Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unravelling the mechanism of cobalt-catalysed remote C–H nitration of 8-aminoquinolinamides and expansion of substrate scope towards 1-naphthylpicolinamide†
Melody
Chu
a,
Oriol
Planas
b,
Anna
Company
 b,
Xavi
Ribas
b,
Xavi
Ribas
 b,
Alex
Hamilton
b,
Alex
Hamilton
 *a and
Christopher J.
Whiteoak
*a and
Christopher J.
Whiteoak
 *a
*a
aDepartment of Biosciences and Chemistry, Sheffield Hallam University, Sheffield, S1 1WB, UK. E-mail: c.whiteoak@shu.ac.uk; a.hamilton@shu.ac.uk
bDepartament de Química, Grup de Química Bioinspirada, Supramolecular i Catàlisi (QBIS-CAT), Institut de Química Computacional i Catàlisi (IQCC), Universitat de Girona, Campus de Montilivi, 17071 Girona, Catalonia, Spain
First published on 18th November 2019
Abstract
Previously, an unexpected Co-catalysed remote C–H nitration of 8-aminoquinolinamide compounds was developed. This report provided a novel reactivity for Co which was assumed to proceed through the mechanistic pathway already known for analogous Cu-catalysed remote couplings of the same substrates. In order to shed light into this intriguing, and previously unobserved reactivity for Co, a thorough computational study has now been performed, which has allowed for a full understanding of the operative mechanism. This study demonstrates that the Co-catalysed remote coupling does not occur through the previously proposed Single Electron Transfer (SET) mechanism, but actually operates through a high-spin induced remote radical coupling mechanism, through a key intermediate with significant proportion of spin density at the 5- and 7-positions of the aminoquinoline ring. Additionally, new experimental data provides expansion of the synthetic utility of the original nitration procedure towards 1-naphthylpicolinamide which unexpectedly appears to operate via a subtly different mechanism despite having a similar chelate environment.
Introduction
The controlled and selective functionalisation of C–H bonds is currently a topic which is attracting a significant amount of interest from the chemical community.1 When successfully applied, C–H functionalisation protocols are generally used to either reduce the length of complex multi-step syntheses or to provide rapid access to new derivatives of biologically active molecules as a late stage functionalisation tool.2 The challenge in the latter application is to regiochemically functionalise a complex compound in a predictable and controlled fashion, whereby there are often a number of other reactive functional groups present. Currently, a majority of the developed C–H functionalisation protocols utilise a chelation directing group strategy for control of product selectivity3 and well established/understood Concerted Metalation Deprotonation (CMD) or Oxidative Addition (OA) steps.4 These key mechanistic approaches are very common when employing second and third-row transition metals in C–H functionalisation. In contrast, the chemistries of first-row transition metals are more diverse than second- and third-row analogues, which provides an exciting potential opportunity to discover and develop novel protocols outside of those using traditional CMD and OA steps (e.g. radical coupling approaches).5 In the case of late stage C–H bond functionalisation, these unique reactivities could be applied to provide access to previously inaccessible analogues of biologically active compounds through new innovative C–C and C–X bond forming protocols.Several first-row transition metals have been shown to provide reactivities amenable for application in C–H functionalisation protocols. However, the already known rich mechanistic diversity of Co in the field of C–H functionalisation makes this metal stand out as an exciting candidate for the basis of novel protocols.6,7
Functionalisation of quinolines beyond traditional C2 modification has recently become a topic of increasing interest8 as quinoline is an important heterocycle found in many biologically active compounds. For example, the 8-aminoquinoline scaffold is the basis of a number of anti-malarial drugs9 and facile modification as a late stage functionalisation tool may provide access to new potent drugs which could have significant impact on one of the world's major causes of death.10
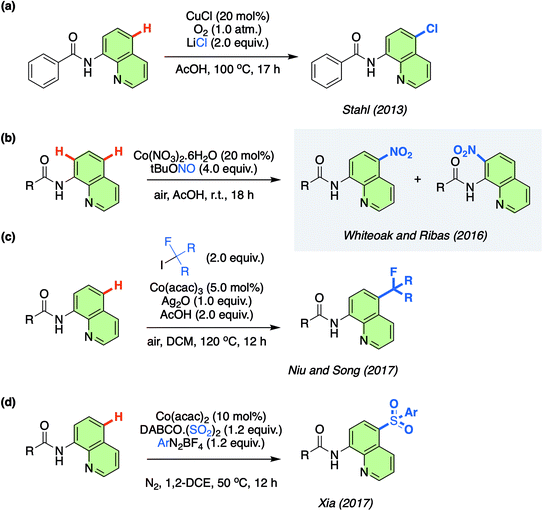
One novel approach to the functionalisation of 8-aminoquinolinamides has been the remote (non-proximate) C–H bond chlorination catalysed by Cu at the C7 position, which was originally reported by Stahl/Ertem and co-workers in 2013 (Scheme 1a).11 Non-proximate C–H bond functionalisation, therefore moving beyond directing group based protocols, is a major challenge in synthetic chemistry with only a limited, but increasing, number of approaches currently available.12 This initial work by Stahl/Ertem and co-workers demonstrated the first example of a metal-catalysed remote C–H functionalisation of 8-aminoquinolinamides and Density Functional Theory (DFT) calculations were employed to elucidate the mechanism, which was found to be based on a key Single Electron Transfer (SET) step. This seminal work has been the inspiration for the development of an ever-expanding number of practical Cu-catalysed remote C–H functionalisation protocols of the same substrate.13 Meanwhile, other metals have also been employed as catalysts for remote C–H functionalisation of these 8-aminoquinolinamides, although to a significantly reduced extent.14
Resulting from the rich aforementioned chemistry of Co, in 2016 some of us reported on the unexpected remote nitration of 8-aminoquinolinamide scaffold using tert-butyl nitrite (TBN) as nitro source (Scheme 1b).15,16 This was the first time that Co had been observed to display this type of reactivity. Since this report, others have also been attracted to the potential of this novel potential for Co in the field of C–H functionalisation (Scheme 1c and d).17 The lack of understanding of this new Co-catalysed approach has now inspired us to examine the mechanism in more detail utilising the open shell DLPNO-CCSD(T) (Domain-based Local Pair-Natural Orbital Coupled Cluster approximations) method, in order that the unique reactivity of Co can be fully understood and as a result be further rationally exploited by others.
