol in the excited state normally lies around 6.7,87 meaning that these probes may non-selectively detect (by quenching of fluorescence) pH in cells. We performed fluorescence pH titrations on AZO-2 and AZO-3, establishing their pKa's as 7.8 and 4.5, respectively (Fig. 1). Protonation quenches the fluorescence of both cinnolines, but AZO-3 provides a pH-independent signal above pH 6. AZO-1's signal is also pH-independent at physiological pH, but we did not pursue it or any variants with X = OMe because of this probe poor reactivity, even with nitrite in acid.
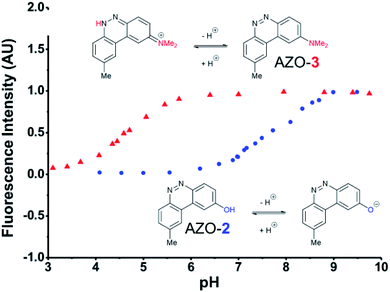 | ||
| Fig. 1 Fluorescence emission pH titration of 5 μM solutions of the cinnoline products of probes 2 (blue) and 3 (red) in 2.5% aqueous DMSO with excitation set at the probes' respective absorbance maxima (Table 1). | ||
We next evaluated the influence of position Y by changing it from methyl to cyano, postulating that the greater conjugation from NMe2 to CN would result in a bathochromic shift of fluorescence wavelengths. The X = NMe2 and Y = CN cinnoline variant (AZO-4) had excitation and emission maxima 35–40 nm longer than AZO-3; however, the quantum yield of AZO-4 was determined to be less than 1%, even lower than AZO-3's quantum yield (2%).
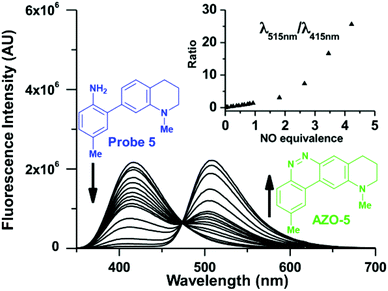
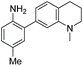
When considering AZO-3 and AZO550's low quantum yield, we speculated that the excitation energy was lost to non-radiative relaxation through rotation about the ring A aryl-NMe2 bond.88,89 This bond rotation decreases significantly when the N-alkyl groups are fused to the aryl ring, so we synthesized N-methyl-tetrahydroquinoline (5) and julolidine (6) variants on ring A, with methyl at Y on ring B. The ring fusion in both variants resulted in a remarkable increase in quantum yield, from 0.02 and 0.11 for non-fused AZO-3 and AZO550 to 0.36 for AZO-6 and 0.62 for AZO-5. In addition, the molar absorptivity for AZO-5 also increased, making it the brightest of the biphenyl series – nearly twenty times brighter than AZO550, albeit at shorter wavelengths. The julolidine variant did produce the longest fluorescence wavelengths of the group, confirming the expected greater planarity of the dialkyl-N to aryl bond. However, a steric clash between the aromatic proton and the julolidine benzylic protons, as depicted in Fig. 2, disrupts the planarity of the entire fluorophore, possibly explaining AZO-6's lower quantum yield and lower molar absorptivity than AZO-5.
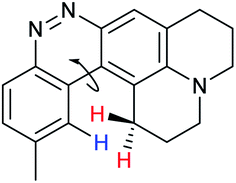 | ||
| Fig. 2 Disruption of planarity in AZO-6 due to A1,3 strain. | ||
Probe 5 proved to be the brightest candidate, but AZO-5's excitation and emission maxima (425 and 508 nm) fall short of the ideal wavelengths for the universal FITC filters. We decided to add ring C with the hypothesis that benzannulation would extend fluorescence wavelengths to those more suitable for the FITC filter set. For a thorough comparison, we studied the N,N-dimethylamino, N-methyltetrahydroquinoline, and julolidine variants at position X on ring A. For each of these variants, H occupied position Y and we derivatized position Z with H, N-acetamide (NHAc), and CN – a total of nine variants.
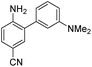
Focusing on brightness first, the results listed in Table 1 (probes 7–15) show that both NMe2 (at X) and CN (at Z) substitutions are detrimental to cinnoline brightness. For the same Z substituent, the NMe2 series members had lower molar absorptivities and quantum yields than their corresponding N-methyl tetrahydroquinoline or julolidine congeners, i.e., cinnolines 7versus10 and 13, 8versus11 and 14, and 9versus12 and 15. This trend is readily explained by the greater non-radiative relaxation rate caused by rotation about the N-aryl bond for NMe2 variants versus the singly fused N-methyl tetrahydroquinoline and doubly fused julolidine variants. Likewise, the Z = CN cinnoline series exhibited lower molar absorptivities than the corresponding Z = NHAc or H series (for the same X substituent). We speculate that the Franck–Condon excited state has significant quinoid character in the Z = CN series, with less quinoid character in the ground state, such that the poor orbital overlap between the two states results in lower probability of photon absorption.
