Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Singlet fission in a hexacene dimer: energetics dictate dynamics†
Samuel N.
Sanders‡
a,
Elango
Kumarasamy‡
a,
Kealan J.
Fallon
 a,
Matthew Y.
Sfeir
a,
Matthew Y.
Sfeir
 *bc and
Luis M.
Campos
*bc and
Luis M.
Campos
 *a
*a
aDepartment of Chemistry, Columbia University, New York, NY 10027, USA. E-mail: lc2730@columbia.edu
bPhotonics Initiative, Advanced Science Research Center, City University of New York, New York, NY 10031, USA. E-mail: msfeir@gc.cuny.edu
cDepartment of Physics, Graduate Center, City University of New York, New York, NY 10016, USA
First published on 9th December 2019
Abstract
Singlet fission (SF) is an exciton multiplication process with the potential to raise the efficiency limit of single junction solar cells from 33% to up to 45%. Most chromophores generally undergo SF as solid-state crystals. However, when such molecules are covalently coupled, the dimers can be used as model systems to study fundamental photophysical dynamics where a singlet exciton splits into two triplet excitons within individual molecules. Here we report the synthesis and photophysical characterization of singlet fission of a hexacene dimer. Comparing the hexacene dimer to analogous tetracene and pentacene dimers reveals that excess exoergicity slows down singlet fission, similar to what is observed in molecular crystals. Conversely, the lower triplet energy of hexacene results in an increase in the rate of triplet pair recombination, following the energy gap law for radiationless transitions. These results point to design rules for singlet fission chromophores: the energy gap between singlet and triplet pair should be minimal, and the gap between triplet pair and ground state should be large.
Introduction
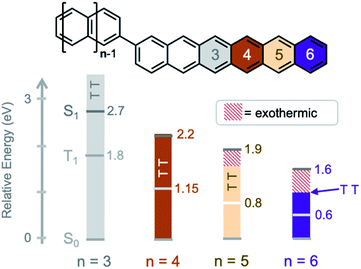
The potential to exploit exciton multiplication in a variety of applications has sparked interest to develop materials to understand intrinsic fundamental details of excited state dynamics.1–7 Singlet fission, where one photon produces two excitons, can occur in organic chromophores with energetically low-lying triplet states.8,9 This process requires electronic interaction between two or more chromophores, and so most research has focused on molecular crystals, polymers, or dimer assemblies in solution.10–18 Dimers serve as model systems to study singlet fission. They represent the fundamental smallest number of chromophores required for SF and varying the connectivity between the chromophores can lead to insightful structure–property relationships of the constrained excitons, from the generation,19–22 separation,23–26 and recombination27–30 of triplet states, to the elucidation of the bound triplet pair state.31–34It has been established that molecular vibrations play a key role in mediating singlet fission in both oligoacene molecular crystals (intermolecular singlet fission, xSF) and in acene intramolecular singlet fission (iSF) compounds.17,35–38 In crystals, it is now commonly accepted that both coherent and incoherent formation of triplet pairs is possible, even within the same system.35,39 Experimental signatures of vibrational coherences have been detected using ultrafast vibrational and 2D electronic spectroscopy, where both inter- and intramolecular vibrational modes have been found to be important.40–44 For incoherent triplet pair formation, the signature of vibrational mediation has been the dependence of the singlet fission rate constant on the energetic driving force ΔES-TT. This driving force increases with n, the number of rings in the oligoacene chromophore, such that tetracene (Tc, n = 4) < pentacene (Pc, n = 5) < hexacene (Hc, n = 6). However, the singlet fission rate constant is not monotonic with ΔES-TT, being maximized when the driving force approaches zero and decreasing for more exo- or endothermic conditions. The rate constant decreases considerably in hexacene, where ΔES-TT is on the order of several molecular vibrations.36,45,46
The important role of vibrations in iSF has also been explored.17 However, no coherent generation of triplet pairs has been reported to date. Rather, singlet fission has been shown to be a purely incoherent process, which can span any time scale that can kinetically compete with decay of the photoexcited singlet. In bridged molecular dimers, singlet fission time constants on the order of 10 ns have been reported.47,48 Recent calculations have suggested that molecular vibrations are essential to bring the energy of the singlet and triplet pair into resonance, enabling fast SF.17 As such, we would expect a similar dependence of the singlet fission rate constant as a function of driving force, i.e., as the energy difference between the singlet and triplet pair increases, the probability of overcoming this energy difference by coupling to molecular vibrations should decrease. However, no iSF materials with a large energetic driving force have been reported to date that would allow us to test this hypothesis.