Herein, we describe our findings and also demonstrate that the protocol can also be successfully transferred to the remote nitration of 1-naphthylpicolinamide analogues which replace the aminoquinolinamide chelate environment with a picolinamide, thus further expanding the applicability of the previously discovered protocol.
Results and discussion
The initial approach in this study was to identify the nature of the starting “Co(II) source” in the mechanism. Given the use of AcOH as solvent, the experimental conditions would allow for extensive counter-ion ligand exchange, leading to a number of potential Co(II) species. Relative free energies for ligand exchange (Table 1) identified Co(OAc)2 as the most thermodynamically stable Co species. This compound was therefore used as the basis for the active catalyst species for the remainder of the reaction mechanism. It should be noted at this point that the observed benefit of using Co(NO3)2 over Co(OAc)2 in the original protocol15 is likely a result of the background nitration of the substrate through traditional nitration utilising in situ formed HNO3 in an acidic environment.| Co(II) species | Relative free energy, ΔG298 (kcal mol−1) |
|---|---|
| Co(NO3)2 | 0.00 |
| Co(NO3) (OAc) | −20.21 |
| Co(OAc)2 | −48.34 |
After this initial study on catalyst species we embarked on full elucidation of the reaction mechanism. As a result of the moderate to large size of the catalytic system being studied, DFT calculations would traditionally be the method of choice. However, due to the potential for different spin states to be involved in the mechanism, which pose a significant challenge for DFT based methods, the recently implemented open shell DLPNO-CCSD(T) method was utilised.18,19 This near linear scaling ab initio electronic structure method has the potential for coupled cluster accuracy for large, synthetically relevant catalysts. Recent work by Chen and co-worker on the same catalyst, but focusing on a different C–H activation protocol, highlighted the importance of these methods due to the complicated multi-state reactivity which has been previously reported.20 In this report Multi-State Reactivity (MSR) was found to be an intriguing feature of the chemistry of high-spin Co(III), where this report revealed the highly complex mechanisms operative in Co-catalyzed C–H activation processes.
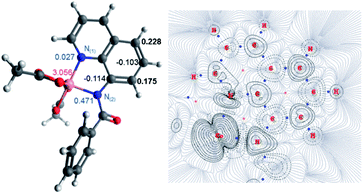
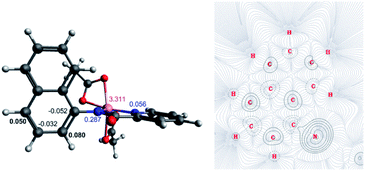
The first step of the reaction is hydrogen abstraction by the in situ formed tBuO˙ radical leading to the radical of the 8-aminoquinolinamide ligand, L1. Coupling L1 to the [Co(AcO)2] pre-catalyst forms the initial catalytic species Int1·L1. This species can exist in three possible spin states; low spin singlet (1Int 1, S = 0), medium spin triplet (3Int 1, S = 1) and high spin quintet (5Int 1, S = 2). With the DLPNO-CCSD(T) method the high spin quintet state (5Int 1·L1) proved to be the ground-state species, which is in full agreement with previous work from Chen and co-worker.20 Examination of the electronic structure of the three potential spin state complexes leads to the conclusion that both 1Int 1·L1 and 3Int 1·L1 are Co(III) complexes whereas the 5Int 1·L1 is actually a high-spin quartet Co(II) species with the radical still residing on the quinoline moiety of the ligand. Cumulative spin densities on the aminoquinoline ligand, calculated using DLPNO-CCSD/def2-tzvp,21 for 1Int 1·L1, 3Int 1·L1 and 5Int 1·L1 are 0, 0.18 and 0.72, respectively. Fig. 1 highlights the increased electron spin density residing at 5- and 7-positions, showing the potential sites for radical addition to 5Int 1·L1. A similar spin population distribution was previously observed by Chen and co-worker leading to the description as a quartet Co(II) ferromagnetically coupled with a ligand radical.20
Topological analysis using Quantum Theory of Atom in Molecules (QTAIM)22 and different bond order descriptors (Table 2) for the three spin state structures highlight a significant decrease in bonding/interaction between the Co centre and the 8-aminoquinolinamide ligand on progression from singlet to quintet state. This is in further agreement with the assignment of 1Int 1·L1 and 3Int 1·L1 being Co(III) whereas 5Int 1·L1 being high spin Co(II) with an associated radical ligand, based on the spin density analysis.