Within the Z = CN series, for the two alicyclic amine variants at X (AZO-12 and AZO-15), the ground state incorporates greater donation of the aligned nitrogen lone pair into the polyaromatic system than from the more freely-rotating X = NMe2 variant (AZO550), leading to better orbital overlap of the ground state with the excited state, as demonstrated by their higher absorptivities. For the Z = H and NHAc variants, both substituents are less electron withdrawing than the quinoid-inducing CN, meaning that their ground and excited states are more similar and their absorptivities higher.90,91 The Z = CN series also produced lower quantum yields. Here we posit that the excited state relaxes through internal charge transfer to the nitrile π* orbital, with subsequent non-radiative relaxation through the increased hydrogen-bonding of this negatively-charged complex with buffer. N-methyl tetrahydroquinoline variants proved brighter than their A1,3-strained julolidine counterparts, as discussed for AZO-6. Interestingly, for AZO-13 the greater electron-withdrawing nature of the NHAc Z-substituent (acceptor) acting upon the dialkyl amine (donor) lone pair electrons countered the A1,3 strain to promote greater planarity and therefore a higher quantum yield, as compared to Z = H for AZO-14.
As anticipated, adding ring C to the biphenyl probes bathochromically shifted fluorescence wavelengths. While benzannulation of fluorones to seminaphthofluorones shifts wavelengths by more than 100 nm, we found only 15–30 nm shift for the cinnolines.92–94 Substituting CN at Z (9, 12, 15) induced the greatest shift, but this substitution prohibitively decreased brightness. Variants substituted with NHAc at Z (7, 10, 13) showed only slight shifts (3–7 nm) to longer wavelengths compared to their corresponding H (8, 11, 14) substituted ones. Varying X in the benzannulated series from NMe2 (7, 8, 9) to N-methyltetrahydroquinoline (10, 11, 12) to julolidine (13, 14, 15) continued the aforementioned pattern in the biphenyl series of red-shifting fluorescence wavelengths with greater ring fusion. Benzannulated julolidine variants therefore produced the longest wavelength probes; unfortunately, julolidine substitution also decreased brightness, as proposed above, due to A1,3 strain.
The spectroscopic data reveal probes 5, 10, 11, and 13 as the brightest in the group. Importantly, as with first generation NO550 and AZO550, the maximum excitation and emission spectra are well resolved from each other for both the probes and their cinnoline products, obviating homo-Förster resonance energy transfer (FRET) that occurs for high intracellular concentrations of ortho-diaminophenyl-type probes.82 Furthermore, in the case of the four brightest probes, the absorption spectra for the probes minimally overlap with the absorption spectra of their corresponding cinnolines (Fig. S1†). Consequently, as described below, in cells the probes can be excited independently from their N2O3 reaction products to compare intracellular distribution or compartmentalization of probe and cinnoline product, as well as to maintain a nearly zero background for detecting product (as previously reported for NO550).
Fluorimetric NO titrations of probes
We next looked at fluorescence titrations with NO of the probes in aerobic buffer. Incremental aliquots of a saturated NO solution (1.9 mM),15,95,96 up to five equivalents of NO, were added to an air-equilibrated 50 μM solution of each probe variant in 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 50 mM PBS/DMSO at pH 7.4 (ESI Fig. S2†). Two equivalents of NO react with a half-equivalent of molecular oxygen to form one equivalent of N2O3 (Scheme 1), a portion of which is degraded to nitrite via hydrolysis.23 To maximize signal, probes should exhibit sufficient nucleophilicity to successfully compete with an excess of water. We started at the biphenyl system with no ring C, with Y as Me, and with X varied as OMe (1), OH (2), and NMe2 (3), their Hammett σ+ substituent constants of −0.78, −0.92, and −1.7, respectively.97 Variant 1 did not produce sufficient cinnoline product to be detected by fluorescence (Fig. 3), whereas 2 and 3 provided ample signal, confirming that X must be sufficiently electron-donating (σ+ < −0.78) to readily promote nucleophilic attack by ring A. Subsequently, we set X as NMe2 and changed the weakly donating methyl (σ+ = −0.311) at Y to a strongly-withdrawing cyano group, (σ+ = 0.659).98 With no cinnoline fluorescence detected, we concluded that the nitrile group inhibits the nucleophilicity of the p-amine to such an extent that it could not N-nitrosate.
1 50 mM PBS/DMSO at pH 7.4 (ESI Fig. S2†). Two equivalents of NO react with a half-equivalent of molecular oxygen to form one equivalent of N2O3 (Scheme 1), a portion of which is degraded to nitrite via hydrolysis.23 To maximize signal, probes should exhibit sufficient nucleophilicity to successfully compete with an excess of water. We started at the biphenyl system with no ring C, with Y as Me, and with X varied as OMe (1), OH (2), and NMe2 (3), their Hammett σ+ substituent constants of −0.78, −0.92, and −1.7, respectively.97 Variant 1 did not produce sufficient cinnoline product to be detected by fluorescence (Fig. 3), whereas 2 and 3 provided ample signal, confirming that X must be sufficiently electron-donating (σ+ < −0.78) to readily promote nucleophilic attack by ring A. Subsequently, we set X as NMe2 and changed the weakly donating methyl (σ+ = −0.311) at Y to a strongly-withdrawing cyano group, (σ+ = 0.659).98 With no cinnoline fluorescence detected, we concluded that the nitrile group inhibits the nucleophilicity of the p-amine to such an extent that it could not N-nitrosate.