While dimers of tetracene and pentacene have been extensively studied, the excited state dynamics of hexacene dimers (and longer oligoacenes, n > 6) remain unknown. This is not surprising given that the stability of the oligoacenes is compromised as their π-system is extended and it has been a major challenge to stabilize heptacene (n = 7)49 as calculations predict the emergence of an open-shell diradical character of the singlet ground-state.50,51
Additionally, oligoacenes with low triplet energies are needed to understand triplet pair decay processes. A large range of triplet pair lifetimes has been observed in iSF compounds. Coupled triplet pairs in contiguous molecular dimers have been shown to undergo a rapid non-radiative geminate decay process, while coupled triplet pairs in bridged iSF compounds have been found to persist for ∼1 μs.52 While recently reported molecular design schemes have shown ways to suppress fast recombination and permit quantitative generation of free triplets in individual molecules,23 the nature of this phenomena is still unexplained. Preliminary data has showed that concerted triplet pair decay follows the energy gap law for radiationless transitions, suggesting that multi-vibration relaxation to the singlet ground state is directly occurring.29 Nonetheless, a wider range of triplet pair energies are needed to establish this fact. Thus, in order to develop general guidelines for the design and synthesis of SF chromophores, it is imperative to understand how the intrinsic energies of the materials impact the formation, dissociation, and recombination of multiple exciton states in individual molecules.
Here, we study the excited state dynamics of a hexacene dimer (bihexacene, BH, n = 6) – the most exothermic known SF chromophore of the oligoacene series. Moreover, we compare the excited state dynamics of bitetracene (BT, n = 4), bipentacene (BP, n = 5), and pentacene–tetracene and pentacene–hexacene heterodimers (PT, PH) that have similar core connectivity and stabilizing/solubilizing groups. Within this series, both the energetic driving force for singlet fission and the total triplet energy changes significantly (Fig. 1) using triplet energies taken from literature (summary in ESI†).53,54 These studies provide fundamental insights into the role of vibrations in mediating both the formation and decay of triplet pairs. We find that an energy gap law55,56 holds for both the formation and decay of triplet pairs, with the magnitude of the rate constants decreasing when an increasing number of molecular vibrations is required to overcome energetic differences between the singlet and triplet pair potential energy surfaces.
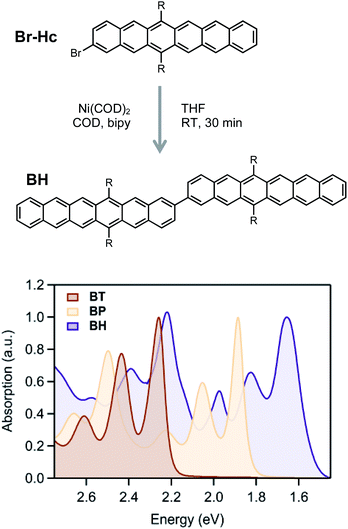
Hexacene derivatives are notoriously unstable and react readily with oxygen or dimerize upon exposure to light. In order to synthesize and characterize these compounds, we adopted the (triisobutyl)silyl acetylene (TIBS) group introduced by Anthony et al., which provides better solubility and stability compared to the more commonly employed triisopropylsilyl acetylene (TIPS) group.46,57,58 Additionally, we connected the dimer at the 2-position in order to compare the excited state dynamics with our previously reported BP16 and BT59,60 with similar connectivity and protecting groups. While Suzuki–Miyaura cross coupling conditions between brominated and analogous borylated acene partners has been the workhorse strategy for preparing acene dimers, it was crucial to simplify the synthetic manipulation of the relatively unstable hexacene derivatives. Therefore, we adopted a mild and step-economical strategy to perform a homocoupling of 2-bromo (TIBS)hexacene using bis(cyclooctadiene)nickel(0) at room temperature (Fig. 2), forming the hexacene dimer in high yield.