| Bonding parameter | 1Int1·L1 | 3Int1·L1 | 5Int1·L1 | |||
|---|---|---|---|---|---|---|
| Co–N(1) | Co–N(2) | Co–N(1) | Co–N(2) | Co–N(1) | Co–N(2) | |
| a Bonding parameters: Co–N(1)amide Co–N(2)pyrrole: distance r in Å, QTAIM parameters in a.u.; ρ electron density, ∇2ρ laplacian of electron density, H(r) local energy density. FBO = Fuzzy Bond Order,24 MBO = Mayer Bond Order,25 LBO = Laplacian Bond Order.26 | ||||||
| r | 1.948 | 1.894 | 1.878 | 1.925 | 1.985 | 2.073 |
| ρ | 0.104 | 0.113 | 0.125 | 0.108 | 0.087 | 0.072 |
| ∇2ρ | 0.408 | 0.563 | 0.441 | 0.474 | 0.477 | 0.356 |
| H(r) | −0.030 | −0.031 | −0.045 | −0.032 | −0.020 | −0.014 |
| FBO | 1.053 | 1.010 | 1.183 | 0.982 | 0.901 | 0.784 |
| MBO | 0.736 | 0.651 | 0.777 | 0.626 | 0.434 | 0.399 |
| LBO | 0.369 | 0.515 | 0.450 | 0.454 | 0.343 | 0.268 |
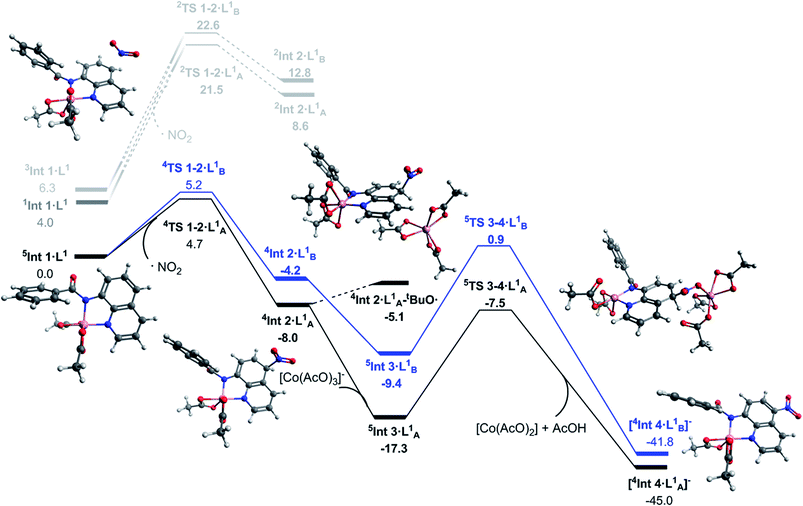
The transition states for the remote NO2 radical coupling at the 5- and 7-positions starting from 5Int 1·L1 lie at 4.7 and 5.2 kcal mol−1 respectively (Fig. 2). This low barrier height is in good agreement with the protocol being operative under ambient conditions. Additionally, the nitration step is also the regioselective transition state with a ΔΔG‡298 K of 0.5 kcal mol−1 in favour of the observed 5-substituted product. This difference results in a calculated regioselectivity of 60%, which is in reasonably good agreement with the experimentally observed 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 product ratio. Calculation of the low spin nitration reaction, via2TS 1–2·LA/B1, showed it to be approximately 17 kcal mol−1 less favourable than the proposed high spin mechanism shown in Fig. 2. This substantial stabilisation of the high-spin mechanism further strengthens the proposed remote radical coupling mechanism via the quintet state. There has been discussion in the literature regarding the potential over-stabilisation of high spin complexes with DLPNO-CCSD(T) methods,23 however the degree of over-stabilisation is in the order of 5 kcal mol−1 at most and therefore as a result we are confident this reaction proceeds via the proposed high spin pathway.
1 product ratio. Calculation of the low spin nitration reaction, via2TS 1–2·LA/B1, showed it to be approximately 17 kcal mol−1 less favourable than the proposed high spin mechanism shown in Fig. 2. This substantial stabilisation of the high-spin mechanism further strengthens the proposed remote radical coupling mechanism via the quintet state. There has been discussion in the literature regarding the potential over-stabilisation of high spin complexes with DLPNO-CCSD(T) methods,23 however the degree of over-stabilisation is in the order of 5 kcal mol−1 at most and therefore as a result we are confident this reaction proceeds via the proposed high spin pathway.