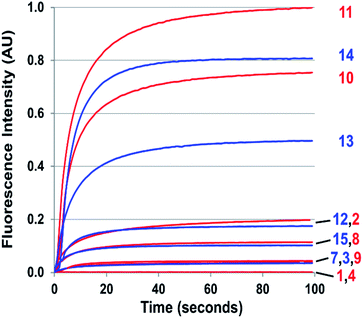
The probes that did form cinnolines from N2O3 (2–3, 5–15) for the most part required less than five minutes to nearly fully react. The high level of fluorescence from species 10, 11, 13, and 14 makes these species the most promising of the group we examined (Fig. 3 and ESI Fig. S2†). More than two equivalents of NO, typically four in most cases, are necessary to reach saturation, however. We postulate that at the starting concentration of probe (50 μM, compared to 55.5 M for water), hydrolysis of N2O3 competes sufficiently as a side-reaction to warrant the excess NO.99 Despite requiring at least twice the theoretical amount of NO for full conversion, these probes surpass DAF-2, the predecessor of DAF-FM in reactivity. The fluorescence intensity of a solution of 3 exposed to two equivalents of NO, when calibrated to varying concentration standards of AZO-3, showed 36% cinnoline formation. In comparison, 27% of DAF-2 converted to the triazole when exposed to 2 equivalents of NO under similar conditions.79
With the high reactivity of these probes and with the creation of signal with virtually no background, excellent detection limits were obtained. We defined the lower detection limit (LDL) as the concentration of NO that produces signal equal to three times the experimental setup noise. The LDL of 9 (NO550) was previously determined to be 30 nM;85 for the brighter 10, the detection limit is 2 nM, comparable to DAF-FM's detection limit of 3 nM.76
The resolution of the probe and cinnoline emission spectra enables ratiometry when the probes and their cinnoline products share an excitation band (Fig. 4). Ratiometry corrects for several experimental factors, including variations in intracellular dye concentrations, in optical path due to cell thickness, and in instrumental noise.100 However, with our probes two factors moderate this benefit of ratiometry. First, the probe excitation band is in a shorter, cell-damaging near-UV range than that for the cinnoline products. Second, the shorter excitation results in little cinnoline emission, instead primarily unreacted probe background fluorescence. The later phenomenon however does not affect one of the most significant attributes of our 2-aminobiphenyl system: i.e. that exciting at the cinnoline absorbance maximum results in virtually no background contribution from the probe.
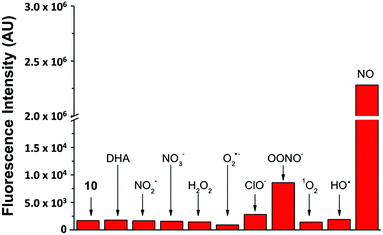
By keeping the 2-aminobiphenyl core, we expected to maintain in our newer probes the selectivity of NO550 for NO over other common biological substrates.85 Indeed this anticipation proved correct; as one example, Fig. 5 demonstrates fluorescence emission for probe 10 in the presence of NO versus that in the presence of various oxidants. Even at one hundred equivalents of peroxynitrite to probe, at most a five-fold increase of fluorescence was observed, as compared with a greater than 1300-fold increase for merely one equivalent of NO. It is important to note that we used purified peroxynitrite for this study. When this is generated in cellulo it involves both super oxide and NO which could potentially activate the fluorescent probe through a free radical mechanism.