The absorption spectra (Fig. 2) show only a modest bathochromic shift, comparing the monomer, Br-HC, to BH, indicating little excited state delocalization over the entire molecule (ESI Fig. S3†), similar to what is observed in BT and BP derivatives. The energy of the singlet excited state was estimated from the absorption onset to be ∼1.55 eV. Given that the energy of the triplet state in solution phase hexacene is estimated to be ∼0.55 eV,50,54 this gives rise to a highly exothermic driving force for fission, approximately double what has been reported for BP, the most exothermic previously reported SF dimer.
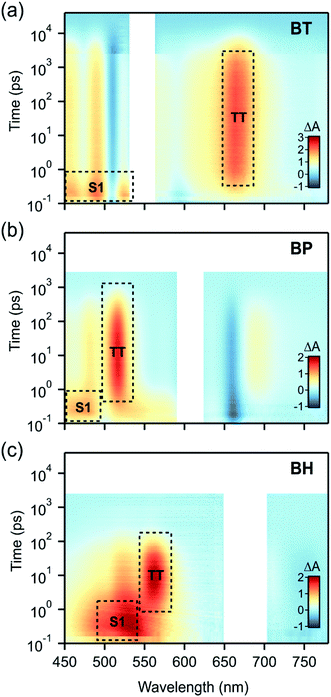
The singlet fission dynamics in BH are established using transient absorption spectroscopy (TAS), following well established techniques.14,16,18,23,27,52 These include comparison of directed photoexcited transient species to triplet sensitization experiments. Spectral decomposition using global analysis techniques allows us to readily extract the time constants for singlet fission and the evolution associated spectra consistent with a sequential decay (S0 → S1 → TT → S0). We note that TAS experiments were carried out in anaerobic conditions, deoxygenating the solution by sparging with argon for 5 min, to prevent photooxidative decomposition. The data was collected after excitation (680 nm pump), using ∼100 fs pulses with a fluence of ∼25 μJ cm−2. The stability of the compound during the experiment was confirmed by linear absorption measurements and by the repeatability of the measurement over multiple transient absorption data sets.
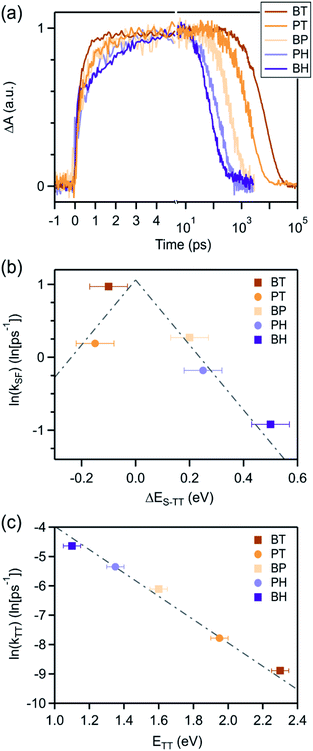
We find that BH undergoes rapid singlet fission to a bound triplet pair, followed by rapid concerted decay of the triplet pair back to the ground state. While these dynamics are qualitatively similar to BP and BT (Fig. 3), differences in the rate constants elucidate the role of molecular vibrations in both the formation and decay of the biexciton. Photoexcitation of BH generates the singlet exciton, which is most readily identified by a characteristic photoinduced absorption (PIA) with a λmax near 530 nm. The decay of this state occurs with a time constant of 2.5 ps and is concurrent with the rise of the triplet pair state, which has a characteristic PIA near 560 nm (annotated in Fig. 3).