To ensure the previously proposed intramolecular Singlet Electron Transfers (SET) mechanism was not involved in the reaction, LR-TD-DFT (Linear Response Time Dependent-Density Functional Theory) calculations were performed on 1Int 1·L1. The first excited state transition, corresponding to the excitation of an electron from the 8-aminoquinolinamide ligand to the Co(III) centre, is found to be in the range of 37–43 kcal mol−1, depending on the choice of DFT functional and amount of HF exchange included (see ESI for full details†). The transition energy is similar to that reported by Stahl/Ertem and co-workers for the related Cu(II) complex.11 Optimisation of the corresponding excited state structure, 1Int 1ES·L1, with the hybrid functional PBE0 provides a value of 24.4 kcal mol−1 above 1Int 1·L1. Although not directly comparable due to the differing methodologies used, both the transition energy and the optimised excited state complex is significantly higher in energy than the transition state on the proposed high-spin mechanism starting from the quintet ground state, 5Int 1·L1. As a result of the requirement for a coupling transition state after 1Int 1ES·L1, this precludes this mechanism on the grounds of it being energetically unfeasible.
Continuing with the high-spin mechanism, the nitration of 5Int 1·L1 results in the formation of the intermediate complex, 4Int 2·L1. This intermediate is 8.0 kcal mol−1 lower in energy than the preceding starting complex. The lower energy pathway leading to nitration at the 5-position forms 4Int 2·LA1 which is 3.8 kcal mol−1 more stable than the corresponding 7-substituted product (4Int 2·LB1), and 16.6 kcal mol−1 more stable than the equivalent low spin doublet mechanism (2Int 2·LA1). A number of different pathways for the deprotonation of 4Int 2·L1 at the 5- and 7-positions were explored. Addition of [Co(AcO)3]− to 4Int 2·L1 forms 5Int 3·L1, this step is exergonic by 9.7 kcal mol−1. Cobalt assisted deprotonation by acetate anion has a barrier of 9.8 kcal mol−1, leading to the final intermediate [4Int 4·L1]−, which undergoes proto-demetallation to form the observed product and regenerates the catalyst, completing the catalytic cycle. Attempts to optimise the transition state for deprotonation by an isolated acetate anion led directly to [4Int 4·L1]−, suggesting this pathway could also be occurring, but no energetic barrier is reported. Due to the reaction conditions allowing for the formation of tBuO˙ (eqn (1)), a hydrogen atom abstraction pathway was also explored. Formation of the adduct complex 4Int 2·LA1–tBuO˙ is endergonic by 2.9 kcal mol−1 and therefore 12.2 kcal mol−1 less favourable than 5In t3·LA1 and 2.4 kcal mol−1 less favourable than 5TS 3–4·L1. Based on these results we propose cobalt assisted acetate deprotonation is the active pathway (Fig. 2). Nitration at the 7-position (route B) is consistently between 3 and 8 kcal mol−1 less favourable throughout the entire mechanism, and in good agreement with the experimental observations for regioselectivity.
| tBuONO → tBuO˙ + NO | (1) |
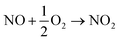 | (2) |
As a result of this new in-depth study, in adjustment to our previously proposed SET mechanism for the Co-catalysed remote nitration of 8-aminoquinolamide substrates, we now propose the mechanism shown in Scheme 2. In the first step, the Co(II) coordination to substrate is promoted by H˙ abstraction from the amide by the tBuO˙ radical generated from in situ decomposition of the TBN. This results in a high spin Co(II) species with partially delocalised radical character on the 5- and 7-positions of the 8-aminoquinolinamide moiety which then reacts with the nitrogen dioxide (NO2) formed from the decomposition of TBN (eqn (1) and (2)).27 Deprotonation of the intermediate coupling product with the in situ formed [Co(OAc)3]− furnishes the chelate species of the final product, which can then undergo proto-demetallation by acetic acid to reform the initial Co(OAc)2 catalyst, releasing the final nitrated 8-aminoquinolinamide product.
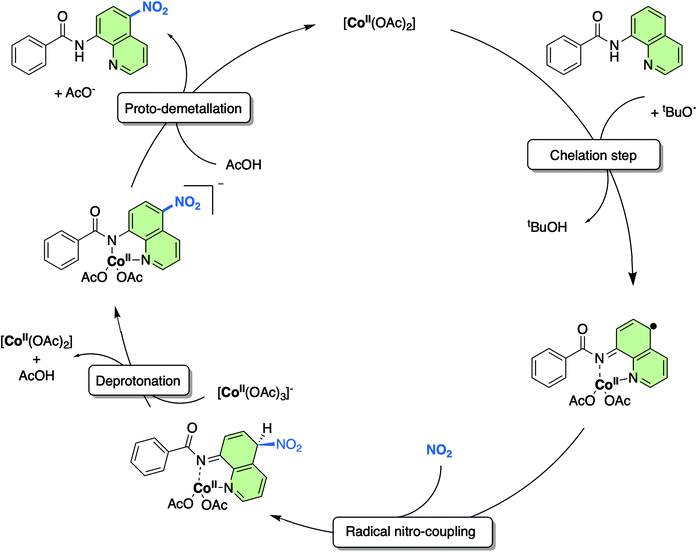 | ||
| Scheme 2 Proposed mechanism for the remote nitration of 8-aminoquinolinamide substrates catalysed by Co(OAc)2 at the 5-position based on the computational studies in from this work. Note: the NO2 is formed in situ from the decomposition of TBN (see eqn (1) and (2)). | ||
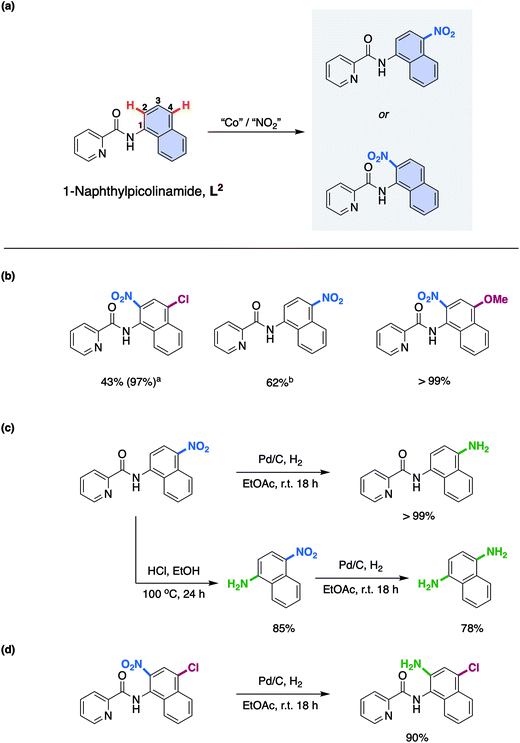
With this understanding in hand, we turned our attention to expanding the scope of this novel reaction mechanism. Rather than focus on addition of different coupling partners at the 5- and 7-positions of the 8-aminoquinolinamide substrate, something which has already been exemplified by both Xia and co-workers17a and Niu and Song and co-workers,17b we choose to focus on selective nitration of substrates that have proven more challenging by more traditional synthetic methods. In an effort to direct experiments a new substrate was rationally proposed which would facilitate selective 1-aminonaphthyl nitration while maintaining the key bi-dentate nitrogen chelation. 1-Naphthylpicolinamide, L2, (Scheme 3a) was chosen to ensure formation of the proposed required Co chelate intermediate. Indeed, Cu examples of remote functionalisation of these substrates are known and have previously been reported.28
As with the 8-aminoquinolinamide substrate, it was possible to convert a number of differently substituted 1-naphthylpicolinamide substrates to the corresponding nitrated products as shown in Scheme 3b. The electron-withdrawing 4-chloro-substituted 1-naphthylpicolinamide, provided clean nitration at the 2-position. It should be noted that in order to get an almost quantitative yield (97%), it was necessary to increase the TBN loading from 4.0 equivalents to 6.0 equivalents.15 The lower reactivity of electron-withdrawing substituted substrates was previously observed with the 8-aminoquinolinamide substrates.15 The parent 1-naphthylpicolinamide substrate with no substituents could also be nitrated, although despite our best efforts it was not possible to successfully isolate the 2-nitration product. As a result of this, the reported yield therefore corresponds to the 4-nitration product only, which was successfully obtained as an analytically pure sample. Finally, the electron-donating methoxy-substituted substrate could also be cleanly converted to the corresponding 2-nitrated product quantitatively.
In order to exemplify the potential for these compounds for further utility, the 1-naphthylpicolinamide with nitration at the 4-position was initially reduced to the corresponding amine using Pd/C and H2 (Scheme 3c). Additionally, the picolinamide bond could be hydrolysed under acid conditions to furnish 1-amino-4-nitronaphthalene in good yield (85%) and thereafter, the 1,4-diaminonaphthalene was prepared in good yield through reduction of this nitro-product using Pd/C and hydrogen (78%; Scheme 3c). Finally, a highly substituted product containing synthetically useful chloro and amine functionalities again obtained through a facile Pd/C and H2 reduction of the nitration product of the 4-chloro-substituted 1-naphthylpicolinamide could be easily prepared (Scheme 3d).
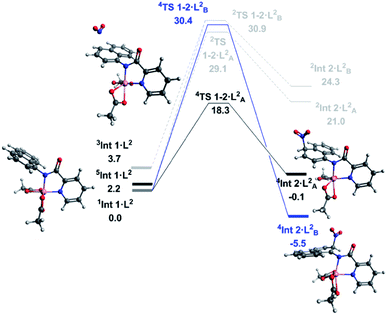
With successful transfer of the original nitration reaction to the 1-naphthylpicolinamide substrates we wondered if it followed the same reaction mechanism. Initially it was assumed that the 1-naphthylpicolinamide substrate would follow a similar reaction path to the 8-aminoquinolinamide example. In contrast to the 8-aminoquinalineamide substrate, the DLPNO-CCSD(T) calculations suggest the ground state of the 1-naphthylpicolinamide complex is the singlet (S = 0) with the quintet (S = 2) and triplet (S = 1) states being 2.2 and 3.7 kcal mol−1 higher in energy respectively (Fig. 3). TDDFT calculations on 1Int 1·L2 gives the first excited state transition, corresponding to the SET mechanism, of 37.7 kcal mol−1. An equivalent to the barrier to this was seen for 1Int 1·L1.
Thereafter spin density analysis of 5Int 1·L2 showed a significant proportion of density at the observed reactive 2- and 4-positions of the 1-aminonaphthyl moiety (Fig. 4), again highlighting the potential for remote radical coupling. Nitration at the 2- and 4-position of the 1-naphthylpicolinamide substrate can then proceed via either a low spin (S = 1/2) or high spin (S = 3/2) pathway. As with the 8-aminoquiolinamide mechanism the high spin pathway, through 4TS 1-2·L2, is significantly more favourable. This suggests a Multi-State Reactivity (MSR) mechanism with crossing between potential energy surfaces,29 from low to high spin, as the nitration step proceeds in order to facilitate the remote radical coupling. The high spin products of the nitration step, 4Int 2·LA/B2, are approximately 25 kcal mol−1 more energetically stable compared to the equivalent low spin intermediate, 2Int 2·LA/B2. Interestingly the observed substitution at the 4-position is kinetically favoured, while the 2-position is the thermodynamic intermediate. This can be accounted for by the additional long range hydrogen bonding (2.45 Å) between the hydrogen at the 2-position of the nitrated naphthyl moiety and the Co bound acetate. The calculated higher energy barriers for 1-naphthylpicolinamide compared to 8-aminoquinolinamide agrees with the experimental requirement for more forcing reaction conditions (increase in number of equivalents of TBN in the case of 4-chloro-substituted 1-naphthylpicolinamide). Increased spin density on the Co and decreased spin density on the 1-aminonaphthyl moiety further support this decrease in reactivity (Fig. 4).
Conclusions
In summary, we have studied the mechanism of the remote nitration of 8-aminoquinolinamides catalysed by Co using the robust DLPNO-CCSD(T) methodology and found there to be a significant difference in mechanism to the well-studied analogous Cu-catalysed remote functionalisation protocols of 8-aminoquinolinamides. The results reported within this work identify an unusual mechanism which does not pass through the initially proposed SET process, but instead, the reaction takes place via a High-Spin Remote Induced Radical Coupling, providing an alternative route for the NO2 to couple compared with a traditional SET process. The high spin reactivity has been successfully transferred to 1-naphthylpicolinamide substrates, further enhancing the applicability of the original protocol, where the computational study has demonstrated that a subtly different mechanism is operative (Multi-State Reactivity). Overall, the distinct mechanistic pathways shown in this work provide a complimentary tool for the remote C–H functionalisation of both 8-aminoquinolinamides and 1-naphthylpicolinamides. This new mechanistic understanding should now inspire others to develop new synthetic protocols for remote functionalisation using Co-catalysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. H., C. J. W. and M. C. would like to thank the Department of Biosciences and Chemistry/Biomolecular Sciences Research Centre (BMRC) at Sheffield Hallam University for funding. X. R. and A. C. thank the Spanish MICINN (CTQ2016-77989-P), and the Catalan DIUE of the Generalitat de Catalunya (2017SGR264). X. R. thanks ICREA for an ICREA Acadèmia award. C. J. W. and X. R. also thank COST Action CHAOS (CA15106). O. P. thanks the Spanish MECD for a pre-doctoral fellowship (FPU13-04099). We thank IQCC for granting access to their computing cluster.Notes and references
-
J. J. Li, C–H Bond Activation in Organic Synthesis, CRC Press, Boca Raton, 1st edn, 2017 Search PubMed
.
- For an overview see:
(a) D. C. Blakemore, L. Castro, I. Churcher, D. C. Rees, A. W. Thomas, D. M. Wilson and A. Wood, Nat. Chem., 2018, 10, 383–394 CrossRef CAS PubMed
; (b) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369–375 CrossRef CAS PubMed
.
- For an overview of directing groups in metal-catalysed C–H functionalisations, see:
(a) C. Sambiagio, D. Schönbauer, R. Blieck, T. Dao-Huy, G. Pototschnig, P. Schaaf, T. Wiesinger, M. F. Zia, J. Wencel-Delord, T. Besset, B. U. W. Maes and M. Schnürch, Chem. Soc. Rev., 2018, 47, 6603–6743 RSC
; (b) M. Zhang, Y. Zhang, X. Jie, H. Zhao, G. Li and W. Su, Org. Chem. Front., 2014, 1, 843–895 RSC
; (c) M. Corbet and F. De Campo, Angew. Chem., Int. Ed., 2013, 52, 9896–9898 CrossRef CAS
.
- For an overview of mechanisms of C–H bond activation see: A. E. Shilov and G. B. Shul'pin, Chem. Rev., 1997, 98, 2879–2932 CrossRef PubMed
.
- H. Yi, G. Zhang, H. Wang, Z. Huang, J. Wang, A. K. Singh and A. Lei, Chem. Rev., 2017, 117, 9016–9085 CrossRef CAS PubMed
.
- For reviews of Co-catalysed C–H functionalisation protocols see:
(a) G. Pototschnig, N. Maulide and M. Schnürch, Chem.–Eur. J., 2017, 23, 9206–9232 CrossRef CAS PubMed
; (b) W. Liu and L. Ackermann, ACS Catal., 2016, 6, 3743–3752 CrossRef CAS
; (c) P. G. Chirila and C. J. Whiteoak, Dalton Trans., 2017, 46, 9721 RSC
; (d) T. Yoshino and S. Matsunaga, Adv. Synth. Catal., 2017, 359, 1245 CrossRef CAS
; (e) S. Wang, S.-Y. Chen and X.-Q. Yu, Chem. Commun., 2017, 53, 3165 RSC
.
- For an overview of some of the many different mechanisms reported for Co-catalysed C–H functionalisation protocols see: O. Planas, P. G. Chirila, C. J. Whiteoak and X. Ribas, Adv. Organomet. Chem., 2018, 69, 209–282 CrossRef
.
- T. Iwai and M. Sawamura, ACS Catal., 2015, 5, 5031–5040 CrossRef CAS
.
-
(a) S. Vandekerckhove and M. D'Hooghe, Bioorg. Med. Chem., 2015, 23, 5098–5119 CrossRef CAS PubMed
; (b) X. Nqoro, N. Tobeka and B. A. Aderibigbe, Molecules, 2017, 22, 2268 CrossRef PubMed
.
- R. Sharma, S. Patil and P. Maurya, SAR QSAR Environ. Res., 2013, 25, 189–203 CrossRef PubMed
.
- A. M. Suess, M. Z. Ertem, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 9797–9804 CrossRef CAS PubMed
.
- For some innovative examples see:
(a) Z. Zhang, K. Tanaka and J.-Q. Yu, Nature, 2017, 543, 538–542 CrossRef CAS PubMed
; (b) J. Luo, S. Preciado and I. Larrosa, J. Am. Chem. Soc., 2014, 136, 4109–4112 CrossRef CAS PubMed
; (c) O. Saidi, J. Marafie, A. E. W. Ledger, P. M. Liu, M. F. Mahon, G. Kociok-Köhn, M. K. Whittlesey and C. G. Frost, J. Am. Chem. Soc., 2011, 133, 19298–19301 CrossRef CAS PubMed
; (d) N. Hofmann and L. Ackermann, J. Am. Chem. Soc., 2013, 135, 5877–5884 CrossRef CAS PubMed
; (e) C. Yuan, L. Zhu, R. Zeng, Y. Lan and Y. Zhao, Angew. Chem., Int. Ed., 2018, 57, 1277–1281 CrossRef CAS PubMed
.
- For some representative examples see:
(a) X.-X. Liu, Z.-Y. Wu, X.-L. Luo, Y.-Q. He, X.-Q. Zhou, Y.-X. Fan and G.-S. Huang, RSC Adv., 2016, 6, 71485–71488 RSC
; (b) J. Xu, C. Shen, X. Zhu, P. Zhang, M. J. Ajitha, K.-W. Huang, Z. An and X. Liu, Chem.–Asian J., 2016, 11, 882–892 CrossRef CAS PubMed
; (c) C. Xia, K. Wang, J. Xu, Z. Wei, C. Shen, G. Duan, Q. Zhu and P. Zhang, RSC Adv., 2016, 6, 37173–37179 RSC
; (d) X. Zhu, L. Qiao, P. Ye, B. Ying, J. Xu, C. Shen and P. Zhang, RSC Adv., 2016, 6, 89979–89983 RSC
; (e) Y. Dou, Z. Xie, Z. Sun, H. Fang, C. Shen, P. Zhang and Q. Zhu, ChemCatChem, 2016, 8, 3570–3574 CrossRef CAS
; (f) C. Xia, K. Wang, J. Xu, C. Shen, D. Sun, H. Li, G. Wang and P. Zhang, Org. Biomol. Chem., 2017, 15, 531–535 RSC
; (g) L. Zhu, R. Qiu, X. Cao, S. Xiao, X. Xu, C.-T. Au and S.-F. Yin, Org. Lett., 2015, 17, 5528–5531 CrossRef CAS PubMed
; (h) H. Saloo, A. Mandal, J. Selvakumarm and M. Baidya, Eur. J. Org. Chem., 2016, 4321–4327 Search PubMed
; (i) H. Li, H. Chen, C. Bian, Z. Tang, A. K. Singh, X. Qi, X. Yue, Y. Lan, J.-F. Lee and A. Lei, Chem. Commun., 2017, 53, 6736–6739 RSC
; (j) S. Han, A. Liang, X. Ren, X. Gao, J. Li, D. Zou, Y. Wu and Y. Wu, Tetrahedron Lett., 2017, 58, 4859–4863 CrossRef CAS
.
- For a comprehensive overview of recent advances in metal catalysed remote C–H functionalisation's of 8-aminoquinolinamides see: B. Khan, H. S. Dutta and D. Koley, Asian J. Org. Chem., 2018, 7, 1270–1297 CrossRef CAS
.
- C. J. Whiteoak, O. Planas, A. Company and X. Ribas, Adv. Synth. Catal., 2016, 358, 1679–1688 CrossRef CAS
.
-
tert-Butyl nitrite has found a variety of uses in organic chemistry and has recently been well reviewed: A. Dahiya, A. K. Sahoo, T. Alam and B. K. Patel, Chem.–Asian J. DOI:10.1002/asia.201901072
.
-
(a) K. Wang, G. Wang, G. Duan and C. Xia, RSC Adv., 2017, 7, 51313–51317 RSC
; (b) J.-F. Suo, X.-M. Zhao, K.-X. Zhang, S.-L. Zhou, J.-L. Niu and M.-P. Song, Synthesis, 2017, 49, 3916–3924 CrossRef CAS
.
- M. Sparta and F. Neese, Review application of closed shell DLPNO-CCSD(T) methods, Chem. Soc. Rev., 2014, 43, 5032–5041 RSC
.
-
(a) C. Riplinger, P. Pinski, U. Becker, E. F. Valeev and F. Neese, J. Chem. Phys., 2016, 144, 024109 CrossRef PubMed
; (b) M. Saitow, U. Becker, C. Riplinger, E. F. Valeev and F. Neese, J. Chem. Phys., 2017, 146, 164105 CrossRef PubMed
.
- P. Ma and H. Chen, ACS Catal., 2019, 9, 1962–1972 CrossRef CAS
.
- L. Goerigk and S. Grimme, J. Chem. Theory Comput., 2011, 7, 291–309 CrossRef CAS PubMed
.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- Q. M. Phung, C. Martin-Fernandez, J. N. Harvey and M. Feldt, J. Chem. Theory Comput., 2019, 15, 4297–4304 CrossRef CAS PubMed
.
- I. Mayer and P. Salvador, Chem. Phys. Lett., 2004, 383, 368–375 CrossRef CAS
.
- A. J. Bridgeman, G. Cavigliasso, L. R. Ireland and J. Rothery, J. Chem. Soc., Dalton Trans., 2001, 2095–2108 RSC
.
- T. Lu and F. Chen, J. Phys. Chem. A, 2013, 117, 3100–3108 CrossRef CAS PubMed
.
-
P. G. Wang, T. B. Cai and N. Taniguchi in Nitric Oxide Donors: for Pharmaceutical and Biological Application, ed. P. G. Wang, T. B. Cai and N. Taniguchi, Wiley-VCH, Weinheim, 2005 Search PubMed
.
- For some recent examples of the remote functionalisation of naphthalylamides see:
(a) J.-N. Han, C. Du, X. Zhu, Z.-L. Wang, Y. Zhu, Z.-Y. Chu, J.-L. Niu and M.-P. Song, Beilstein J. Org. Chem., 2018, 14, 2090–2097 CrossRef CAS PubMed
; (b) Y. Dong, B. Yin, P. Zhang and Q. Zhu, Eur. J. Org. Chem., 2018, 4571–4576 Search PubMed
; (c) G. You, K. Wang, X. Wang, G. Wang, J. Sun, G. Duan and C. Xia, Org. Lett., 2018, 20, 4005–4009 CrossRef CAS PubMed
; (d) H. Zhu, S. Sun, H. Qiao, F. Yang, Y. Wu and Y. Wu, Org. Lett., 2018, 20, 620–623 CrossRef CAS PubMed
; (e) J.-M. Li, Y.-H. Wang, Y. Yu, R.-B. Wu, J. Weng and G. Lu, ACS Catal., 2017, 7, 2661 CrossRef CAS
; (f) P. Bai, S. Sun, Z. Li, H. Qiao, X. Su, Y. Wu and Y. Wu, J. Org. Chem., 2017, 82, 12119–12127 CrossRef CAS PubMed
.
- For selected examples of multi-state reactivity in other systems, see:
(a) N. Agenet, V. Gandon, K. P. C. Vollhardt, M. Malacria and C. Aubert, J. Am. Chem. Soc., 2007, 129, 8860–8871 CrossRef CAS PubMed
; (b) D. Schröder, S. Shaik and H. Schwarz, Acc. Chem. Res., 2000, 33, 139–145 CrossRef PubMed
; (c) Y. Sun, H. Tang, K. Chen, L. Hu, J. Yao, S. Shaik and H. Chen, J. Am. Chem. Soc., 2016, 138, 3715–3730 CrossRef CAS PubMed
; (d) L. Hu and H. Chen, J. Am. Chem. Soc., 2017, 139, 15564–15567 CrossRef CAS PubMed
; (e) Ref. 20 of this manuscript.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental; experimental protocols, characterization data, and NMR spectra of all new compounds. DFT studies; details of methods used and coordinates for all complexes. See DOI: 10.1039/c9sc05076k |
| This journal is © The Royal Society of Chemistry 2020 |