Probe performance in cells
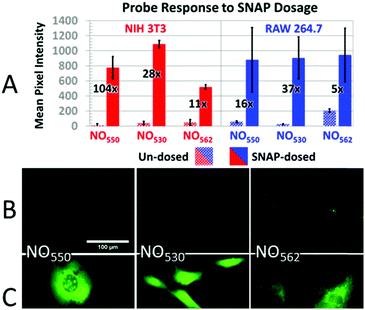
Taking into consideration the probes' spectral properties and response to NO, we decided to focus on probes 10 (henceforth named NO530), and 13 (NO562) and to compare them to the first-generation probe 9 (NO550) and commercial probe DAF-FM. In the current studies, we examined the response of these probes to NO in an NIH-3T3 murine fibroblast cell line and a RAW 264.7 murine macrophage cell line because, while NO530 and NO562 readily reacted with NO in solution, most important is their performance in live cells. We first pre-incubated cells with the probes and then exposed cells to either exogenous NO-donor S-nitrosopenicillamine (SNAP) or to a NO solution. Next, we compared the specificity of NO530, NO562, and DAF-FM probes for the detection of endogenous NO produced in RAW 264.7 cells. Finally, we evaluated the cytotoxicity of NO530.NIH-3T3 and RAW 264.7 cells were pre-loaded with 10 μM solutions of NO550, NO530, and NO562, and exposed to 1 mM and 200 μM SNAP solutions, respectively (Fig. 6). Microscopic observations revealed cell-associated blue fluorescence, characteristic of the unreacted probes (ESI Fig. S3†). In both cell types, the unreacted NO550, NO530, and NO562 produced homogenous cytoplasmic staining visible in the blue (DAPI) channel (ESI Fig. S4†). When exposed to SNAP, NO550, NO530, and NO562 converted to their respective cinnolines as demonstrated by increased green fluorescence signal in the FITC channel corresponding to cinnoline emission spectra (ESI Fig. S3†). In SNAP-dosed cells compared to the untreated cells, NO562 produced the smallest signal increase among the three tested probes (Fdosed/Fun-dosed, Fig. 6A). NO562 also produced higher background than the two other probes and generated lower signal in SNAP-treated NIH-3T3 cells. The observed increased background fluorescence of NO562 in untreated cells could be explained by the use of FITC filter set, which has shorter than necessary window for excitation/emission and thus may result in a bleed-through of the signal from the unreacted probe (Table 1 and ESI Fig. S3†). However, we also observed high background of NO562 reaction with NO endogenously produced in RAW264.7 cells using an IN Cell Analyzer imaging system (INCA 2200, GE Healthcare) with appropriate Cy3 filters (data not shown). In contrast, NO530 generated somewhat more SNAP-dependent signal than NO550 in NIH-3T3 cells, although it provided a several-fold lower increase relative to unstimulated cells (28-fold vs. 104-fold). In RAW 264.7 cells, NO530 and NO550 stimulated cell fluorescence was not significantly different, although NO530 provided somewhat higher fractional increase in fluorescence as a result of lower background (Fig. 6A blue). It is worth noting here, hat the production of N2O3 is dependent on O2 concentration in cells, and that higher nitrosation levels will occur when cells are treated with higher than normal O2 levels.
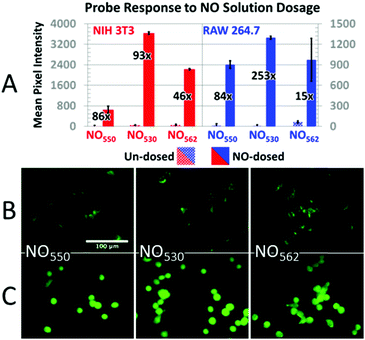
In addition, cells loaded with each probe were exposed to 200 μM NO (NIH-3T3 cells) or 320 μM NO (RAW 264.7 cells) by diluting 1.9 mM saturated NO solution into the cell medium (Fig. 7). In NIH-3T3 cells, NO530 produced the highest signal, while NO550 yielded the smallest change (although a similar fractional change in signal to NO530). All three probes yielded similar signal in RAW 264.7 cells; NO530 provided the best fractional change in signal because of substantially lower background before addition of NO. Taken together, these findings suggest that NO530 was the most promising probe to investigate endogenous production of NO in RAW 264.7 cells.
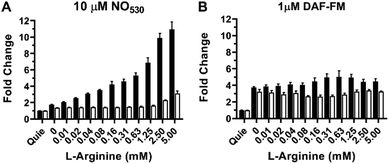
The RAW 264.7 cell line is known to express inducible NOS (iNOS), an enzyme that can be stimulated by a combination of lipopolysaccharide (LPS) and interferon-γ (IFN-γ) to produce NO in the presence of NO-synthase substrate L-arginine. We tested NO530 response in both quiescent and LPS/IFN-activated RAW 264.7 cells, and compared results to those obtained using DAF-FM DA. Data were obtained using cell imaging instrument INCA 2200. Cells were exposed to L-arginine in the presence of NO probes as described in the ESI.† In the presence of NO530, quiescent RAW 264.7 cells produced low fluorescence signal that did not measurably change after treatment with arginine. In the absence of exogenous arginine, LPS/IFN-activated RAW 264.7 cells produced approximately two-fold higher fluorescence signal than un-activated cells; cellular fluorescence further increased in response to arginine in a concentration-dependent manner (Fig. 8A, black bars). Pre-incubation of RAW 264.7 cells with pan-NOS inhibitor L-NG-monomethyl arginine (L-NMMA) blocked most of the signal in cells loaded with NO530, indicating the high NO specificity of the signal (Fig. 8A, white bars). In contrast, DAF-FM strongly reacted with activated RAW 264.7 cells, but both cells and media produced high fluorescence signal, requiring two iterative PBS washes to remove media fluorescence. Nevertheless, no arginine-concentration-dependence of the cell-associated signal could be observed and the pre-treatment with L-NMMA had little effect on DAF-FM-mediated fluorescence (Fig. 8B). The current observation confirms previously reported observations that 4,5-diaminofluorescein-based probes are prone to non-specific interactions with reactive species present in cells and cell culture media, exhibiting low selectivity towards cellular NO at physiologically relevant conditions.101,102
Finally, we did not observe signs of cytotoxicity when using our family of probes, even after 18 hour of incubation. Since NO530 was the best performer in terms of fluorescence ratio in NO-dosing studies, we evaluated its cytotoxicity in RAW 264.7 cells at various concentrations with a Calcein Blue/propidium iodide viability assay. For 1.25, 2.5, 5, 10, 20, and 40 μM loading concentrations of NO530, greater than 93% of the cells stained a viable blue (ESI Fig. S5†), similar to 95% viability in controls where either only DMSO or no additional solution was added.