To verify that triplet pairs are formed via singlet fission, spectral decomposition of transient absorption data are compared to triplet-sensitization experiments on BH. For triplet sensitization, neat solvent is replaced by a 20 mM solution of anthracene, which upon photoexcitation at 360 nm rapidly generates triplets via intersystem crossing. Due its high concentration, essentially all absorption occurs in the anthracene, followed by collisional transfer of triplet excitons to BH (data in ESI†). The singlet fission triplet pair spectra (from spectral decomposition) and sensitized spectra are indistinguishable, validating our assignment of the triplet state (ESI Fig. S2†). However, we find that the product of singlet decay is triplet pairs that do not dephase into free triplets in these compounds. The triplet pair can be differentiated from an individual triplet because, despite its spectral similarity, it decays remarkably fast, with a recombination time constant of 104 ps. In contrast, the intrinsic lifetime of a lone triplet state on BH was estimated to be 11 μs. These data reflect the dominance of the 1(TT) → S0 concerted decay process that has been observed in other contiguous dimer systems, and indicates a system in the strong exchange coupling limit.29
We can compare the overall singlet fission dynamics in BH, to a set of analogous contiguous dimers with identical connectivity – BP, BT, PT, and PH (Fig. 4, summary Table in ESI†). The 2.5 ps time constant for singlet fission in BH is considerably slower than the time constants observed in all other contiguous dimers: BP (0.76 ps), BT (0.38 ps), PT (0.83 ps), and PH (1.2 ps). This slower time constant in BH is indeed consistent with a multi-vibrational dissipation process for the excess energy driving singlet fission, similar to the role of optical phonons in crystalline solids. Unlike analogous molecular crystals, we do not observe instantaneous triplet pair formation via a coherent process.41 Still, the dependence of the singlet fission rate on the exoergicity implies that vibrations play a large role in the determining the dynamics of the incoherent generation process. In hexacene, electron-phonon coupling proceeds through a few dominant vibrational modes, with energies of ∼180–200 meV.36,41 This can be clearly seen in the vibronic overtones in the linear optical absorption spectrum (Fig. 2), with an energy spacing approximately matching this energy. For compounds with singlet–triplet pair energy gaps exceeding 400 meV (as is expected in hexacene), this corresponds to several molecular vibrations and results in a slower overall singlet fission process. The dependence of the singlet fission rate constant (kSF) on the driving force is not monotonic and peaks for BT (Fig. 4b), which has the smallest difference between the singlet and triplet pair. We note that PT, which is slightly more endothermic, has a slower time constant. The trend from BT > BP > BH is similar to what is observed in molecular crystals.36 While uncertainty in the absolute triplet energies53,54 (error bars in Fig. 4b and c) precludes a deeper analysis, we find that a simplified rate scaling adopted from Busby et al.36 – kSF ∼ exp(−ΔES-TT) – satisfactorily reproduces the observed trend (dotted lines, Fig. 4b).
Similarly, the triplet pair recombination process appears to depend only on the total energy of the triplet pair, and follows the same simple kTT ∼ exp(−ΔETT-S0) scaling behavior (Fig. 4c) observed for kSF. Here, the wider energy range allows us to confirm that the behavior is dictated by a simple energy gap law, which has previously been observed for other radiationless transition processes, including free triplets.55,56,61 The manifestation of the energy gap law here again indicates the importance of molecular vibrations in the overall singlet fission process. We can use evidence from our previous work on molecular dimers to help explain this phenomenon. Contiguous dimers have shown that the singlet and triplet pair energy manifolds are mixed, with allowed TT-Sn optical transitions.32,62 Furthermore, radiationless decay of triplet pairs has been shown to slow down as chromophore proximity decreases. From this, we suggest that the triplet pair decays through coupling to singlet vibronic modes, permitting a rapid and spin-allowed route back to the ground state. We note that the triplet pair lifetime is less than the photoluminescence lifetime of the monomers in all cases, despite the similar energy of the triplet pair to the singlet for BT and BP.16,60,63,64 This supports our assertion of a very distinct recombination process for the triplet pair.
Conclusions
In conclusion, we report a 2,2′-hexacene dimer BH, synthesized through a mild Ni-mediated homocoupling procedure. This compound exhibits sufficient stability for structural and spectroscopic characterization. Using ultrafast transient absorption spectroscopy, we discover relatively slow rates of singlet fission which we attribute to the excessive exothermicity of the hexacene dimer. The resulting triplet pair state is short-lived, explained by the very low ∼1.0 eV energy of the bound state. Our results add clarity to the energy gap law and sheds light on the importance of molecular design as a tool for creating materials with tailored rates of singlet fission and triplet pair recombination.Conflicts of interest
There are no conflicts to declare.Acknowledgements
L. M. C. acknowledges support from the Office of Naval Research Young Investigator Program (Award N00014-15-1-2532) and Cottrell Scholar Award. S. N. S. thanks the NSF for GRFP (DGE 11-44155). This research used resources of the Center for Functional Nanomaterials, which is a U.S. DOE Office of Science Facility, at Brookhaven National Laboratory under Contract No. DE-SC0012704.Notes and references
- Y. Zhao, Y. Guo and Y. Liu, Adv. Mater., 2013, 25, 5372–5391 CrossRef CAS PubMed.
- T. N. Singh-Rachford and F. N. Castellano, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- J. Lee, P. Jadhav, P. D. Reusswig, S. R. Yost, N. J. Thompson, D. N. Congreve, E. Hontz, T. Van Voorhis and M. A. Baldo, Acc. Chem. Res., 2013, 46, 1300–1311 CrossRef CAS PubMed.
- B. D. Ravetz, A. B. Pun, E. M. Churchill, D. N. Congreve, T. Rovis and L. M. Campos, Nature, 2019, 565, 343–346 CrossRef CAS PubMed.
- Q. Zhao, F. Li and C. Huang, Chem. Soc. Rev., 2010, 39, 3007–3030 RSC.
- Q. Zhao, C. Huang and F. Li, Chem. Soc. Rev., 2011, 40, 2508–2524 RSC.
- M. B. Smith and J. Michl, Annu. Rev. Phys. Chem., 2013, 64, 361–386 CrossRef CAS PubMed.
- M. B. Smith and J. Michl, Chem. Rev., 2010, 110, 6891–6936 CrossRef CAS PubMed.
- J. J. Burdett and C. J. Bardeen, Acc. Chem. Res., 2013, 46, 1312–1320 CrossRef CAS PubMed.
- A. M. Müller, Y. S. Avlasevich, W. W. Schoeller, K. Müllen and C. J. Bardeen, J. Am. Chem. Soc., 2007, 129, 14240–14250 CrossRef PubMed.
- A. M. Müller, Y. S. Avlasevich, K. Müllen and C. J. Bardeen, Chem. Phys. Lett., 2006, 421, 518–522 CrossRef.
- J. Zirzlmeier, R. Casillas, S. R. Reddy, P. B. Coto, D. Lehnherr, E. T. Chernick, I. Papadopoulos, M. Thoss, R. R. Tykwinski and D. M. Guldi, Nanoscale, 2016, 8, 10113–10123 RSC.
- J. Zirzlmeier, D. Lehnherr, P. B. Coto, E. T. Chernick, R. Casillas, B. S. Basel, M. Thoss, R. R. Tykwinski and D. M. Guldi, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 5325–5330 CrossRef CAS PubMed.
- T. Sakuma, H. Sakai, Y. Araki, T. Mori, T. Wada, N. V. Tkachenko and T. Hasobe, J. Phys. Chem. A, 2016, 120, 1867–1875 CrossRef CAS PubMed.
- S. N. Sanders, E. Kumarasamy, A. B. Pun, M. T. Trinh, B. Choi, J. Xia, E. J. Taffet, J. Z. Low, J. R. Miller, X. Roy, X.-Y. Zhu, M. L. Steigerwald, M. Y. Sfeir and L. M. Campos, J. Am. Chem. Soc., 2015, 137, 8965–8972 CrossRef CAS PubMed.
- E. G. Fuemmeler, S. N. Sanders, A. B. Pun, E. Kumarasamy, T. Zeng, K. Miyata, M. L. Steigerwald, X.-Y. Zhu, M. Y. Sfeir, L. M. Campos and N. Ananth, ACS Cent. Sci., 2016, 2, 316–324 CrossRef CAS PubMed.
- E. Busby, J. Xia, Q. Wu, J. Z. Low, R. Song, J. R. Miller, X. Zhu, L. M. Campos and M. Y. Sfeir, Nat. Mater., 2015, 14, 426–433 CrossRef CAS PubMed.
- F. S. Conrad-Burton, T. Liu, F. Geyer, R. Costantini, A. P. Schlaus, M. S. Spencer, J. Wang, R. Hernández Sánchez, B. Zhang, Q. Xu, M. L. Steigerwald, S. Xiao, H. Li, C. Nuckolls and X. Zhu, J. Am. Chem. Soc., 2019, 141, 13143–13147 CrossRef CAS PubMed.
- K. J. Fallon, P. Budden, E. Salvadori, A. M. Ganose, C. N. Savory, L. Eyre, S. Dowland, Q. Ai, S. Goodlett, C. Risko, D. O. Scanlon, C. W. M. Kay, A. Rao, R. H. Friend, A. J. Musser and H. Bronstein, J. Am. Chem. Soc., 2019, 141, 13867–13876 CrossRef CAS PubMed.
- P. M. Zimmerman, Z. Zhang and C. B. Musgrave, Nat. Chem., 2010, 2, 648–652 CrossRef CAS PubMed.
- S. W. Eaton, L. E. Shoer, S. D. Karlen, S. M. Dyar, E. A. Margulies, B. S. Veldkamp, C. Ramanan, D. A. Hartzler, S. Savikhin, T. J. Marks and M. R. Wasielewski, J. Am. Chem. Soc., 2013, 135, 14701–14712 CrossRef CAS PubMed.
- A. B. Pun, A. Asadpoordarvish, E. Kumarasamy, M. J. Y. Tayebjee, D. Niesner, D. R. McCamey, S. N. Sanders, L. M. Campos and M. Y. Sfeir, Nat. Chem., 2019, 11, 821–828 CrossRef CAS PubMed.
- L. M. Yablon, S. N. Sanders, H. Li, K. R. Parenti, E. Kumarasamy, K. J. Fallon, M. J. A. Hore, A. Cacciuto, M. Y. Sfeir and L. M. Campos, J. Am. Chem. Soc., 2019, 141, 9564–9569 CrossRef CAS PubMed.
- H. Kim and P. M. Zimmerman, Phys. Chem. Chem. Phys., 2018, 20, 30083–30094 RSC.
- I. Breen, R. Tempelaar, L. A. Bizimana, B. Kloss, D. R. Reichman and D. B. Turner, J. Am. Chem. Soc., 2017, 139, 11745–11751 CrossRef CAS PubMed.
- S. N. Sanders, E. Kumarasamy, A. B. Pun, K. Appavoo, M. L. Steigerwald, L. M. Campos and M. Y. Sfeir, J. Am. Chem. Soc., 2016, 138, 7289–7297 CrossRef CAS PubMed.
- H. Sakai, R. Inaya, H. Nagashima, S. Nakamura, Y. Kobori, N. V. Tkachenko and T. Hasobe, J. Phys. Chem. Lett., 2018, 9, 3354–3360 CrossRef CAS PubMed.
- S. N. Sanders, E. Kumarasamy, A. B. Pun, M. L. Steigerwald, M. Y. Sfeir and L. M. Campos, Angew. Chem., Int. Ed., 2016, 55, 3373–3377 CrossRef CAS PubMed.
- E. Kumarasamy, S. N. Sanders, A. B. Pun, S. A. Vaselabadi, J. Z. Low, M. Y. Sfeir, M. L. Steigerwald, G. E. Stein and L. M. Campos, Macromolecules, 2016, 49, 1279–1285 CrossRef CAS.
- M. Chen, M. D. Krzyaniak, J. N. Nelson, Y. J. Bae, S. M. Harvey, R. D. Schaller, R. M. Young and M. R. Wasielewski, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 8178–8183 CrossRef CAS PubMed.
- M. T. Trinh, A. Pinkard, A. B. Pun, S. N. Sanders, E. Kumarasamy, M. Y. Sfeir, L. M. Campos, X. Roy and X.-Y. Zhu, Sci. Adv., 2017, 3(7), e1700241 CrossRef PubMed.
- B. D. Folie, J. B. Haber, S. Refaely-Abramson, J. B. Neaton and N. S. Ginsberg, J. Am. Chem. Soc., 2018, 140, 2326–2335 CrossRef CAS PubMed.
- S. N. Sanders, A. B. Pun, K. R. Parenti, E. Kumarasamy, L. M. Yablon, M. Y. Sfeir and L. M. Campos, Chem, 2019, 5, 1988–2005 CAS.
- H. L. Stern, A. Cheminal, S. R. Yost, K. Broch, S. L. Bayliss, K. Chen, M. Tabachnyk, K. Thorley, N. Greenham, J. M. Hodgkiss, J. Anthony, M. Head-Gordon, A. J. Musser, A. Rao and R. H. Friend, Nat. Chem., 2017, 9, 1205 CrossRef CAS PubMed.
- E. Busby, T. C. Berkelbach, B. Kumar, A. Chernikov, Y. Zhong, H. Hlaing, X. Y. Zhu, T. F. Heinz, M. S. Hybertsen, M. Y. Sfeir, D. R. Reichman, C. Nuckolls and O. Yaffe, J. Am. Chem. Soc., 2014, 136, 10654–10660 CrossRef CAS PubMed.
- A. J. Musser, M. Liebel, C. Schnedermann, T. Wende, T. B. Kehoe, A. Rao and P. Kukura, Nat. Phys., 2015, 11, 352 Search PubMed.
- A. F. Morrison and J. M. Herbert, J. Phys. Chem. Lett., 2017, 8, 1442–1448 Search PubMed.
- N. R. Monahan, D. Sun, H. Tamura, K. W. Williams, B. Xu, Y. Zhong, B. Kumar, C. Nuckolls, A. R. Harutyunyan, G. Chen, H.-L. Dai, D. Beljonne, Y. Rao and X.-Y. Zhu, Nat. Chem., 2016, 9, 341 CrossRef PubMed.
- A. A. Bakulin, S. E. Morgan, T. B. Kehoe, M. W. B. Wilson, A. W. Chin, D. Zigmantas, D. Egorova and A. Rao, Nat. Chem., 2015, 8, 16 CrossRef PubMed.
- G.-H. Deng, Q. Wei, J. Han, Y. Qian, J. Luo, A. R. Harutyunyan, G. Chen, H. Bian, H. Chen and Y. Rao, J. Chem. Phys., 2019, 151, 54703 CrossRef.
- K. Bera, C. J. Douglas and R. R. Frontiera, J. Phys. Chem. Lett., 2017, 8, 5929–5934 CrossRef CAS PubMed.
- C. Schnedermann, A. M. Alvertis, T. Wende, S. Lukman, J. Feng, F. A. Y. N. Schröder, D. H. P. Turban, J. Wu, N. D. M. Hine, N. C. Greenham, A. W. Chin, A. Rao, P. Kukura and A. J. Musser, Nat. Commun., 2019, 10, 4207 CrossRef PubMed.
- R. Tempelaar and D. R. Reichman, J. Chem. Phys., 2018, 148, 244701 CrossRef PubMed.
- Y. D. Zhang, Y. Wu, Y. Xu, Q. Wang, K. Liu, J. W. Chen, J. J. Cao, C. Zhang, H. Fu and H. L. Zhang, J. Am. Chem. Soc., 2016, 138, 6739–6745 CrossRef CAS PubMed.
- J. Lee, M. J. Bruzek, N. J. Thompson, M. Y. Sfeir, J. E. Anthony and M. A. Baldo, Adv. Mater., 2013, 25, 1445–1448 CrossRef CAS PubMed.
- E. Kumarasamy, S. N. Sanders, M. J. Y. Tayebjee, A. Asadpoordarvish, T. J. H. Hele, E. G. Fuemmeler, A. B. Pun, L. M. Yablon, J. Z. Low, D. W. Paley, J. C. Dean, B. Choi, G. D. Scholes, M. L. Steigerwald, N. Ananth, D. R. McCamey, M. Y. Sfeir and L. M. Campos, J. Am. Chem. Soc., 2017, 139, 12488–12494 CrossRef CAS PubMed.
- B. S. Basel, J. Zirzlmeier, C. Hetzer, B. T. Phelan, M. D. Krzyaniak, S. R. Reddy, P. B. Coto, N. E. Horwitz, R. M. Young, F. J. White, F. Hampel, T. Clark, M. Thoss, R. R. Tykwinski, M. R. Wasielewski and D. M. Guldi, Nat. Commun., 2017, 8, 15171 CrossRef CAS PubMed.
- Z. Sun, Q. Ye, C. Chi and J. Wu, Chem. Soc. Rev., 2012, 41, 7857–7889 RSC.
- Y. Yang, E. R. Davidson and W. Yang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E5098–E5107 CrossRef CAS PubMed.
- M. Bendikov, H. M. Duong, K. Starkey, K. N. Houk, E. A. Carter and F. Wudl, J. Am. Chem. Soc., 2004, 126, 7416–7417 CrossRef CAS PubMed.
- E. Kumarasamy, M. J. Y. Tayebjee, L. M. Campos, S. N. Sanders, D. R. McCamey and M. Y. Sfeir, Nat. Phys., 2016, 13, 182–188 Search PubMed.
- C. K. Yong, A. J. Musser, S. L. Bayliss, S. Lukman, H. Tamura, O. Bubnova, R. K. Hallani, A. Meneau, R. Resel, M. Maruyama, S. Hotta, L. M. Herz, D. Beljonne, J. E. Anthony, J. Clark and H. Sirringhaus, Nat. Commun., 2017, 8, 15953 Search PubMed.
- H. Angliker, E. Rommel and J. Wirz, Chem. Phys. Lett., 1982, 87, 208–212 CrossRef CAS.
- J. V Caspar and T. J. Meyer, J. Phys. Chem., 1983, 87, 952–957 CrossRef.
- R. Englman and J. Jortner, Mol. Phys., 1970, 18, 145–164 CrossRef CAS.
- M. M. Payne, S. R. Parkin and J. E. Anthony, J. Am. Chem. Soc., 2005, 127, 8028–8029 CrossRef CAS PubMed.
- J. E. Anthony, D. L. Eaton and S. R. Parkin, Org. Lett., 2002, 4, 15–18 CrossRef CAS PubMed.
- A. B. Pun, S. N. Sanders, M. Y. Sfeir, L. M. Campos and D. N. Congreve, Chem. Sci., 2019, 10, 3969–3975 RSC.
- A. B. Pun, S. N. Sanders, E. Kumarasamy, M. Y. Sfeir, D. N. Congreve and L. M. Campos, Adv. Mater., 2017, 29, 1701416 CrossRef PubMed.
- J. S. Wilson, N. Chawdhury, M. R. A. Al-Mandhary, M. Younus, M. S. Khan, P. R. Raithby, A. Köhler and R. H. Friend, J. Am. Chem. Soc., 2001, 123, 9412–9417 CrossRef CAS PubMed.
- S. Khan and S. Mazumdar, J. Phys. Chem. Lett., 2017, 8, 4468–4478 CrossRef CAS PubMed.
- N. V. Korovina, J. Joy, X. Feng, C. Feltenberger, A. I. Krylov, S. E. Bradforth and M. E. Thompson, J. Am. Chem. Soc., 2018, 140, 10179–10190 CrossRef CAS PubMed.
- N. Nijegorodov, V. Ramachandran and D. P. Winkoun, Spectrochim. Acta, Part A, 1997, 53, 1813–1824 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc05066c |
| ‡ SNS and EK contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |