Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecularly engineered hole-transport material for low-cost perovskite solar cells†
Babak
Pashaei
a,
Sebastiano
Bellani
b,
Hashem
Shahroosvand
 *a and
Francesco
Bonaccorso
*a and
Francesco
Bonaccorso
 *bc
*bc
aGroup for Molecular Engineering of Advanced Functional Materials (GMA), Chemistry Department, University of Zanjan, Zanjan, Iran. E-mail: Shahroos@znu.ac.ir
bGraphene Labs, Istituto Italiano di Tecnologia, via Morego 30, 16163 Genova, Italy. E-mail: Francesco.bonaccorso@iit.it
cBeDimensional SpA, Via Albisola 121, 16163 Genova, Italy
First published on 13th January 2020
Abstract
Triphenylamine-N-phenyl-4-(phenyldiazenyl)aniline (TPA-AZO) is synthesized via a facile CuI-catalyzed reaction and used as a hole transport material (HTM) in perovskite solar cells (PSCs), as an alternative to the expensive spiro-type molecular materials, including commercial 2,2′,7,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene (spiro-OMeTAD). Experimental and computational investigations reveal that the highest occupied molecular orbital (HOMO) level of TPA-AZO is deeper than that of spiro-OMeTAD, and optimally matches with the conduction band of the perovskite light absorber. The use of TPA-AZO as a HTM results in PSC prototypes with a power conversion efficiency (PCE) approaching that of the spiro-OMeTAD-based reference device (17.86% vs. 19.07%). Moreover, the use of inexpensive starting reagents for the synthesis of TPA-AZO makes the latter a new affordable HTM for PSCs. In particular, the cost of 1 g of TPA-AZO ($22.76) is significantly lower compared to that of spiro-OMeTAD ($170–475). Overall, TPA-AZO-based HTMs are promising candidates for the implementation of viable PSCs in large-scale production.
Introduction
During recent years, the growth of power conversion efficiency (PCE) in perovskite solar cells (PSCs) has stunned the photovoltaic community.1 From 2008 to 2019, the record-high PCE of PSCs has increased from 3.8% (ref. 2) to beyond 25.2%,3 which is an unprecedented trend in the history of photovoltaics. To boost the PCE of PSCs, the perovskite photoactive layer must work synergistically with the other functional components of the cell, such as the charge transport layer (CTL) and the current collector.4 In particular, the photo-generated charges have to be extracted by the perovskite absorber and directed towards the current collector through the CTL.5 Consequently, the appropriate tailoring of energy levels at the interfaces is of paramount importance to eliminate/reduce the energy barrier and/or the interfacial structural/morphological defects, which are the cause of charge losses,6 hysteresis phenomena7 and instability effects.8 While several effective electron transport materials (ETMs) are available,9 including metal oxides10 (e.g., TiO2,11 SnO2,12 ZnO,13 ZrO2 (ref. 14)), metal chalcogenides15 and organic materials16 (e.g., fullerene17 and graphene18), the development of efficient, stable and low-cost hole transport materials (HTMs) is a major challenge for the further progress of the PSC technology.19Two different categories of HTMs, namely organic20 and inorganic materials,21 have been extensively investigated and introduced into PSCs. Inorganic HTMs are especially promising because of their low intrinsic chemical stability and moderate costs,22 directly competing with the so-called carbon-based PSCs (i.e., PSCs based on carbon HTMs and/or carbon electrodes).23 In fact, efficient PSCs based on inorganic HTMs including CuI,24 CuSCN,25 CoOx,26 NiOx,27 Cu2ZnSnS4,28 CuCrO2,29 V2O5,30 MoOx and WOx,31 CuAlO2,32 CuGaO2,33 CuS34 and CuOx35 have been successfully reported. Typically, these HTMs do not require the use of dopants, which can be the source of degradation effects and additional costs. Meanwhile, organic molecular HTMs, such as 2,2′,7,7′-tetrakis[N,N-di(4-methoxyphenyl)amino]-9,9′-spirobifluorene (spiro-OMeTAD) and poly[bis(4-phenyl)(2,4,6-trimethylphenyl)amine] (PTAA), have been established in PSCs with record-breaking PCEs,36 which are typically superior to those achieved using inorganic HTMs.22a,b However, these HTMs have both technical and economic issues. In fact, on one hand, they often need hygroscopic dopants (such as bis(trifluoromethylsulphonyl)imide (LiTFSI) and 4-tert-butylpyridine (TBP), as well as Co(III) complexes) that trigger degradation of the perovskite layer.37 On the other hand, their high cost represents a fundamental hurdle to scale up the manufacturing of PSCs. For example, the cost of spiro-OMeTAD is between 170 and 475$ g−1,38 contributing to ∼10% of the overall cost of perovskite solar modules.39 The PTAA cost is more than twice that of spiro-OMeTAD.40 The high cost of organic HTMs is mainly related to their production via multi-step synthetic routes, as well as several purification rounds, which need expensive starting reagents and/or catalysts. Actually, the synthesis of these HTMs involves the Pd-catalyzed amination of aryl halides,41 which is significantly more complex compared to the Heck,42 Stille,43 and Suzuki44 reactions frequently used in the synthesis of organic molecules.45 For example, prototypical HTMs based on spiro-organic derivatives are typically synthesized via Pd-catalyzed cross-coupling reactions, mainly contributing to the final cost of the materials. Therefore, tremendous effort has been directed to developing new molecularly engineered organic HTMs through inexpensive synthetic protocols.46 To the best of our knowledge, two of the cheapest HTMs reported in the literature are the EDOT-OMeTPA (a small molecule based on triphenylamine (TPA) and 3,4-ethylenedioxythiophene (EDOT) moieties) (∼$10 g−1)38 and the spiro[fluorene-9,9′-xanthene] (SFX) derivatives (∼$16 g−1).47 Unfortunately, PSCs based on these HTMS have reached PCEs inferior to 13%, pointing out the need to further optimize such HTMs to reach the state-of-the-art performance of PSCs. Recently, poly(3-hexylthiophene) (P3HT) has also been used as a dopant-free organic HTM, allowing PSCs to reach a certified PCE of 22.7%. This impressive result was achieved using a new device architecture based on a wide-bandgap halide perovskite formed on top of a narrow-bandgap light-absorbing layer by an in situ reaction of n-hexyl trimethyl ammonium bromide on the perovskite surface.36e This strategy was the key to significantly increase the PCE of PSCs based on P3HT as HTMs (typically between 16% and 18% for doped P3HT,48 and lower than 15% for pristine P3HT48a). It is noteworthy that advanced architectures, showing superior stability and/or PCEs, have also been achieved by coupling organic HTMs with inorganic interlayers, such as transition metal dichalcogenides (e.g., MoS2),5b,49 indicating the possibility of improving the performance of PSCs currently fabricated using organic HTMs.
Among the viable organic HTMs, TPA derivatives represent promising candidates to replace the aforementioned expensive benchmarking organic materials. Beyond their facile and cheap synthesis, their TPA moiety is an electron donor unit,50 showing the ability to transport positive charges efficiently.51 Moreover, their low ionization potential, which results from the amine nitrogen atom,51b can minimize the hole oxidizability injection barrier from the transparent anodes used in optoelectronic devices.52 Experimental results have proven that TPA derivatives display chemical and thermal stabilities.53 Lastly, their high solubility in organic solvents allows them to be processed through established printing/coating techniques.53 Not by a chance, the TPA unit has been effectively investigated in various optoelectronic materials used for PSCs,54 dye-sensitized solar cells (DSSCs),55 organic light-emitting diodes (OLEDs),56 and organic field-effect transistors (OFETs).57
The TPA molecule also represents a versatile platform for the incorporation of different substituents at different positions. Therefore, it can be an ideal building block for the construction of HTMs based on pseudo-three dimensional conjugated architectures, whose tunable energy levels can efficiently collect/block the photogenerated holes/electrons from the perovskite layer.58 In this context, aromatic AZO compounds, which include an Ar–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–Ar moiety (in which Ar is an aromatic ring and the –N
N–Ar moiety (in which Ar is an aromatic ring and the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– is the AZO functional group), represent interesting functional groups for the TPA unit. In fact, they display distinctive photochemical and photophysical properties, including chemical and thermal stability, as well as their manifestation in two cis–trans isomeric forms, which easily and reversibly convert into one another.59 Consequently, aromatic AZO compounds have been already exploited in the chemical industry as dyes/pigments,60 therapeutic agents,61 radical reaction initiators,62 drug delivery systems,63 nonlinear optics,64 photochemical molecular switches,65 molecular shuttles,66 nanotubes,67 and eye glasses and optical filters.68 Specifically for PSCs, the cis–trans isomerisation of aromatic AZO derivatives could be exploited to modulate the hole mobility of TPA-based HTMs.69 However, the combination of TPA and AZO into a new HTM for PSCs has not been reported yet.
N– is the AZO functional group), represent interesting functional groups for the TPA unit. In fact, they display distinctive photochemical and photophysical properties, including chemical and thermal stability, as well as their manifestation in two cis–trans isomeric forms, which easily and reversibly convert into one another.59 Consequently, aromatic AZO compounds have been already exploited in the chemical industry as dyes/pigments,60 therapeutic agents,61 radical reaction initiators,62 drug delivery systems,63 nonlinear optics,64 photochemical molecular switches,65 molecular shuttles,66 nanotubes,67 and eye glasses and optical filters.68 Specifically for PSCs, the cis–trans isomerisation of aromatic AZO derivatives could be exploited to modulate the hole mobility of TPA-based HTMs.69 However, the combination of TPA and AZO into a new HTM for PSCs has not been reported yet.
Herein, we report the synthesis of aromatic AZO-functionalized TPA (TPA-AZO) to be investigated as a possible low-cost HTM in PSCs. Our results show the possibility to achieve PCEs approaching those of benchmarking devices based on organic HTMs, but in a cost-effective way.
Results and discussion
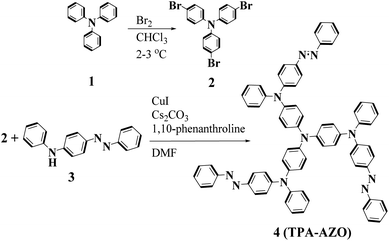
The synthetic route and molecular structure of TPA-AZO are shown in Fig. 1, while the procedure details are reported in the Experimental section. Briefly, TPA-AZO was synthesized through a two-step reaction, including: (1) the bromination of TPA in CHCl3 solution70 (see ESI, Fig. S1†) and (2) the amination reaction between tribromotriarylamine (t-Br-TPA) and N-phenyl-4-(phenyldiazenyl)aniline. All the compounds were purified through recrystallization and column chromatography. TPA-AZO was characterized by spectroscopic, electrochemical and thermoanalytical techniques, and the results were compared with those measured for spiro-OMeTAD, hereafter assumed as the HTM benchmark. The absence of the singlet at 6.07 ppm in the 1HNMR spectra of TPA-AZO (Fig. S2†), which is related to N–H of N-phenyl-4-(phenyldiazenyl)aniline, confirms the successful synthesis of TPA-AZO. Fig. 2a shows the ultraviolet-visible (UV-vis) absorption and photoluminescence (PL) spectra of the investigated HTMs.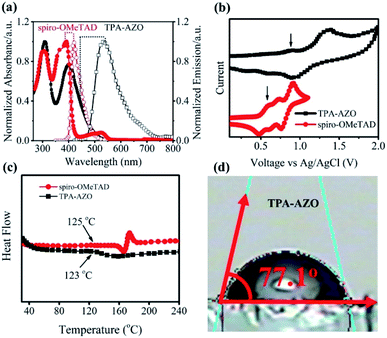 | ||
| Fig. 2 (a) UV-vis absorption and PL emission spectra of TPA-AZO and spiro-OMeTAD. (b) CV curves of spiro-OMeTAD and TPA-AZO. (c) DSC curves of TPA-AZO and spiro-OMeTAD. (d) Contact angles of TPA-AZO. | ||
The UV-vis absorption spectra of TPA-AZO and spiro-OMeTAD exhibit two main bands attributed to π → π* and n → π* transitions.71 The second band of TPA-AZO is red-shifted compared to that of spiro-OMeTAD. The PL emission of TPA-AZO is also red-shifted (by 115 nm) relative to that of spiro-OMeTAD. The optical band gap (Eo–o) of the materials can be qualitatively estimated by the energy corresponding to the intersection between the UV-vis absorption and the PL spectra. Therefore, the Eo–o of TPA-AZO is smaller than that of spiro-OMeTAD. However, the intensity of the n → π* band in the spectrum of TPA-AZO is lower than the corresponding one of spiro-OMeTAD. This indicates that the synthesized TPA-AZO can minimize light absorption in the visible spectral region. Thus, TPA-AZO can in principle reduce the absorption of visible light reflected by the back contact, as well as it can be effectively used in bifacial PSCs.59,66
Cyclic voltammetry (CV) analysis was carried out to determine the highest occupied molecular orbital (HOMO) levels of TPA-AZO and spiro-OMeTAD (Fig. 2b).72 The CV curve of TPA-AZO shows two reversible oxidation peaks, while the CV curve of spiro-OMeTAD exhibits three reversible oxidation peaks, in agreement with previous studies.73 Notably, the oxidation/reduction potentials of TPA-AZO are higher than those of spiro-OMeTAD (data are summarized in Table 1). The estimated values for the HOMO energies (EHOMO) of TPA-AZO and spiro-OMeTAD are −5.39 eV and −5.20 eV, respectively (according to the equation: EHOMO = −(Eox (vs. Fc/Fc+) + 4.8 eV)). The lowest unoccupied molecular orbital (LUMO) energies (ELUMO) of the materials, as calculated by ELUMO = EHOMO + Eo–o, are −2.55 eV and −2.16 eV for TPA-AZO and spiro-OMeTAD, respectively.
| HTM | λ abs [nm] | λ em [nm] | E o–o [eV] | E OX [V] | E HOMO [eV] | E LUMO [eV] | T g [°C] | η quench | Hole mobility (pristine state) [cm2 V−1 s−1] | Hole mobility (doped state) [cm2 V−1 s−1] |
|---|---|---|---|---|---|---|---|---|---|---|
| a UV-Vis and photoluminescence spectra were measured in CHCl3 solution. b E o–o was estimated by the energy corresponding to the intersection of the UV-Vis absorption and PL spectra. c From CV measurements, E1/2 = 1/2(Epa + Epc); 0.1 M chloroform/tetrabutylammonium perchlorate (TBAP) versus Ag/AgCl at scan rate of 80 mV s−1. d E HOMO = −(Eox (vs. Fc/Fc+) + 4.8 eV). e E LUMO = EHOMO + Eo–o. | ||||||||||
| TPA-AZO | 308, 401 | 535 | 2.84 | 0.79 | −5.39 | −2.55 | 123 | 0.83 | 9.4 × 10−5 | 1.2 × 10−4 |
| Spiro-OMeTAD | 304, 389 | 421 | 3.04 | 0.6 | −5.20 | −2.16 | 124 | 0.94 | 2.6 × 10−5 | 2.0 × 10−4 |
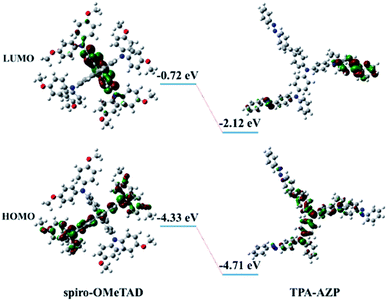
Density functional theory (DFT) calculations for the energy levels of TPA-AZO and spiro-OMeTAD (Fig. 3) also indicate that the HOMO level of TPA-AZO is deeper than that of spiro-OMeTAD, evidencing its optimal matching with the HOMO level of the perovskite layer (−5.43 eV).38
Differential scanning calorimetry (DSC) analysis was carried out to investigate the thermal stability of TPA-AZO, which was compared with that of spiro-OMeTAD (Fig. 2c). The DSC curves indicate that TPA-AZO has a glass transition temperature (Tg) of about 123 °C, which is very close to the Tg of spiro-OMeTAD (124 °C).74 These results indicate that TPA-AZO shows a similar thermal stability behaviour to spiro-OMeTAD, which undergoes a severe morphological deformation at temperature higher than 80 °C.75 One of the main strategies to enhance the stability of PSCs is the use of hydrophobic HTMs to prevent the diffusion of water and moisture into the photoactive layer of the cells.76 In fact, hydrophobic HTMs can act as barrier layers to avoid the water-/moisture-induced decomposition of CH3NH3PbI3 to CH3NH3I and PbI2.77 Therefore, water contact angle measurements were carried out on the TPA-AZO surface to examine its hydrophobicity. As shown in Fig. 2d, the measured water contact angle for TPA-AZO is 77°. This result indicates that TPA-AZO is hydrophobic and might prevent the penetration of water into the perovskite layer, thus limiting degradation effects.76c
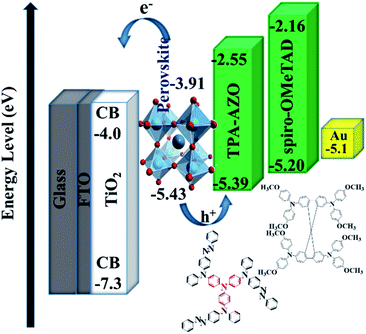
As suggested by its spectroscopic, electrochemical and analytical characterization, TPA-AZO was subsequently used as a HTM in PSCs. The cells were fabricated by using the following mesoscopic architecture:37b glass-FTO/TiO2 (compact layer)/TiO2 (mesoporous layer)/(FAPbI3)0.85(MAPbBr3)0.15/TPA-AZO or spiro-OMeTAD/Au (Fig. 4). The layered structure of the PSC architecture was confirmed by scanning electron microscopy (SEM) (Fig. 4b).
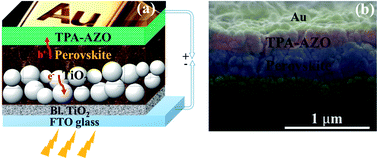 | ||
| Fig. 4 (a) Sketch of the mesoscopic architecture adopted for the investigated PSCs. (b) SEM cross section of a representative PSC based on the TPA-AZO HTM. | ||
The energy level diagram in Fig. 5 shows that the estimated HOMO level of TPA-AZO optimally matches that of the perovskite. In particular, the energy difference between the HOMO levels of the perovskite and TPA-AZO (ΔHOMOperovskite–HTM) ( ∼0.04 eV) is lower than that between the HOMO levels of the perovskite and spiro-OMeTAD (∼0.23 eV). This means that TPA-AZO reduces the energy barrier related to hole extraction relative to the case of spiro-OMeTAD.
Effective charge carrier transport is another important feature determining the design of efficient HTMs.78 The hole mobilities of the HTMs were investigated by means of the space-charge limited current (SCLC) method, in agreement with previous literature.79 Thus, the mobility values were extracted from the J–V curves (Fig. S3†) measured for each HTM. The estimated data are reported in Table 1 for both TPA-AZO and spiro-OMeTAD. The estimated hole mobility of pristine TPA-AZO is 9.8 × 10−4 cm2 V−1 s−1, which is more than one order of magnitude higher than that of spiro-OMeTAD (2.6 × 10−5 cm2 V−1 s−1). After doping the HTMs with LiTFSI and TBP, the hole mobility increases compared to that of the pristine samples, reaching values of 1.2 × 10−4 cm2 V−1 s−1 and 2.0 × 10−4 cm2 V−1 s−1 for doped TPA-AZO and doped spiro-OMeTAD, respectively. However, the hole mobility data and the conductivity values measured for the pristine HTMs are useful information. In fact, the elimination of the use of dopant would increase the stability of the PSCs, while reducing their overall cost.80
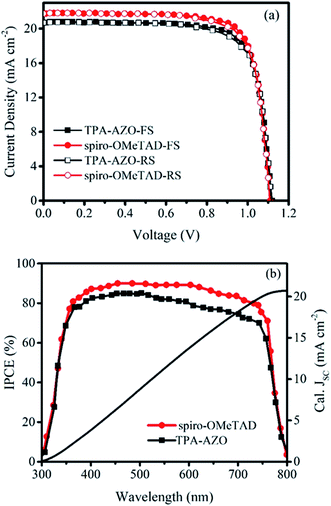
The photovoltaic performances of the PSCs based on the doped TPA-AZO and spiro-OMeTAD were investigated by measuring their current density–voltage (J–V) curves under AM1.5G illumination (Fig. 6a). The corresponding photovoltaic parameters are summarized in Table 2, which also reports the data obtained for the PSCs using the dopant-free HTMs, whose J–V curves are reported in Fig. S4.†
| HTM | J SC [mA cm−2] | V OC [V] | FF [%] | PCE [%] |
|---|---|---|---|---|
| Dopant-free TPA-AZO | 17.01 | 0.94 | 63 | 10.07 |
| TPA-AZO-forward scan (FS) | 20.72 | 1.12 | 77 | 17.86 |
| TPA-AZO-reverse scan (RS) | 20.67 | 1.12 | 76 | 17.59 |
| Dopant-free spiro-OMeTAD | 14.69 | 0.79 | 47 | 5.45 |
| Spiro-OMeTAD-FS | 21.75 | 1.11 | 79 | 19.07 |
| Spiro-OMeTAD-RS | 21.73 | 1.11 | 77 | 18.57 |
The PSC based on TPA-AZO shows a short-circuit current density (JSC) of 20.72 mA cm−2, an open-circuit voltage (VOC) of 1.12 V, and a fill factor (FF) of 0.77, leading to a PCE of 17.86% (forward scan –FS–). This PCE value is inferior to that measured for the reference PSC, i.e., the spiro-OMeTAD one, PCE = 19.07% (FS). However, the PCE achieved by using TPA-AZO is significantly superior to those previously achieved by using low-cost organic HTMs in conventional architectures (e.g., 11% for EDOT-OMeTPA38 and 12.4% for SFX derivatives47). As shown in Fig. 6a, the photovoltaic parameters obtained by measuring J–V curves in reverse scan (RS) mode were similar to those measured with FS. The statistical analyses of the photovoltaic parameters measured for 30 samples of each doped HTM-based PSC are reported in Fig. S4,† showing average PCEs of 17.17% and 15.93% for the PSCs based on spiro-OMeTAD and TPA-AZO, respectively. In addition, the PSCs based on dopant-free TPA-AZO yielded a PCE of 10.07%, which is significantly higher than that of dopant-free spiro-OMeTAD (5.45%) (Table 2, Fig. S5†). As shown in Fig. S6,† the dopants do not affect the film morphology of TPA-AZO, while they are needed to homogenize the morphology of spiro-OMeTAD, in agreement with previous studies.76c,81 These results indicate that our dopant-free TPA-AZO is promising to be used in advanced PSC architecture, as for example shown with P3HT,36e for which its doping is instead recommended for conventional architectures.48a
The difference between the JSC of our optimized PSCs is the major reason for the lower PCE of the TPA-AZO-based PSC compared to that of the spiro-OMeTAD-based reference. Fig. 6b shows the incident photon to current efficiency (IPCE) measurements of the investigated PSCs. The integrated current density (calculated from the IPCE data in the 300–800 nm range under AM1.5G conditions) for the TPA-AZO-based PSC is consistent with the J–V curve data (i.e., Jsc, calc. ∼ Jsc). In particular, the IPCE of the TPA-AZO-based PSC is lower compared to the spiro-OMeTAD-based reference one in the 450–600 nm spectral range.82
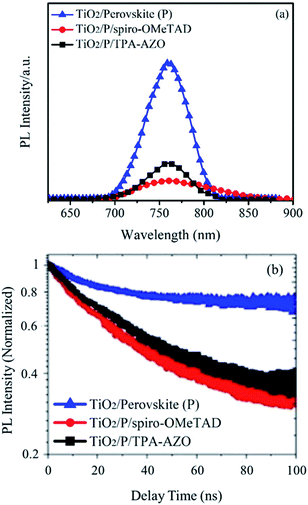
In order to understand the photocurrent losses, steady-state and time-resolved PL spectroscopy measurements were performed to specifically evaluate the capability of the HTMs to extract the photogenerated holes from the perovskite layer. In fact, the hole extraction process hinders radiative charge recombination in the absorber material,89,90 which consequently shows a PL quenching.91 As shown by the steady-state PL spectra (Fig. 7a), spiro-OMeTAD quenches the PL of the perovskite more effectively than AZO-TPA.83 The calculated PL quenching factor (ηquench) for the spiro-OMeTAD-based structure is ∼10% higher than that of TPA-AZO (see values in Table 1).
Time-resolved PL spectroscopy data (Fig. 7b) indicate that the photo-charge lifetime (τ2) in the presence of spiro-OMeTAD (7.8 ns, as estimated by fitting the PL decay traces with the bi-exponential decay model) is slightly lower than that in the presence of TPA-AZO (9.2 ns). It is noteworthy that both these values are significantly lower than that measured for the HTM-free structures (16.7 ns),84 confirming that both the HTMs effectively quench the PL of the perovskite absorber by extracting the photo-generated holes.85 Overall, the PL measurements show that, despite the optimal matching between the HOMO levels of TPA-AZO and the perovskite, spiro-OMeTAD is still more efficient to extract holes from the perovskite compared to TPA-AZO.86 Nonetheless, the addition of more electron donating groups such as methoxy groups could prospectively improve the performance of TPA-AZO as the HTM.
The stability of the photovoltaic performance is another crucial aspect of investigation for PSCs, which still suffer from severe degradation over time under realistic operating conditions. As shown in Fig. 8, the photovoltaic performance stability of the PSC based on TPA-AZO HTM is similar to that recorded for the spiro-OMeTAD-based reference. This suggests that degradation effects are attributed to causes not directly ascribed to the HTMs.
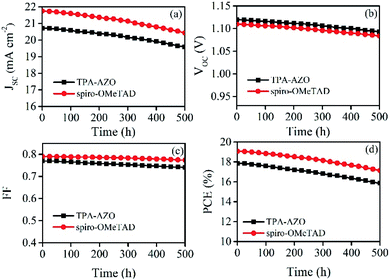 | ||
| Fig. 8 Stability of the photovoltaic characteristics over time for the PSCs based on TPA-AZO and spiro-OMeTAD: (a) JSC; (b) VOC; (c) FF; (d) PCE. | ||
In addition, a huge advantage of our newly synthesized TPA-AZO compared with spiro-OMeTAD is related to the preparation costs (much lower for the case of TPA-AZO). In fact, the estimated costs of TPA-AZO and spiro-OMeTAD are $22.76 g−1 and $273.62 g−1, respectively (Tables S1 and S2†).38,85,87 This difference in price is related to the following factors: (1) six experimental steps have to be carried out to produce pure spiro-OMeTAD, while only two steps are needed for TPA-AZO; (2) the catalyst used for the synthesis of TPA-AZO (CuI/Cs2CO3/phenanthroline) is significantly cheaper than that used for the synthesis of spiro-OMeTAD (Pd/bis(diphenylphosphino)-l,l′-binaphthyl (BINAP)). Noteworthy, the additional costs associated with dopants used for our optimized PSCs are reported in Table S3,† showing that they only marginally contribute to the overall cost of TPA-AZO.
In addition to spiro-OMeTAD, other small organic HTMs can be compared with our TPA-AZO HTM in terms of synthesis cost and PCE of their corresponding cells (Table S4†).73,80a,88 For example, the use of star-shaped HTMs, based on TPA with diphenyl ethenyl side arms, resulted in PCEs up to 11.8%.89 The use of p-methoxy side groups further increased the PCE to 13.63%.88g In order to increase their charge transport properties, prevent aggregation and improve their stability, the side arms of these materials were modified with a bis-dimethylfluorenyl amino moiety. The resulting star-shaped structures allowed their PSCs to reach PCE ∼ 18%.77d Other interesting examples of organic HTMs based on TPA are bis[(4-methoxyphenyl)aminophenyl]ethene (EtheneDTPA) and tetrakis[(4-me-thoxyphenyl)aminophenyl]ethene (EtheneTTPA), which have the lowest cost for the synthesis of 1 g (101.34 and 52.59$ per g, respectively) among the HTMs reported in Table S4† beyond our TPA-AZO. However, the PCE of their corresponding PSCs is ∼15%, which is lower than those of PSCs based on other low-cost HTMs.37b,38,88a,90 Lastly, various building blocks, such as spirobifluorenes,91 thiophenes,37b,38,80a,92 triazatruxenes,73,93 azulenes94 and other small organic HTMs,4a,88e,95 have been used as the core of several HTMs. Despite their remarkable PCE values, all these HTMs have higher synthesis costs compared to that of TPA-AZO. Thus, our TPA-AZO is still overall advantageous compared to the aforementioned organic HTMs, as shown in Fig. 9.
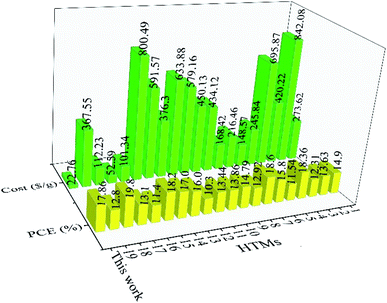 | ||
| Fig. 9 Comparison between costs of organic HTMs (green histograms) and PCEs of the corresponding PSCs (red histograms), as reported in our work and other relevant studies. The HTMs reported in previous literature are named with numbers from 1 to 19. Their full names and schemes are given in ESI, Table S4.† | ||
Our TPA-AZO was further compared with other dopant-free inorganic and polymeric HTMs. In spite of worthy properties of dopant-free inorganic HTMs, including thermal and chemical stability towards moisture exposure, they suffer from several issues. For example, the solvent used for their deposition on the perovskite layer can dissolve the perovskite, thereby affecting the stability of the PSCs.25b,96 In addition, such HTMs can be low band-gap materials with limited optical transparency,97 and/or can have expensive deposition processes (e.g., magnetron sputtering).98 Lastly, bare polymers can suffer from complex or costly purification procedures, and/or difficult infiltration into nanostructured materials.96 As shown in Table S5,† our PSCs based on dopant-free TPA-AZO exhibit PCEs that are superior to those achieved by several dopant-free HTMs, although high-performance inorganic and polymeric HTMs have been demonstrated.24,25b,28,33,99 Overall, the distinctive balance between low-cost synthesis of our TPA-AZO and the satisfactory PCE of its corresponding PSCs is promising for the realization of high-efficiency and viable PSCs.
Conclusion
In conclusion, our results shed light on the engineering of low-cost and effective HTMs based on TPA. In particular, our AZO-functionalized TPA (TPA-AZO) is a promising material candidate to replace the spiro-OMeTAD benchmark. The spectroscopic and electrochemical analyses and the DFT calculations support that the HOMO of TPA-AZO is optimally tuned with that of the perovskite layer. The TPA-AZO-based PSCs achieved a PCE as high as 17.86%, close to that of spiro-OMeTAD. Moreover, the cost of 1 g of TPA-AZO is about one-thirty of that of spiro-OMeTAD. This makes our TPA-AZO one of the cheapest effective HTMs for PSCs. Based on our current knowledge, we believe that the commercialization of TPA-AZO-based PSCs could more affordable than that of spiro-OMeTAD-based PSCs.Experimental
Materials
All starting materials were purchased from Aldrich Chemical and Merck Companies and used without further purification.Characterization of materials
1H NMR spectra were recorded on Bruker Advance 250 MHz spectrometers, locked on deuterated solvents. Chemical shifts were calibrated against tetramethylsilane as an internal standard. UV-vis absorption and PL spectra were measured using an Ultrospec 3100 pro spectrophotometer and AvaSpec-125 spectrophotometer, respectively. The electrochemical studies were accomplished by using a SAMA500 potentiostat electrochemical analyzer in a three-electrode cell configuration. A Pt disk and a Pt wire were used as the working electrode and the counter electrode, respectively. A KCl-saturated Ag/AgCl electrode was used as reference. The CV measurements were performed in chloroform solvent, using 0.1 M TBAP as the supporting electrolyte.Synthesis of triphenylamine-N-phenyl-4-(phenyldiazenyl)aniline (TPA-AZO)
Tribromotriarylamine (t-Br-TPA) was prepared according to the procedure reported in literature.70 Then, a mixture of CuI (0.050 g), 1,10-phenantholine (0.10 g), Cs2CO3 (2.00 g), t-Br-TPA (0.482 g, 1 mmol), N-phenyl-4-(phenyldiazenyl)aniline (0.982 g, 3.6 mmol) and 5 ml of dimethylformamide (DMF) was heated under reflux for 2 days. After cooling to ambient temperature, the product was extracted by using CHCl3 and H2O. The combined organic phases were dried over anhydrous MgSO4 and evaporated. The crude product was purified by column chromatography (CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane 4
hexane 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) (45%). 1H NMR (CDCl3, 250 MHz): 7.05–7.25 (m, 9H), 7.31–7.49 (m, 5H), 7.86–7.89 (m, 4H). CHN: anal. calcd for C72H54N10 (%): C, 81.643; H, 5.144; N, 13.227. Found (%): C, 81.649; H, 5.15; N, 13.233. ESI-MS: m/z 1057.10, [M − H]+.
7) (45%). 1H NMR (CDCl3, 250 MHz): 7.05–7.25 (m, 9H), 7.31–7.49 (m, 5H), 7.86–7.89 (m, 4H). CHN: anal. calcd for C72H54N10 (%): C, 81.643; H, 5.144; N, 13.227. Found (%): C, 81.649; H, 5.15; N, 13.233. ESI-MS: m/z 1057.10, [M − H]+.
Fabrication of solar cells
Perovskite solar cells were fabricated on fluorine-doped tin oxide (FTO) coated glass substrates. Part of the glass substrate coated with FTO was etched with Zn powder and 2 M HCl solution in ethanol. Subsequently, the substrates were washed with distilled water, detergent, acetone, ethanol and isopropanol. The samples were then treated with an ultraviolet/O3 cleaner for 15 min. On these substrates, a solution of HCl and titanium isopropoxide (TTIP) in anhydrous ethanol (a solution of HCl (35 μl) in ethanol (2.53 ml) was added drop by drop to a solution of TTIP (369 μl) in ethanol (2.53 ml)) was deposited by spin-coating at 2000 rpm for 30 s. Subsequently, the substrates were heated at 500 °C for 30 min and cooled down to room temperature. Mesoporous TiO2 diluted in ethanol was deposited by spin-coating at 2000 rpm for 10 s to achieve a 300–400 nm-thick layer. Afterward, the substrates were sintered at 500 °C for 30 min. The PbI2 solution was coated on the mesoporous TiO2 layer for 5 s at 6500 rpm and dried at 70 °C. The mixed perovskite precursor solution was prepared by dissolving PbI2 (1.15 M), formamidinium iodide (FAI) (1.10 M), PbBr2 (0.2 M), and methylammonium bromide (MABr) (0.2 M) in an anhydrous solvent DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dimethyl sulfoxide (DMSO) = 4
dimethyl sulfoxide (DMSO) = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (volume ratio). The perovskite solution was spin-coated in a two-step procedure at 1000 and 6000 rpm for 10 and 30 s, respectively. Next, TPA-AZO or spiro-OMeTAD were deposited by spin-coating their solutions at 4000 rpm for 20 s. The HTM solutions were prepared by dissolving the HTMs in chlorobenzene at a concentration of 78 mM, with the addition of 18 μl LiTFSI (from a stock solution in acetonitrile with a concentration of 1.0 M) and 29 μl tert-butyl pyridine (from a stock solution in chlorobenzene with a concentration of 1.0 M). Finally, an 80 nm-thick Au electrode was deposited by thermal evaporation under high vacuum (∼10−5 Pa).37b,85
1 (volume ratio). The perovskite solution was spin-coated in a two-step procedure at 1000 and 6000 rpm for 10 and 30 s, respectively. Next, TPA-AZO or spiro-OMeTAD were deposited by spin-coating their solutions at 4000 rpm for 20 s. The HTM solutions were prepared by dissolving the HTMs in chlorobenzene at a concentration of 78 mM, with the addition of 18 μl LiTFSI (from a stock solution in acetonitrile with a concentration of 1.0 M) and 29 μl tert-butyl pyridine (from a stock solution in chlorobenzene with a concentration of 1.0 M). Finally, an 80 nm-thick Au electrode was deposited by thermal evaporation under high vacuum (∼10−5 Pa).37b,85
Characterization of solar cells
The J–V curves were measured using a solar simulator (Newport, Oriel Class A, 91195A) with a source meter (Keithley 2420) under 100 mA cm−2 illumination (AM 1.5G) and a calibrated Si-reference cell certified by the National Renewable Energy Laboratory (NREL). The J–V curves of all devices were measured by masking the active area with a metal mask of area 0.096 cm2. The SEM apparatus with the MIRA III model (TESCAN Co.) was used for the acquisition of the SEM images.Mobility measurements
Charge transport in the HTMs was investigated by the SCLC method reported in the literature.79b Experimentally, the FTO coated glass substrates were prepared according to the “Solar cells fabrication” section. After spin-coating the poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT:PSS) layer onto the substrates, the HTM films were deposited by spin-coating the HTM solutions in anhydrous chlorobenzene (10 mg ml−1) at 2000 rpm for 20 s. Finally, an Au layer was evaporated onto the active layer under high vacuum (∼10−5 Pa). The hole mobility was estimated using Mott–Gurney's equation.Computational methods
The ground-state geometries were optimized using density functional theory (DFT) with the B3LYP hybrid functional at the basis set level of 6-31G*, and the frontier molecular orbitals were drawn using an isovalue of 0.03 a.u. All the calculations were performed using the Gaussian 09 package in the PowerLeader workstation. The molecular orbitals were visualized using Gauss View 5.0.8.Conflicts of interest
There are no conflicts to declare.Acknowledgements
B. P and H. Sh thank the University of Zanjan for financial support.Notes and references
- (a) M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643 CrossRef CAS PubMed; (b) F. De Angelis, D. Meggiolaro, E. Mosconi, A. Petrozza, M. K. Nazeeruddin and H. J. Snaith, ACS Energy Lett., 2017, 2, 857 CrossRef CAS.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050 CrossRef CAS PubMed.
- Best Research-Cell Efficiencies (NREL), https://www.nrel.gov/pv/cell-efficiency.html.
- (a) N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, J. Am. Chem. Soc., 2013, 135, 19087 CrossRef CAS PubMed; (b) P. K. Nayak, S. Mahesh, H. J. Snaith and D. Cahen, Nat. Rev. Mater., 2019, 4, 269 CrossRef CAS.
- (a) P. Schulz, E. Edri, S. Kirmayer, G. Hodes, D. Cahen and A. Kahn, Energy Environ. Sci., 2014, 7, 1377 RSC; (b) L. Najafi, B. Taheri, B. Martín-García, S. Bellani, D. Di Girolamo, A. Agresti, R. Oropesa-Nuñez, S. Pescetelli, L. Vesce, E. Calabrò, M. Prato, A. E. Del Rio Castillo, A. Di Carlo and F. Bonaccorso, ACS Nano, 2018, 12, 10736 CrossRef CAS PubMed; (c) A. Agresti, S. Pescetelli, A. L. Palma, B. Martin-Garcia, L. Najafi, S. Bellani, I. Moreels, M. Prato, F. Bonaccorso and A. Di Carlo, ACS Energy Lett., 2019, 4, 1862 CrossRef CAS; (d) Y. Bai, X. Meng and S. Yang, Adv. Energy Mater., 2018, 8, 1701883 CrossRef.
- (a) A. Fakharuddin, L. Schmidt-Mende, G. Garcia-Belmonte, R. Jose and I. Mora-Sero, Adv. Energy Mater., 2017, 7, 1700623 CrossRef; (b) P. Schulz, ACS Energy Lett., 2018, 3, 1287 CrossRef CAS.
- (a) T. S. Sherkar, C. Momblona, L. n. Gil-Escrig, J. Ávila, M. Sessolo, H. J. Bolink and L. J. A. Koster, ACS Energy Lett., 2017, 2, 1214 CrossRef CAS PubMed; (b) M. Pantaler, K. T. Cho, V. I. Queloz, I. s. García Benito, C. Fettkenhauer, I. Anusca, M. K. Nazeeruddin, D. C. Lupascu and G. Grancini, ACS Energy Lett., 2018, 3, 1781 CrossRef CAS; (c) J. Peng, Y. Wu, W. Ye, D. A. Jacobs, H. Shen, X. Fu, Y. Wan, N. Wu, C. Barugkin and H. T. Nguyen, Energy Environ. Sci., 2017, 10, 1792 RSC.
- (a) M. Lira-Cantú, Nat. Energy, 2017, 2, 17115 CrossRef; (b) J. A. Christians, P. Schulz, J. S. Tinkham, T. H. Schloemer, S. P. Harvey, B. J. T. de Villers, A. Sellinger, J. J. Berry and J. M. Luther, Nat. Energy, 2018, 3, 68 CrossRef CAS; (c) R. Wang, M. Mujahid, Y. Duan, Z. K. Wang, J. Xue and Y. Yang, Adv. Funct. Mater., 2019, 1808843 CrossRef CAS.
- (a) K. Mahmood, S. Sarwar and M. T. Mehran, RSC Adv., 2017, 7, 17044 RSC; (b) G. Yang, H. Tao, P. Qin, W. Ke and G. Fang, J. Mater. Chem. A, 2016, 4, 3970 RSC; (c) M. F. M. Noh, C. H. Teh, R. Daik, E. L. Lim, C. C. Yap, M. A. Ibrahim, N. A. Ludin, A. R. bin Mohd Yusoff, J. Jang and M. A. M. Teridi, J. Mater. Chem. C, 2018, 6, 682 RSC.
- J. You, L. Meng, T.-B. Song, T.-F. Guo, Y. M. Yang, W.-H. Chang, Z. Hong, H. Chen, H. Zhou and Q. Chen, Nat. Nanotechnol., 2016, 11, 75 CrossRef CAS PubMed.
- T. Leijtens, G. E. Eperon, S. Pathak, A. Abate, M. M. Lee and H. J. Snaith, Nat. Commun., 2013, 4, 2885 CrossRef PubMed.
- (a) Z. Zhu, Y. Bai, X. Liu, C. C. Chueh, S. Yang and A. K. Y. Jen, Adv. Mater., 2016, 28, 6478 CrossRef CAS; (b) Q. Dong, Y. Shi, K. Wang, Y. Li, S. Wang, H. Zhang, Y. Xing, Y. Du, X. Bai and T. Ma, J. Phys. Chem. C, 2015, 119, 10212 CrossRef CAS.
- (a) R. Azmi, W. T. Hadmojo, S. Sinaga, C. L. Lee, S. C. Yoon, I. H. Jung and S. Y. Jang, Adv. Energy Mater., 2018, 8, 1870022 CrossRef; (b) M. M. Tavakoli, R. Tavakoli, Z. Nourbakhsh, A. Waleed, U. S. Virk and Z. Fan, Adv. Mater. Interfaces, 2016, 3, 1500790 CrossRef.
- A. Priyadarshi, A. Bashir, J. T. Gunawan, L. J. Haur, A. Bruno, Z. Akhter, N. Mathews and S. G. Mhaisalkar, Energy Technol., 2017, 5, 1866 CrossRef CAS.
- (a) J. Liu, C. Gao, L. Luo, Q. Ye, X. He, L. Ouyang, X. Guo, D. Zhuang, C. Liao and J. Mei, J. Mater. Chem. A, 2015, 3, 11750 RSC; (b) L. Wang, W. Fu, Z. Gu, C. Fan, X. Yang, H. Li and H. Chen, J. Mater. Chem. C, 2014, 2, 9087 RSC.
- (a) O. Malinkiewicz, A. Yella, Y. H. Lee, G. M. Espallargas, M. Graetzel, M. K. Nazeeruddin and H. J. Bolink, Nat. Photonics, 2014, 8, 128 CrossRef CAS; (b) A. A. Said, J. Xie and Q. Zhang, Small, 2019, 15, 1900854 CrossRef PubMed; (c) M. Ulfa, T. Zhu, F. Goubard and T. Pauporté, J. Mater. Chem. A, 2018, 6, 13350 RSC.
- (a) L. L. Deng, S. Y. Xie and F. Gao, Adv. Electron. Mater., 2018, 4, 1700435 CrossRef; (b) E. Castro, J. Murillo, O. Fernandez-Delgado and L. Echegoyen, J. Mater. Chem. C, 2018, 6, 2635 RSC; (c) T. Gatti, E. Menna, M. Meneghetti, M. Maggini, A. Petrozza and F. Lamberti, Nano Energy, 2017, 41, 84 CrossRef CAS.
- (a) A. Agresti, S. Pescetelli, B. Taheri, A. E. Del Rio Castillo, L. Cinà, F. Bonaccorso and A. Di Carlo, ChemSusChem, 2016, 9, 2609 CrossRef CAS PubMed; (b) A. Agresti, S. Pescetelli, A. L. Palma, A. E. Del Rio Castillo, D. Konios, G. Kakavelakis, S. Razza, L. Cinà, E. Kymakis and F. Bonaccorso, ACS Energy Lett., 2017, 2, 279 CrossRef CAS; (c) P. O'Keeffe, D. Catone, A. Paladini, F. Toschi, S. Turchini, L. Avaldi, F. Martelli, A. Agresti, S. Pescetelli, A. E. Del Rio Castillo, F. Bonaccorso and A. Di Carlo, Nano Lett., 2019, 19, 684 CrossRef PubMed; (d) B. Taheri, N. Y. Nia, A. Agresti, S. Pescetelli, C. Ciceroni, A. E. D. R. Castillo, L. Cinà, S. Bellani, F. Bonaccorso and A. Di Carlo, 2D Materials, 2018, 5, 045034 CrossRef CAS; (e) F. Biccari, F. Gabelloni, E. Burzi, M. Gurioli, S. Pescetelli, A. Agresti, A. E. Del Rio Castillo, A. Ansaldo, E. Kymakis and F. Bonaccorso, Adv. Energy Mater., 2017, 7, 1701349 CrossRef.
- B. Pashaei, H. Shahroosvand, M. Ameri, E. Mohajerani and M. K. Nazeeruddin, J. Mater. Chem. A, 2019, 7, 21867 RSC.
- P. Qin, N. Tetreault, M. I. Dar, P. Gao, K. L. McCall, S. R. Rutter, S. D. Ogier, N. D. Forrest, J. S. Bissett, M. J. Simms, A. J. Page, R. Fisher, M. Grätzel and M. K. Nazeeruddin, Adv. Energy Mater., 2015, 5, 1400980 CrossRef.
- (a) S. Ito, in Organic–inorganic halide perovskite photovoltaics: From fundamentals to device architectures, Springer, 2016, ch. 14, p. 343 Search PubMed; (b) Z. Yu and L. Sun, Small Methods, 2018, 2, 1700280 CrossRef.
- (a) P.-K. Kung, M.-H. Li, P.-Y. Lin, Y.-H. Chiang, C.-R. Chan, T.-F. Guo and P. Chen, Adv. Mater. Interfaces, 2018, 5, 1800882 CrossRef; (b) R. Singh, P. K. Singh, B. Bhattacharya and H.-W. Rhee, Applied Materials Today, 2019, 14, 175 CrossRef; (c) N. Arora, M. I. Dar, A. Hinderhofer, N. Pellet, F. Schreiber, S. M. Zakeeruddin and M. Grätzel, Science, 2017, 358, 768 CrossRef CAS PubMed; (d) J. Chen and N.-G. Park, J. Phys. Chem. C, 2018, 122, 14039 CrossRef CAS.
- (a) F. Wang, M. Endo, S. Mouri, Y. Miyauchi, Y. Ohno, A. Wakamiya, Y. Murata and K. Matsuda, Nanoscale, 2016, 8, 11882 RSC; (b) K. Aitola, K. Sveinbjörnsson, J.-P. Correa-Baena, A. Kaskela, A. Abate, Y. Tian, E. M. Johansson, M. Grätzel, E. I. Kauppinen and A. Hagfeldt, Energy Environ. Sci., 2016, 9, 461 RSC; (c) R. He, X. Huang, M. Chee, F. Hao and P. Dong, Carbon Energy, 2019, 1, 109–123 CrossRef; (d) F. Meng, A. Liu, L. Gao, J. Cao, Y. Yan, N. Wang, M. Fan, G. Wei and T. Ma, J. Mater. Chem. A, 2019, 7, 8690 RSC; (e) Q. Q. Chu, B. Ding, J. Peng, H. Shen, X. Li, Y. Liu, C. X. Li, C. J. Li, G. J. Yang, T. P. White and K. R. Catchpole, J. Mater. Sci. Technol., 2019, 35, 987 CrossRef; (f) G. Grancini, C. Roldán-Carmona, I. Zimmermann, E. Mosconi, X. Lee, D. Martineau, S. Narbey, F. Oswald, F. De Angelis, M. Graetzel and M. K. Nazeeruddin, Nat. Commun., 2017, 8, 15684 CrossRef CAS; (g) H. Chen, Z. Wei, H. He, X. Zheng, K. S. Wong and S. Yang, Adv. Energy Mater., 2016, 6, 1502087 CrossRef; (h) X. Chang, W. Li, L. Zhu, H. Liu, H. Geng, S. Xiang, J. Liu and H. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 33649 CrossRef CAS.
- J. A. Christians, R. C. Fung and P. V. Kamat, J. Am. Chem. Soc., 2013, 136, 758 CrossRef PubMed.
- (a) S. Chavhan, O. Miguel, H.-J. Grande, V. Gonzalez-Pedro, R. S. Sánchez, E. M. Barea, I. Mora-Seró and R. Tena-Zaera, J. Mater. Chem. A, 2014, 2, 12754 RSC; (b) P. Qin, S. Tanaka, S. Ito, N. Tetreault, K. Manabe, H. Nishino, M. K. Nazeeruddin and M. Grätzel, Nat. Commun., 2014, 5, 3834 CrossRef CAS.
- A. Bashir, S. Shukla, J. H. Lew, S. Shukla, A. Bruno, D. Gupta, T. Baikie, R. Patidar, Z. Akhter and A. Priyadarshi, Nanoscale, 2018, 10, 2341 RSC.
- (a) J. Y. Jeng, K. C. Chen, T. Y. Chiang, P. Y. Lin, T. D. Tsai, Y. C. Chang, T. F. Guo, P. Chen, T. C. Wen and Y. J. Hsu, Adv. Mater., 2014, 26, 4107 CrossRef CAS PubMed; (b) F. Xie, C.-C. Chen, Y. Wu, X. Li, M. Cai, X. Liu, X. Yang and L. Han, Energy Environ. Sci., 2017, 10, 1942 RSC; (c) S. Yue, K. Liu, R. Xu, M. Li, M. Azam, K. Ren, J. Liu, Y. Sun, Z. Wang and D. Cao, Energy Environ. Sci., 2017, 10, 2570 RSC.
- Q. Wu, C. Xue, Y. Li, P. Zhou, W. Liu, J. Zhu, S. Dai, C. Zhu and S. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 28466 CrossRef CAS PubMed.
- W. A. Dunlap-Shohl, T. B. Daunis, X. Wang, J. Wang, B. Zhang, D. Barrera, Y. Yan, J. W. Hsu and D. B. Mitzi, J. Mater. Chem. A, 2018, 6, 469 RSC.
- (a) M. Cheng, Y. Li, M. Safdari, C. Chen, P. Liu, L. Kloo and L. Sun, Adv. Energy Mater., 2017, 7, 1602556 CrossRef; (b) Z. Liu, T. He, K. Liu, Q. Zhi and M. Yuan, RSC Adv., 2017, 7, 26202 RSC.
- Z.-L. Tseng, L.-C. Chen, C.-H. Chiang, S.-H. Chang, C.-C. Chen and C.-G. Wu, Sol. Energy, 2016, 139, 484 CrossRef CAS.
- F. Igbari, M. Li, Y. Hu, Z.-K. Wang and L.-S. Liao, J. Mater. Chem. A, 2016, 4, 1326 RSC.
- H. Zhang, H. Wang, W. Chen and A. K. Y. Jen, Adv. Mater., 2017, 29, 1604984 CrossRef PubMed.
- H. Rao, W. Sun, S. Ye, W. Yan, Y. Li, H. Peng, Z. Liu, Z. Bian and C. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 7800 CrossRef CAS PubMed.
- (a) C. Zuo and L. Ding, Small, 2015, 11, 5528 CrossRef CAS PubMed; (b) H. Rao, S. Ye, W. Sun, W. Yan, Y. Li, H. Peng, Z. Liu, Z. Bian, Y. Li and C. Huang, Nano Energy, 2016, 27, 51 CrossRef CAS.
- (a) K. Do, H. Choi, K. Lim, H. Jo, J. W. Cho, M. K. Nazeeruddin and J. Ko, Chem. Commun., 2014, 50, 10971 RSC; (b) D. Bi, C. Yi, J. Luo, J.-D. Décoppet, F. Zhang, S. M. Zakeeruddin, X. Li, A. Hagfeldt and M. Grätzel, Nat. Energy, 2016, 1, 16142 CrossRef CAS; (c) J. Feng, X. Zhu, Z. Yang, X. Zhang, J. Niu, Z. Wang, S. Zuo, S. Priya, S. Liu and D. Yang, Adv. Mater., 2018, 30, 1801418 CrossRef; (d) W. S. Yang, B.-W. Park, E. H. Jung, N. J. Jeon, Y. C. Kim, D. U. Lee, S. S. Shin, J. Seo, E. K. Kim and J. H. Noh, Science, 2017, 356, 1376 CrossRef CAS PubMed; (e) E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T.-Y. Yang, J. H. Noh and J. Seo, Nature, 2019, 567, 511 CrossRef CAS PubMed.
- (a) N. Onozawa-Komatsuzaki, T. Funaki, T. N. Murakami, S. Kazaoui, M. Chikamatsu and K. Sayama, Electrochemistry, 2017, 85, 226 CrossRef CAS; (b) M. Saliba, S. Orlandi, T. Matsui, S. Aghazada, M. Cavazzini, J.-P. Correa-Baena, P. Gao, R. Scopelliti, E. Mosconi and K.-H. Dahmen, Nat. Energy, 2016, 1, 15017 CrossRef CAS; (c) J. Ye, L. Zhou, L. Zhu, X. Zhang, Z. Shao, X. Pan and S. Dai, RSC Adv., 2016, 6, 17354 RSC.
- M. Petrus, T. Bein, T. Dingemans and P. Docampo, J. Mater. Chem. A, 2015, 3, 12159 RSC.
- A. Connell, Z. Wang, Y.-H. Lin, P. C. Greenwood, A. A. Wiles, E. W. Jones, L. Furnell, R. Anthony, C. P. Kershaw, G. Cooke, H. J. Snaith and P. J. Holliman, J. Mater. Chem. C, 2019, 7, 5235 RSC.
- J. H. Heo, D. S. Lee, D. H. Shin and S. H. Im, J. Mater. Chem. A, 2019, 7, 888 RSC.
- U. K. Singh, E. R. Strieter, D. G. Blackmond and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 14104 CrossRef CAS PubMed.
- (a) I. P. Beletskaya and A. V. Cheprakov, Chem. Rev., 2000, 100, 3009 CrossRef CAS PubMed; (b) C. Amatore, G. Broeker, A. Jutand and F. Khalil, J. Am. Chem. Soc., 1997, 119, 5176 CrossRef CAS; (c) S. Bräse and A. De Meijere, Met.-Catal. Cross-Coupling React., 1998, p. 98 Search PubMed.
- (a) T. N. Mitchell, Met.-Catal. Cross-Coupling React., 1998, p. 167 Search PubMed; (b) A. L. Casado, P. Espinet, A. M. Gallego and J. M. Martínez-Ilarduya, Chem. Commun., 2001, 339 RSC; (c) A. Ricci, F. Angelucci, M. Bassetti and C. Lo Sterzo, J. Am. Chem. Soc., 2002, 124, 1060 CrossRef CAS PubMed.
- (a) A. Suzuki, in Metal-catalyzed cross-coupling reactions, ed. F. Diederich and P. J. Stang, Wiley-VCH, Weinheim, 1998, ch. 2, p. 49 Search PubMed; (b) A. O. Aliprantis and J. W. Canary, J. Am. Chem. Soc., 1994, 116, 6985 CrossRef CAS.
- R. Franzén, Can. J. Chem., 2000, 78, 957 CrossRef.
- (a) L. Zhang, T. Liu, L. Liu, M. Hu, Y. Yang, A. Mei and H. Han, J. Mater. Chem. A, 2015, 3, 9165 RSC; (b) C. H. Teh, R. Daik, E. L. Lim, C. C. Yap, M. A. Ibrahim, N. A. Ludin, K. Sopian and M. A. M. Teridi, J. Mater. Chem. A, 2016, 4, 15788 RSC; (c) J. Urieta-Mora, I. García-Benito, A. Molina-Ontoria and N. Martín, Chem. Soc. Rev., 2018, 47, 8541 RSC.
- M. Maciejczyk, A. Ivaturi and N. Robertson, J. Mater. Chem. A, 2016, 4, 4855 RSC.
- (a) P. Zhou, T. Bu, S. Shi, L. Li, Y. Zhang, Z. Ku, Y. Peng, J. Zhong, Y.-B. Cheng and F. Huang, J. Mater. Chem. C, 2018, 6, 5733 RSC; (b) N. Y. Nia, F. Matteocci, L. Cina and A. Di Carlo, ChemSusChem, 2017, 10, 3854 CrossRef CAS PubMed.
- G. Kakavelakis, I. Paradisanos, B. Paci, A. Generosi, M. Papachatzakis, T. Maksudov, L. Najafi, A. E. Del Rio Castillo, G. Kioseoglou, E. Stratakis, F. Bonaccorso and E. Kymakis, Adv. Energy Mater., 2018, 8, 1702287 CrossRef.
- K. Zhang, L. Wang, Y. Liang, S. Yang, J. Liang, F. Cheng and J. Chen, Synth. Met., 2012, 162, 490 CrossRef CAS.
- (a) M. Thelakkat, Macromol. Mater. Eng., 2002, 287, 442 CrossRef CAS; (b) P. Cias, C. Slugovc and G. Gescheidt, J. Phys. Chem. A, 2011, 115, 14519 CrossRef CAS PubMed.
- (a) J. H. Burroughes, D. D. Bradley, A. Brown, R. Marks, K. Mackay, R. H. Friend, P. Burns and A. Holmes, Nature, 1990, 347, 539 CrossRef CAS; (b) M. Meier, E. Buchwald, S. Karg, P. Pösch, M. Greczmiel, P. Strohriegl and W. Rieβ, Synth. Met., 1996, 76, 95 CrossRef CAS; (c) X. C. Li, T. M. Yong, F. Cacialli, J. Grüner, R. H. Friend, A. B. Holmes, M. Giles and S. C. Moratti, Adv. Mater., 1995, 7, 898 CrossRef CAS; (d) W. Ishikawa, K. Noguchi, Y. Kuwabaru and Y. Shirota, Adv. Mater., 1993, 5, 559 CrossRef CAS.
- P. Agarwala and D. Kabra, J. Mater. Chem. A, 2017, 5, 1348 RSC.
- (a) S. Lv, L. Han, J. Xiao, L. Zhu, J. Shi, H. Wei, Y. Xu, J. Dong, X. Xu and D. Li, Chem. Commun., 2014, 50, 6931 RSC; (b) J. Wang, S. Wang, X. Li, L. Zhu, Q. Meng, Y. Xiao and D. Li, Chem. Commun., 2014, 50, 5829 RSC.
- (a) N. Zhou, K. Prabakaran, B. Lee, S. H. Chang, B. Harutyunyan, P. Guo, M. R. Butler, A. Timalsina, M. J. Bedzyk and M. A. Ratner, J. Am. Chem. Soc., 2015, 137, 4414 CrossRef CAS PubMed; (b) I. R. Perera, T. Daeneke, S. Makuta, Z. Yu, Y. Tachibana, A. Mishra, P. Bäuerle, C. A. Ohlin, U. Bach and L. Spiccia, Angew. Chem., Int. Ed., 2015, 54, 3758 CrossRef CAS PubMed.
- (a) Y. Shirota, J. Mater. Chem., 2005, 15, 75 RSC; (b) Y. Tao, Q. Wang, C. Yang, C. Zhong, J. Qin and D. Ma, Adv. Funct. Mater., 2010, 20, 2923 CrossRef CAS.
- (a) Y. Song, C. a. Di, X. Yang, S. Li, W. Xu, Y. Liu, L. Yang, Z. Shuai, D. Zhang and D. Zhu, J. Am. Chem. Soc., 2006, 128, 15940 CrossRef CAS PubMed; (b) A. Cravino, S. Roquet, O. Alévêque, P. Leriche, P. Frère and J. Roncali, Chem. Mater., 2006, 18, 2584 CrossRef CAS.
- Z. Ning and H. Tian, Chem. Commun., 2009, 5483 RSC.
- E. Merino, Chem. Soc. Rev., 2011, 40, 3835 RSC.
- K. Hunger, Industrial dyes: chemistry, properties, applications, John Wiley & Sons, 2007 Search PubMed.
- P. Ashutosh and J. Mehrotra, Colourage, 1979, 26, 25 Search PubMed.
- R. D. Athey, Eur. Coat. J., 1998, 146 CAS.
- A. Jain, Y. Gupta and S. K. Jain, Crit. Rev. Ther. Drug Carrier Syst., 2006, 23, 349 CrossRef CAS PubMed.
- T. Ikeda and O. Tsutsumi, Science, 1995, 268, 1873 CrossRef CAS PubMed.
- B. L. Feringa, R. A. V. Delden, N. Koumura and E. M. Geertsema, Chem. Rev., 2000, 100, 1789 CrossRef CAS PubMed.
- H. Murakami, A. Kawabuchi, K. Kotoo, M. Kunitake and N. Nakashima, J. Am. Chem. Soc., 1997, 119, 7605 CrossRef CAS.
- I. A. Banerjee, L. Yu and H. Matsui, J. Am. Chem. Soc., 2003, 125, 6542 Search PubMed.
- J. C. Crano and R. J. Guglielmetti, Organic Photochromic and Thermochromic Compounds: Volume 2: Physicochemical Studies, Biological Applications, and Thermochromism, Springer Science & Business Media, 1999 Search PubMed.
- T. Muraoka, K. Kinbara and T. Aida, Nature, 2006, 440, 512 CrossRef CAS PubMed.
- S. Tang, W. Li, F. Shen, D. Liu, B. Yang and Y. Ma, J. Mater. Chem., 2012, 22, 4401 RSC.
- (a) I. Neogi, S. Jhulki, M. Rawat, R. Anand, T. J. Chow and J. N. Moorthy, RSC Adv., 2015, 5, 26806 RSC; (b) X. Liu, J. You, Y. Xiao, S. Wang, W. Gao, J. Peng and X. Li, Dyes Pigm., 2016, 125, 36 CrossRef CAS.
- (a) R. S. Ashraf, M. Shahid, E. Klemm, M. Al-Ibrahim and S. Sensfuss, Macromol. Rapid Commun., 2006, 27, 1454 CrossRef CAS; (b) C. D. Sunesh, G. Mathai and Y. Choe, Org. Electron., 2014, 15, 667 CrossRef CAS; (c) M. Mandoc, L. Koster and P. Blom, Appl. Phys. Lett., 2007, 90, 133504 CrossRef.
- K. Rakstys, A. Abate, M. I. Dar, P. Gao, V. Jankauskas, G. n. Jacopin, E. Kamarauskas, S. Kazim, S. Ahmad and M. Grätzel, J. Am. Chem. Soc., 2015, 137, 16172 CrossRef CAS PubMed.
- B. Hailegnaw, S. Kirmayer, E. Edri, G. Hodes and D. Cahen, J. Phys. Chem. Lett., 2015, 6, 1543 CrossRef CAS PubMed.
- A. K. Jena, Y. Numata, M. Ikegami and T. Miyasaka, J. Mater. Chem. A, 2018, 6, 2219 RSC.
- (a) T. Swetha and S. P. Singh, J. Mater. Chem. A, 2015, 3, 18329 RSC; (b) T. Xu, L. Chen, Z. Guo and T. Ma, Phys. Chem. Chem. Phys., 2016, 18, 27026 RSC; (c) L. Zheng, Y.-H. Chung, Y. Ma, L. Zhang, L. Xiao, Z. Chen, S. Wang, B. Qu and Q. Gong, Chem. Commun., 2014, 50, 11196 RSC.
- (a) M.-C. Jung and Y. Qi, Org. Electron., 2016, 31, 71 CrossRef CAS; (b) M.-C. Jung, S. R. Raga, L. K. Ono and Y. Qi, Sci. Rep., 2015, 5, 9863 CrossRef CAS PubMed; (c) A. M. Leguy, Y. Hu, M. Campoy-Quiles, M. I. Alonso, O. J. Weber, P. Azarhoosh, M. Van Schilfgaarde, M. T. Weller, T. Bein and J. Nelson, Chem. Mater., 2015, 27, 3397 CrossRef CAS; (d) H. Choi, J. W. Cho, M.-S. Kang and J. Ko, Chem. Commun., 2015, 51, 9305 RSC.
- J. Zhang, B. Xu, L. Yang, C. Ruan, L. Wang, P. Liu, W. Zhang, N. Vlachopoulos, L. Kloo and G. Boschloo, Adv. Energy Mater., 2018, 8, 1701209 CrossRef.
- (a) H. J. Snaith and M. Grätzel, Appl. Phys. Lett., 2006, 89, 262114 CrossRef; (b) T. Leijtens, I.-K. Ding, T. Giovenzana, J. T. Bloking, M. D. McGehee and A. Sellinger, ACS Nano, 2012, 6, 1455 CrossRef CAS PubMed; (c) B. Xu, E. Sheibani, P. Liu, J. Zhang, H. Tian, N. Vlachopoulos, G. Boschloo, L. Kloo, A. Hagfeldt and L. Sun, Adv. Mater., 2014, 26, 6629 CrossRef CAS PubMed.
- (a) P. Ganesan, K. Fu, P. Gao, I. Raabe, K. Schenk, R. Scopelliti, J. Luo, L. H. Wong, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 1986 RSC; (b) A. Abate, S. Paek, F. Giordano, J.-P. Correa-Baena, M. Saliba, P. Gao, T. Matsui, J. Ko, S. M. Zakeeruddin, K. H. Dahmen, A. Hagfeldt, M. Grätzel and M. K. Nazeeruddin, Energy Environ. Sci., 2015, 8, 2946 RSC; (c) Z. Hawash, L. K. Ono, S. R. Raga, M. V. Lee and Y. Qi, Chem. Mater., 2015, 27, 562 CrossRef CAS.
- F. Zhang, X. Zhao, C. Yi, D. Bi, X. Bi, P. Wei, X. Liu, S. Wang, X. Li and S. M. Zakeeruddin, Dyes Pigm., 2017, 136, 273 CrossRef CAS.
- Y. H. Lee, J. Luo, R. Humphry-Baker, P. Gao, M. Grätzel and M. K. Nazeeruddin, Adv. Funct. Mater., 2015, 25, 3925 CrossRef CAS.
- Z. Zhu, Y. Bai, H. K. H. Lee, C. Mu, T. Zhang, L. Zhang, J. Wang, H. Yan, S. K. So and S. Yang, Adv. Funct. Mater., 2014, 24, 7357 CrossRef CAS.
- D. Markov, J. Hummelen, P. Blom and A. Sieval, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 045216 CrossRef.
- K. Rakstys, M. Saliba, P. Gao, P. Gratia, E. Kamarauskas, S. Paek, V. Jankauskas and M. K. Nazeeruddin, Angew. Chem., 2016, 128, 7590 CrossRef.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316 CrossRef CAS PubMed.
- T. P. Osedach, T. L. Andrew and V. Bulović, Energy Environ. Sci., 2013, 6, 711 RSC.
- (a) D. Bi, B. Xu, P. Gao, L. Sun, M. Grätzel and A. Hagfeldt, Nano Energy, 2016, 23, 138 CrossRef CAS; (b) H. Choi, K. Do, S. Park, J. S. Yu and J. Ko, Chem.–Eur. J., 2015, 21, 15919 CrossRef CAS PubMed; (c) A. Molina-Ontoria, I. Zimmermann, I. Garcia-Benito, P. Gratia, C. Roldán-Carmona, S. Aghazada, M. Graetzel, M. K. Nazeeruddin and N. Martín, Angew. Chem., Int. Ed., 2016, 55, 6270 CrossRef CAS PubMed; (d) M. Franckevičius, A. Mishra, F. Kreuzer, J. Luo, S. M. Zakeeruddin and M. Grätzel, Mater. Horiz., 2015, 2, 613 RSC; (e) S. Do Sung, M. S. Kang, I. T. Choi, H. M. Kim, H. Kim, M. Hong, H. K. Kim and W. I. Lee, Chem. Commun., 2014, 50, 14161 RSC; (f) P. Gratia, A. Magomedov, T. Malinauskas, M. Daskeviciene, A. Abate, S. Ahmad, M. Grätzel, V. Getautis and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2015, 54, 11409 CrossRef CAS PubMed; (g) H. Choi, S. Paek, N. Lim, Y. H. Lee, M. K. Nazeeruddin and J. Ko, Chem.–Eur. J., 2014, 20, 10894 CrossRef CAS PubMed.
- H. Choi, S. Park, S. Paek, P. Ekanayake, M. K. Nazeeruddin and J. Ko, J. Mater. Chem. A, 2014, 2, 19136 RSC.
- (a) B. Xu, D. Bi, Y. Hua, P. Liu, M. Cheng, M. Grätzel, L. Kloo, A. Hagfeldt and L. Sun, Energy Environ. Sci., 2016, 9, 873 RSC; (b) M. Daskeviciene, S. Paek, Z. Wang, T. Malinauskas, G. Jokubauskaite, K. Rakstys, K. T. Cho, A. Magomedov, V. Jankauskas and S. Ahmad, Nano Energy, 2017, 32, 551 CrossRef CAS; (c) X. Jiang, Z. Yu, Y. Zhang, J. Lai, J. Li, G. G. Gurzadyan, X. Yang and L. Sun, Sci. Rep., 2017, 7, 42564 CrossRef CAS PubMed.
- M.-H. Li, C.-W. Hsu, P.-S. Shen, H.-M. Cheng, Y. Chi, P. Chen and T.-F. Guo, Chem. Commun., 2015, 51, 15518 RSC.
- (a) H. Wang, A. D. Sheikh, Q. Feng, F. Li, Y. Chen, W. Yu, E. Alarousu, C. Ma, M. A. Haque and D. Shi, ACS Photonics, 2015, 2, 849 CrossRef CAS; (b) H. Li, K. Fu, P. P. Boix, L. H. Wong, A. Hagfeldt, M. Grätzel, S. G. Mhaisalkar and A. C. Grimsdale, ChemSusChem, 2014, 7, 3420 CrossRef CAS.
- F. J. Ramos, K. Rakstys, S. Kazim, M. Grätzel, M. K. Nazeeruddin and S. Ahmad, RSC Adv., 2015, 5, 53426 RSC.
- H. Nishimura, N. Ishida, A. Shimazaki, A. Wakamiya, A. Saeki, L. T. Scott and Y. Murata, J. Am. Chem. Soc., 2015, 137, 15656 CrossRef CAS PubMed.
- (a) L. Cabau, I. Garcia-Benito, A. Molina-Ontoria, N. F. Montcada, N. Martin, A. Vidal-Ferran and E. Palomares, Chem. Commun., 2015, 51, 13980 RSC; (b) A. Krishna, D. Sabba, H. Li, J. Yin, P. P. Boix, C. Soci, S. G. Mhaisalkar and A. C. Grimsdale, Chem. Sci., 2014, 5, 2702 RSC; (c) M. S. Kang, S. D. Sung, I. T. Choi, H. Kim, M. Hong, J. Kim, W. I. Lee and H. K. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 22213 CrossRef CAS.
- L. Calió, S. Kazim, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2016, 55, 14522 CrossRef PubMed.
- S. Nandy, A. Banerjee, E. Fortunato and R. Martins, Reviews in Advanced Sciences and Engineering, 2013, 2, 273 CrossRef.
- E. Fortunato, V. Figueiredo, P. Barquinha, E. Elamurugu, R. Barros, G. Gonçalves, S.-H. K. Park, C.-S. Hwang and R. Martins, Appl. Phys. Lett., 2010, 96, 192102 CrossRef.
- (a) A. S. Subbiah, A. Halder, S. Ghosh, N. Mahuli, G. Hodes and S. K. Sarkar, J. Phys. Chem. Lett., 2014, 5, 1748 CrossRef CAS PubMed; (b) M. I. Hossain, F. H. Alharbi and N. Tabet, Sol. Energy, 2015, 120, 370 CrossRef CAS; (c) B. A. Nejand, V. Ahmadi, S. Gharibzadeh and H. R. Shahverdi, ChemSusChem, 2016, 9, 302 CrossRef CAS PubMed; (d) M. Lv, J. Zhu, Y. Huang, Y. Li, Z. Shao, Y. Xu and S. Dai, ACS Appl. Mater. Interfaces, 2015, 7, 17482 CrossRef CAS PubMed; (e) G. Sfyri, C. V. Kumar, Y.-L. Wang, Z.-X. Xu, C. Krontiras and P. Lianos, Appl. Surf. Sci., 2016, 360, 767 CrossRef CAS; (f) G. Sfyri, C. V. Kumar, G. Sabapathi, L. Giribabu, K. S. Andrikopoulos, E. Stathatos and P. Lianos, RSC Adv., 2015, 5, 69813 RSC; (g) M. Sun, S. Wang, Y. Xiao, Z. Song and X. Li, J. Energy Chem., 2015, 24, 756 CrossRef; (h) H. Lu, Y. Ma, B. Gu, W. Tian and L. Li, J. Mater. Chem. A, 2015, 3, 16445 RSC; (i) S. Ryu, J. H. Noh, N. J. Jeon, Y. C. Kim, W. S. Yang, J. Seo and S. I. Seok, Energy Environ. Sci., 2014, 7, 2614 RSC; (j) Y. Xiao, G. Han, Y. Chang, H. Zhou, M. Li and Y. Li, J. Power Sources, 2014, 267, 1 CrossRef CAS; (k) B. Cai, Y. Xing, Z. Yang, W.-H. Zhang and J. Qiu, Energy Environ. Sci., 2013, 6, 1480 RSC; (l) P. Nagarjuna, K. Narayanaswamy, T. Swetha, G. H. Rao, S. P. Singh and G. Sharma, Electrochim. Acta, 2015, 151, 21 CrossRef CAS; (m) J. W. Lee, S. Park, M. J. Ko, H. J. Son and N. G. Park, ChemPhysChem, 2014, 15, 2595 CrossRef CAS PubMed; (n) A. Dubey, N. Adhikari, S. Venkatesan, S. Gu, D. Khatiwada, Q. Wang, L. Mohammad, M. Kumar and Q. Qiao, Sol. Energy Mater. Sol. Cells, 2016, 145, 193 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9sc05694g |
| This journal is © The Royal Society of Chemistry 2020 |