Conclusions
The 2-aminobiphenyl core selectively creates a longer-wavelength fluorophore when it reacts with oxidized NO. We found that attaching electron-donating groups, primarily dialkyl amines, on the nucleophilic aryl group, and avoiding the conjugation of electron withdrawing groups to the 2-amino group, renders the probes sufficiently nucleophilic to readily scavenge NO in acellular media. Furthermore, fusion of the 4′-aminoalkyl groups to the nucleophilic aryl group increased both fluorescence wavelengths and the fluorescence quantum yield of the cinnoline product. Benzannulation of the 2-amino aryl group also provoked a bathochromic shift in fluorescence and opened a functionalizable position, removed from the non-alkylated amine, for attachment of groups with desired properties via an amide linkage. When considering the propensity of the probe to react with NO, the brightness of the cinnoline product, and its low cytoxocity, NO530 (10) stands as a promising candidate for live cell imaging. Due to its greater response to stimuli, higher selectivity for NO, and the ability to image both reacted and unreacted probe, NO530 provides a attractive option as a nitric oxide probe and a potentially valuable alternative to DAF-FM in various applications.Conflicts of interest
DYF and AVK are employees of Creative Scientist, Inc.Acknowledgements
JBS acknowledges support from Welch Foundation Grant F-1331 for these studies. AVK is a recipient of SBIR Phase I grant 1R43ES027716 from the National Institute of Environmental Health Sciences. EVA acknowledges financial support from HHMI Professorship Program, and the Welch Regents Chair (F-0046). YY acknowledges the National Natural Science Foundation of China (No. 21572061, 21602181, and 21822805).Notes and references
- W. P. Arnold, C. K. Mittal, S. Katsuki and F. Murad, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 3203 CrossRef CAS PubMed.
- R. F. Furchgott and J. V. Zawadzki, Nature, 1980, 288, 373 CrossRef CAS PubMed.
- L. J. Ignarro, G. M. Buga, K. S. Wood, R. E. Byrns and G. Chaudhuri, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 9265 CrossRef CAS PubMed.
- C. K. Mittal and F. Murad, Proc. Natl. Acad. Sci. U. S. A., 1977, 74, 4360 CrossRef CAS PubMed.
- R. M. J. Palmer, A. G. Ferrige and S. Moncada, Nature, 1987, 327, 524 CrossRef CAS PubMed.
- S. B. Abramson, Osteoarthr. Cartil., 2008, 16, S15–S20 CrossRef PubMed.
- A. A. Andreadis, S. L. Hazen, S. A. A. Comhair and S. C. Erzurum, Free Radical Biol. Med., 2003, 35, 213–225 CrossRef CAS PubMed.
- C. Bogdan, Nat. Immunol., 2001, 2, 907 CrossRef CAS PubMed.
- C. Bogdan, Trends Immunol., 2015, 36, 161–178 CrossRef CAS PubMed.
- A. R. Butler and D. L. H. Williams, Chem. Soc. Rev., 1993, 22, 233–241 RSC.
- L. J. Ignarro, J. Physiol. Pharmacol., 2002, 53, 503–514 CAS.
- L. J. Ignarro and B. A. Freeman, Nitric Oxide Biology and Pathobiology, Elsevier, 3rd edn, 2017 Search PubMed.
- L. Leon, J.-F. Jeannin and A. Bettaieb, Nitric Oxide, 2008, 19, 77–83 CrossRef CAS PubMed.
- B. Leonard and M. Maes, Neurosci. Biobehav. Rev., 2012, 36, 764–785 CrossRef CAS PubMed.
- K.-H. Lim, B. B. Ancrile, D. F. Kashatus and C. M. Counter, Nature, 2008, 452, 646 CrossRef CAS PubMed.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271–284 CrossRef CAS PubMed.
- F. Mangialasche, M. C. Polidori, R. Monastero, S. Ercolani, C. Camarda, R. Cecchetti and P. Mecocci, Ageing Res. Rev., 2009, 8, 285–305 CrossRef CAS PubMed.
- A. Martínez-Ruiz, S. Cadenas and S. Lamas, Free Radicals Biol. Med., 2011, 51, 17–29 CrossRef PubMed.
- S. Mocellin, V. Bronte and D. Nitti, Med. Res. Rev., 2006, 27, 317–352 CrossRef PubMed.
- S. Moncada, R. M. Palmer and E. A. Higgs, Pharmacol. Rev., 1991, 43, 109 CAS.
- C. F. Nathan and J. B. Hibbs, Curr. Opin. Immunol., 1991, 3, 65–70 CrossRef CAS PubMed.
- P. Pacher, J. S. Beckman and L. Liaudet, Physiol. Rev., 2007, 87, 315–424 CrossRef CAS PubMed.
- D. D. Thomas, L. A. Ridnour, J. S. Isenberg, W. Flores-Santana, C. H. Switzer, S. Donzelli, P. Hussain, C. Vecoli, N. Paolocci, S. Ambs, C. A. Colton, C. C. Harris, D. D. Roberts and D. A. Wink, Free Radicals Biol. Med., 2008, 45, 18–31 CrossRef CAS PubMed.
- J. C. Toledo and O. Augusto, Chem. Res. Toxicol., 2012, 25, 975–989 Search PubMed.
- R. Zelis, Am. J. Med., 1983, 74, 3–12 CrossRef CAS PubMed.
- F. Bedioui, A. Ismail and S. Griveau, Curr. Opin. Electrochem., 2018, 12, 42–50 CrossRef CAS.
- J. Elliott, J. Duay, O. Simoska, J. B. Shear and K. J. Stevenson, Anal. Chem., 2017, 89, 1267–1274 CrossRef CAS PubMed.
- S. Griveau and F. Bedioui, Anal. Bioanal. Chem., 2013, 405, 3475–3488 CrossRef CAS PubMed.
- S. Jiang, R. Cheng, X. Wang, T. Xue, Y. Liu, A. Nel, Y. Huang and X. Duan, Nat. Commun., 2013, 4, 2225 CrossRef PubMed.
- H. Liu, L. Weng and C. Yang, Microchim. Acta, 2017, 184, 1267–1283 CrossRef CAS.
- Y.-L. Liu, X.-Y. Wang, J.-Q. Xu, C. Xiao, Y.-H. Liu, X.-W. Zhang, J.-T. Liu and W.-H. Huang, Chem. Sci., 2015, 6, 1853–1858 RSC.
- T. Malinski and Z. Taha, Nature, 1992, 358, 676 CrossRef CAS PubMed.
- B. J. Privett, J. H. Shin and M. H. Schoenfisch, Chem. Soc. Rev., 2010, 39, 1925–1935 RSC.
- P. Vallance, K. Bhagat, R. MacAllister, S. Patton, T. Malinski, M. Radomski and S. Moncada, Lancet, 1995, 346, 153–154 CrossRef CAS.
- M. J. Davies, Methods, 2016, 109, 21–30 CrossRef CAS PubMed.
- H. Fujii and J. Berliner Lawrence, Magn. Reson. Med., 1999, 42, 599–602 CrossRef CAS PubMed.
- N. Hogg, J. Zielonka and B. Kalyanaraman, in Nitric Oxide, ed. B. A. Freeman, Academic Press, 3rd edn, 2017, pp. 23–44 Search PubMed.
- L. B. Maia and J. J. G. Moura, in Plant Nitric Oxide: Methods and Protocols, ed. K. J. Gupta, Springer New York, New York, NY, 2016, pp. 81–102 Search PubMed.
- T. Yoshimura, H. Yokoyama, S. Fujii, F. Takayama, K. Oikawa and H. Kamada, Nat. Biotechnol., 1996, 14, 992 CrossRef CAS PubMed.
- L. C. Green, D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok and S. R. Tannenbaum, Anal. Biochem., 1982, 126, 131–138 CrossRef CAS PubMed.
- R. W. Nims, J. C. Cook, M. C. Krishna, D. Christodoulou, C. M. B. Poore, A. M. Miles, M. B. Grisham and D. A. Wink, in Methods in Enzymology, Academic Press, 1996, vol. 268, pp. 93–105 Search PubMed.
- A. J. Dunham, R. M. Barkley and R. E. Sievers, Anal. Chem., 1995, 67, 220–224 CrossRef CAS PubMed.
- K. Kikuchi, T. Nagano, H. Hayakawa, Y. Hirata and M. Hirobe, Anal. Chem., 1993, 65, 1794–1799 CrossRef CAS PubMed.
- M. M. Pelletier, P. Kleinbongard, L. Ringwood, R. Hito, C. J. Hunter, A. N. Schechter, M. T. Gladwin and A. Dejam, Free Radicals Biol. Med., 2006, 41, 541–548 CrossRef CAS PubMed.
- M. Sato, T. Nakajima, M. Goto and Y. Umezawa, Anal. Chem., 2006, 78, 8175–8182 CrossRef CAS PubMed.
- E. Eroglu, S. Charoensin, H. Bischof, J. Ramadani, B. Gottschalk, M. R. Depaoli, M. Waldeck-Weiermair, W. F. Graier and R. Malli, Free Radicals Biol. Med., 2018, 128, 50–58 CrossRef CAS PubMed.
- E. Eroglu, B. Gottschalk, S. Charoensin, S. Blass, H. Bischof, R. Rost, C. T. Madreiter-Sokolowski, B. Pelzmann, E. Bernhart, W. Sattler, S. Hallström, T. Malinski, M. Waldeck-Weiermair, W. F. Graier and R. Malli, Nat. Commun., 2016, 7, 10623 CrossRef CAS PubMed.
- E. Eroglu, S. Hallström, H. Bischof, M. Opelt, K. Schmidt, B. Mayer, M. Waldeck-Weiermair, W. F. Graier and R. Malli, Nitric Oxide, 2017, 70, 59–67 CrossRef CAS PubMed.
- N. Kumar, V. Bhalla and M. Kumar, Coord. Chem. Rev., 2013, 257, 2335–2347 CrossRef CAS.
- R. Leggett, P. Thomas, M. J. Marin, J. Gavrilovic and D. A. Russell, Analyst, 2017, 142, 4099–4105 RSC.
- H. Li and A. Wan, Analyst, 2015, 140, 7129–7141 RSC.
- H. Li, D. Zhang, M. Gao, L. Huang, L. Tang, Z. Li, X. Chen and X. Zhang, Chem. Sci., 2017, 8, 2199–2203 RSC.
- S. Ma, D.-C. Fang, B. Ning, M. Li, L. He and B. Gong, Chem. Commun., 2014, 50, 6475–6478 RSC.
- L. E. McQuade and S. J. Lippard, Curr. Opin. Chem. Biol., 2010, 14, 43–49 CrossRef CAS PubMed.
- J. Miao, Y. Huo, X. Lv, Z. Li, H. Cao, H. Shi, Y. Shi and W. Guo, Biomaterials, 2016, 78, 11–19 CrossRef CAS PubMed.
- J. Tang, Z. Guo, Y. Zhang, B. Bai and W.-H. Zhu, Chem. Commun., 2017, 53, 10520–10523 RSC.
- P. Wardman, Free Radicals Biol. Med., 2007, 43, 995–1022 CrossRef CAS PubMed.
- H.-W. Yao, J.-B. Chen, X.-F. Guo and H. Wang, Nitric Oxide, 2017, 67, 30–38 CrossRef CAS PubMed.
- H. Zheng, G.-Q. Shang, S.-Y. Yang, X. Gao and J.-G. Xu, Org. Lett., 2008, 10, 2357–2360 CrossRef CAS PubMed.
- U. Förstermann and W. C. Sessa, Eur. Heart J., 2012, 33, 829–837 CrossRef PubMed.
- J. N. Armor, J. Chem. Eng. Data, 1974, 19, 82–84 CrossRef CAS.
- J. R. Lancaster, Nitric Oxide, 1997, 1, 18–30 CrossRef CAS PubMed.
- A. W. Shaw and A. J. Vosper, J. Chem. Soc., Faraday Trans. 1, 1977, 73, 1239–1244 RSC.
- R. S. Wade and C. E. Castro, Chem. Res. Toxicol., 1990, 3, 289–291 Search PubMed.
- K. A. Broniowska, A. R. Diers and N. Hogg, Biochim. Biophys. Acta, Gen. Subj., 2013, 1830, 3173–3181 CrossRef CAS PubMed.
- B. C. Smith and M. A. Marletta, Curr. Opin. Chem. Biol., 2012, 16, 498–506 CrossRef CAS PubMed.
- S. Carballal, S. Bartesaghi and R. Radi, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 768–780 CrossRef CAS PubMed.
- K. L. Haas and K. J. Franz, Chem. Rev., 2009, 109, 4921–4960 CrossRef CAS PubMed.
- M. H. Lim, Nat. Protoc., 2007, 2, 408 CrossRef CAS PubMed.
- M. H. Lim and S. J. Lippard, Acc. Chem. Res., 2007, 40, 41–51 CrossRef CAS PubMed.
- M. H. Lim, D. Xu and S. J. Lippard, Nat. Chem. Biol., 2006, 2, 375 CrossRef CAS PubMed.
- L. E. McQuade and S. J. Lippard, Inorg. Chem., 2010, 49, 7464–7471 CrossRef CAS PubMed.
- M. D. Pluth, L. E. McQuade and S. J. Lippard, Org. Lett., 2010, 12, 2318–2321 CrossRef CAS PubMed.
- M. Strianese and C. Pellecchia, Coord. Chem. Rev., 2016, 318, 16–28 CrossRef CAS.
- H. Kojima, N. Nakatsubo, K. Kikuchi, S. Kawahara, Y. Kirino, H. Nagoshi, Y. Hirata and T. Nagano, Anal. Chem., 1998, 70, 2446–2453 CrossRef CAS PubMed.
- H. Kojima, Y. Urano, K. Kikuchi, T. Higuchi, Y. Hirata and T. Nagano, Angew. Chem., Int. Ed., 1999, 38, 3209–3212 CrossRef CAS PubMed.
- S. Lepiller, V. Laurens, A. Bouchot, P. Herbomel, E. Solary and J. Chluba, Free Radicals Biol. Med., 2007, 43, 619–627 CrossRef CAS PubMed.
- T. Nagano, J. Clin. Biochem. Nutr., 2009, 45, 111–124 CrossRef CAS PubMed.
- E. Sasaki, H. Kojima, H. Nishimatsu, Y. Urano, K. Kikuchi, Y. Hirata and T. Nagano, J. Am. Chem. Soc., 2005, 127, 3684–3685 CrossRef CAS PubMed.
- D. Jourd'heuil, Free Radicals Biol. Med., 2002, 33, 676–684 CrossRef.
- M.-C. Broillet, O. Randin and J.-Y. Chatton, FEBS Lett., 2001, 491, 227–232 CrossRef CAS PubMed.
- J. Rodriguez, V. Specian, R. Maloney, D. Jourd'heuil and M. Feelisch, Free Radicals Biol. Med., 2005, 38, 356–368 CrossRef CAS PubMed.
- T. Wang, E. F. Douglass, K. J. Fitzgerald and D. A. Spiegel, J. Am. Chem. Soc., 2013, 135, 12429–12433 CrossRef CAS PubMed.
- X. Zhang, W.-S. Kim, N. Hatcher, K. Potgieter, L. L. Moroz, R. Gillette and J. V. Sweedler, J. Biol. Chem., 2002, 277, 48472–48478 CrossRef CAS PubMed.
- Y. Yang, S. K. Seidlits, M. M. Adams, V. M. Lynch, C. E. Schmidt, E. V. Anslyn and J. B. Shear, J. Am. Chem. Soc., 2010, 132, 13114–13116 CrossRef CAS PubMed.
- Y. T. Ghebremariam, N. F. Huang, S. Kambhampati, K. S. Volz, G. G. Joshi, E. V. Anslyn and J. P. Cooke, J. Vasc. Res., 2014, 51, 68–79 CrossRef CAS PubMed.
- S. Smith and W. Pretorius, Water SA, 2002, 28, 395–402 CAS.
- M. Vogel, W. Rettig, R. Sens and K. H. Drexhage, Chem. Phys. Lett., 1988, 147, 452–460 CrossRef CAS.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- M. Segado, I. Gomez and M. Reguero, Phys. Chem. Chem. Phys., 2016, 18, 6861–6874 RSC.
- J.-K. Lee, T. Fujiwara, W. G. Kofron, M. Z. Zgierski and E. C. Lim, J. Chem. Phys., 2008, 128, 164512 CrossRef PubMed.
- Y. Yang, M. Lowry, X. Xu, J. O. Escobedo, M. Sibrian-Vazquez, L. Wong, C. M. Schowalter, T. J. Jensen, F. R. Fronczek, I. M. Warner and R. M. Strongin, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8829 CrossRef CAS PubMed.
- G. Lee Linda, M. Berry Gillian and C. H. Chen, Cytometry, 1989, 10, 151–164 CrossRef PubMed.
- J. E. Whitaker, R. P. Haugland and F. G. Prendergast, Anal. Biochem., 1991, 194, 330–344 CrossRef CAS PubMed.
- W. Gerrard, in Gas Solubilities, ed. W. Gerrard, Pergamon, 1980, pp. 342–360, DOI:10.1016/B978-0-08-025248-3.50020-2.
- I. G. Zacharia and W. M. Deen, Ann. Biomed. Eng., 2005, 33, 214–222 CrossRef PubMed.
- The sigma plus substituent constant for a substituent X was determined by taking the negative logarithm of the ratio of the rate constant for the solvolysis of 2-chloro-2-para-X-phenylpropane versus that for 2-chloro-2-phenylpropane (X = H for the latter). A negative sigma plus value means that the substituent enhanced the rate of solvolysis by electron-donating resonance stabilization. The more negative the value, the more electron donating is a substituent.
- M. Lewis, C. Bagwill, L. K. E. Hardebeck and S. Wireduaah, Comput. Struct. Biotechnol. J., 2012, 1, e201204004 CrossRef PubMed.
- B. C. Challis and S. Kyrtopoulos, J. Chem. Soc., Perkin Trans., 1978, 2, 1296–1302 RSC.
- N. O'Connor and R. B. Silver, in Methods in Cell Biology, ed. G. Sluder and D. E. Wolf, Academic Press, 2013, vol. 114, pp. 387–406 Search PubMed.
- Z. Lacza, E. M. Horváth, E. Pankotai, A. Csordás, M. Kollai, C. Szabó and D. W. Busija, J. Pharmacol. Toxicol. Methods, 2005, 52, 335–340 CrossRef CAS PubMed.
- K. Uhlenhut and P. Högger, Free Radicals Biol. Med., 2012, 52, 2266–2275 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc04304g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |