Open Access Article
Open Access ArticleFast surface immobilization of native proteins through catalyst-free amino-yne click bioconjugation†
Yiru
Zhang‡
a,
Jianlei
Shen‡
 a,
Rong
Hu
a,
Xiujuan
Shi
a,
Rong
Hu
a,
Xiujuan
Shi
 b,
Xianglong
Hu
b,
Xianglong
Hu
 b,
Benzhao
He
b,
Anjun
Qin
b,
Benzhao
He
b,
Anjun
Qin
 *a and
Ben Zhong
Tang
*a and
Ben Zhong
Tang
 *ab
*ab
aState Key Laboratory of Luminescent Materials and Devices, Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates, Center for Aggregation-Induced Emission, South China University of Technology, Guangzhou 510640, China. E-mail: msqinaj@scut.edu.cn
bDepartment of Chemistry, Hong Kong Branch of Chinese National Engineering Research Centre for Tissue Restoration and Reconstruction, Institute for Advanced Study, and Department of Chemical and Biological Engineering, The Hong Kong University of Science & Technology, Clear Water Bay, Kowloon, Hong Kong, China. E-mail: tangbenz@ust.hk
First published on 18th March 2020
Abstract
Surface immobilization provides a useful platform for biosensing, drug screening, tissue engineering and other chemical and biological applications. However, some of the used reactions are inefficient and/or complicated, limiting their applications in immobilization. Herein, we use a spontaneous and catalyst-free amino-yne click bioconjugation to generate activated ethynyl group functionalized surfaces for fast immobilization of native proteins and cells. Biomolecules, such as bovine serum albumin (BSA), human IgG and a peptide of C(RGDfK), could be covalently immobilized on the surfaces in as short as 30 min. Notably, the bioactivity of the anchored biomolecules remains intact, which is verified by efficiently capturing target antibodies and cells from the bulk solutions. This strategy represents an alternative for highly efficient surface biofunctionalization.
Introduction
Surface modification has been an effective technique to fabricate functional surfaces with various properties, such as hydrophilicity, charged surface, biocompatibility and reactivity to provide a useful platform for biosensing,1–6 tissue engineering7,8 and other chemical and biological applications.9,10 Meanwhile, an ideal reaction used for surface bio-functionalization should meet the requirements of simplicity, efficiency, universality, and biocompatibility, etc. Present techniques for surface biofunctionalization can be classified as nonspecific adsorption11,12 and covalent interactions.13–17 In comparison to nonspecific adsorption, chemical ligation of molecules on a surface can greatly shorten the time and facilitate the formation of a more stable layer on the surface.18 Therefore, the development of a powerful ligation reaction with high efficiency and simple operation is highly desirable.Many chemical reactions have been reported for surface immobilization, such as NHS (N-hydroxysuccinimide) ester-amino ligation,19 aldehyde-assisted ligation,20 epoxy-amino ligation,21 thiol–maleimide ligation,22 Staudinger ligation15 and photoactive bioconjugations.23 However, some of them are inefficient, easily hydrolyzed or complicated, limiting their applications in bioconjugate immobilization (Table S1, ESI†). Recently, the click reaction has attracted more attentions in surface modification because of its fast, efficient and simple features. Several click reactions, such as Cu(I)-catalyzed azide–alkyne cycloaddition,24–26 strain-promoted azide–alkyne cycloaddition,27 and Diels–Alder ligation,16 have been used to immobilize biomolecules on surfaces. Nevertheless, the biomolecules need to be pre-modified by introduction of functional groups, resulting in tedious and complicated processes. Thereby, to develop a new technique for immobilization without pre-modification is in great demand.
In our previous work, we have developed a spontaneous amino-yne click polymerization that enjoys the advantages of atom economy, mild reaction and catalyst-free conditions, and stereo- and regioselectivity, etc.28–32 More recently, the amino-yne click reaction was proved to hold great potential for bioconjugation applications.33,34 Attracted by this highly efficient spontaneous amino-yne click reaction, herein, we applied it in functionalizing surfaces and sequentially immobilizing native bioconjugates for the first time. Excitingly, the amino group coated glass surfaces could be successfully functionalized with ester activated ethynyl groups through the catalyst-free amino-yne click reaction, which could be further modified with organic dyes, native proteins and cells in one step (Fig. 1). Furthermore, we combined the amino-yne click reaction with a micro contact printing (μCP) technique to seed HeLa cells and mesenchymal stem cells (MSCs) on the surfaces with well-defined patterns, which is promising for application in tissue engineering.
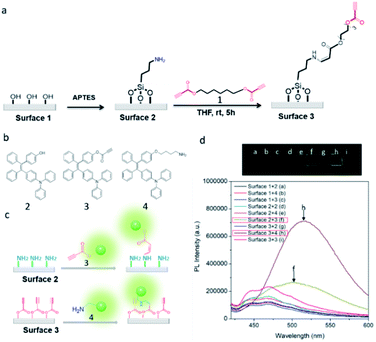
 | ||
| Fig. 1 The illustration of the spontaneous amino-yne click reaction (a) and (b) and its application in surface biofunctionalization (c). | ||
Results and discussion
The glass slides were chosen as the model substrate because of their wide applications in bioassay. Briefly, the glass surfaces were first treated with 3-aminopropyltriethoxysilane (APTES) to readily generate amino group coated surfaces,35 followed by the excessive addition of ester activated diyne 1 (Fig. 2a). Then, an ester activated ethynyl group functionalized layer on the glass surface was fabricated. To verify the reaction between the activated ethynyl groups of diyne 1 and amino groups on the surface, we studied the reaction of APTES with diyne 1 in tetrahydrofuran (THF) solution by using Fourier-transform infrared spectroscopy (FT-IR). The results showed that a new peak at 1610 cm−1 corresponding to the newly formed vinyl groups appeared (Fig. S1, ESI†), indicative of the successful reaction of APTES with diyne 1.30 This result suggests that the ethynyl groups of 1 can react with amino groups of APTES on the surface effectively.To further confirm the success of the modification, the water contact angles of surfaces with different treatments were measured. As listed in Table S2 (ESI†), a distinct change in hydrophobicity could be observed after APTES treatment of the surface. The subsequent reaction with diyne 1 further increased the hydrophobicity of the surface with Δθ changing from 9.55° to 18.42° due to the introduction of the hydrophobic alkyl chains. Meanwhile, X-ray photoelectron spectroscopy (XPS) measurements of the surfaces before and after the modification of diyne 1 further proved the occurrence of the amino-yne click reaction. As shown in Fig. S2 (ESI†), a new peak assignable to the C–O band appeared after the treatment of surface 2 with diyne 1. Furthermore, the data of the element percent ratio of C/N revealed that the values of surface 2 increased compared with those of surface 3 (Table S3, ESI†). Based on the above analysis, we could conclude that the activated ethynyl groups have been covalently introduced on the surfaces of the glass slides. Importantly, unlike the commercial aldehyde modified surfaces that exhibit strong autofluorescence under 488 nm light excitation, the ethynyl functionalized surfaces don't have any autofluorescence under the same conditions, which is more desirable for immobilization (Fig. S3, ESI†).
With the activated ethynyl group modified surfaces in hand, we treated them with functional molecules through the amino-yne click reaction. First, hydroxyl-, ethynyl- and amino-containing luminogens 2, 3 and 4 were designed and synthesized (Fig. 2b and Schemes S1–S3, ESI†). Notably, these luminogens feature the unique aggregation-induced emission (AIE) characteristics (Fig. S4, ESI†). According to the AIE mechanism of restriction of intramolecular motion (RIM),36 once the AIE-active luminogens (AIEgens) are immobilized on the surface, it would be emissive upon excitation. We then treated the surfaces 1–3 with the AIEgens of 2–4, respectively. As shown in Fig. 2c and d, intense fluorescence can only be detected on the surfaces 2 and 3 upon treating with 3 and 4 under UV lamp irradiation after a general washing process with THF, respectively. This phenomenon could be well explained: AIEgen 3 could covalently be immobilized on the amino group functionalized surface 2, whereas, 4 could be covalently bound on the ethynyl groupcontaining surface 3 through the spontaneous amino-yne click reaction, and the RIM mechanism enables the surfaces emissive. However, the treatment of 2–4 with surface 1, 2 and 4 with surface 2 as well as 2 and 3 with surface 3 resulted in no emission upon UV irradiation because the AIEgens could be washed away due to the noncovalent binding between the molecules and the surfaces. These results further confirmed that ethynyl groups had been well-functionalized on the surface 3, and the amino-yne click reaction could be performed successfully for immobilization applications.
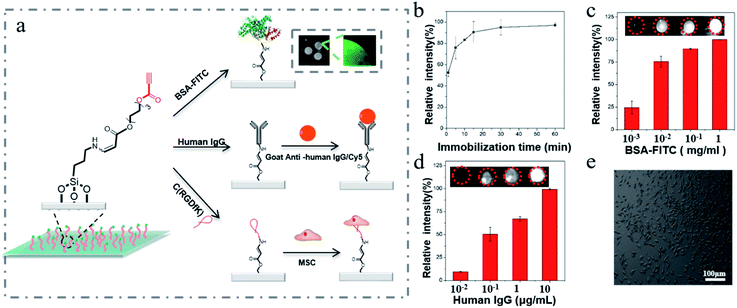
Given that the proteins are full of aliphatic amino groups with low isoelectric points,37 and according to our study that the aliphatic amino groups could efficiently react with the activated ethynyl groups via the spontaneous amino-yne click reaction, we next tried to immobilize native proteins on the ethynyl group functionalized surfaces via this reaction (Fig. 3a). Bovine serum albumin (BSA) was employed due to its wide applications in surface biomodification. The ethynyl group functionalized surfaces were incubated with FITC-labelled BSA (BSA-FITC) followed with washing to remove the unreacted ones. Then, the immobilization rate of BSA was evaluated using a gel imaging system. As shown in Fig. 3a, a strong fluorescence signal on the surface was observed after treatment with BSA-FITC. Time-lapse imaging of the immobilization process of BSA-FITC on the surface indicated that the reaction could complete within 30 min (Fig. 3b). Moreover, by increasing the concentration of BSA-FITC, the fluorescence intensity of the formed spots on the surface was enhanced (Fig. 3c) under the same treatment conditions because more BSA-FITC could be immobilized on the surface.
It is worth noting that the immobilization of BSA-FITC on the activated ethynyl group functionalized surfaces is much more efficient in terms of the reaction time and protein density than that on the commercial aldehyde modified surfaces (Fig. S5, ESI†). As shown above, the immobilization time on the former is only 30 min, while on the latter is at least 180 min.20,38 More importantly, the immobilization of BSA-FITC on activated ethynyl functionalized surfaces is much more stable than that on the commercial aldehyde modified surfaces because the enamine groups generated from the reaction of ethynyl and amino groups are more stable than the imine ones formed via the reaction of aldehyde and amino groups.39 As shown in Fig. S6 (ESI†), when treating the two surfaces in buffer solutions containing acetic acid with a pH value of 3 after the immobilization of BSA-FITC, we found plenty of proteins released from the aldehyde modified surface after 1 h, while the majority proteins on the activated ethynyl functionalized surface remained. This result confirms that the linkage between native proteins and the activated ethynyl functionalized surface is quite stable, which is potentially more suitable for practical applications.
Encouraged by the successful, efficient and stable immobilization of BSA on the ethynyl group functionalized surfaces, we further tried to fabricate a functional biochip, and human IgG was selected as a model antibody. We first investigated its immobilization efficiency and bioactivity on the surfaces. After spotting the human IgG solution on the surface 3 for 30 min at room temperature, the functionalized surface was washed with PBS buffer containing 0.2% sodium dodecyl sulfate to remove the unreacted antibody, and then blocked with BSA to inhibit the nonspecific adsorption. Next, we added Cy5-labeled goat anti-human IgG (goat anti-human IgG/Cy5), the secondary antibody, on the surface to test the bioactivity of human IgG.40 As shown in Fig. 3d, by increasing the concentration of the antibody, the fluorescent signal of the spots increased synchronously. In sharp contract, the sole BSA coated surface showed no observable fluorescent signal after treatment with the antibody under the same experimental conditions (Fig. S7, ESI†). This result well excluded the non-specific physical adsorption of the secondary antibody on the surface.
Furthermore, we immobilized cells on the surface because of the important roles of cell adhesion in tissue engineering. Herein, MSCs were chosen for surface adhesion due to their broad applications in bone regeneration.41 To achieve this goal, C(RGDfK), a short peptide that can enhance cell adhesion through an integrin-mediated interaction, was first immobilized on the surface 3 via the amino-yne click reaction. Then, MSCs were incubated with the C(RGDfK) modified surface for 3 h followed by a mild washing process. For comparison, surfaces 1–3 were also used to treat with MSCs under the same conditions. As shown in Fig. 3e and S8 (ESI†), only the C(RGDfK) modified surface could greatly promote the adhesion of MSCs. In addition, the cells on both the ethynyl group and C(RGDfK) functionalized surfaces exhibit a normal cell morphology, indicative of the good biocompatibility of these two kinds of surfaces.
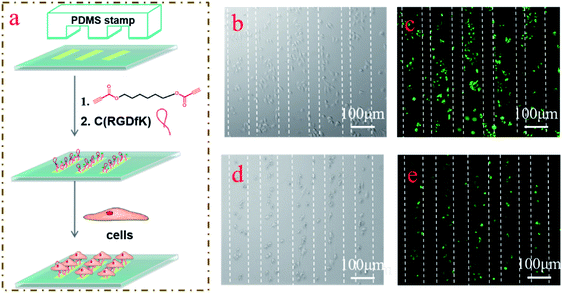
It has been demonstrated that certain patterns can promote the differentiation of MSCs.42 To further exploit the versatility of the amino-yne click reaction in surface modification, we combined a micro contact printing (μCP) technique with the amino-yne reaction to form well defined functional patterns on the surface (Fig. 4a). The patterned surfaces were prepared using the similar procedures to those used in the previous process except that the APTES reagents were transferred to the glass slides via the mediation of PDMS stamps. HeLa cells were incubated with the patterned surface, and the results showed that they could selectively adsorb on the patterned surfaces (Fig. 4b and c and S6, ESI†). Similar results were observed by using MSCs for pattern preparation (Fig. 4d and e, and S9, ESI†), although the adhesion efficiency was not as high as that of HeLa cells because HeLa cells generally have a stronger adhesion force than MSCs.43
Conclusions
In conclusion, we generated a new type of surface for biomolecule immobilization by using the efficient and spontaneous amino-yne click reaction. This kind of ligation tool does not require a catalyst or harsh reaction conditions, and the reaction between ethynyl group functionalized surfaces and biomolecules can be finished within 30 min, which is much shorter than that between aldehyde modified surfaces and biomolecules. Through this technique, we successfully immobilized native proteins and cells on the surfaces with their bioactivity intact. Moreover, we also used a μCP technique and the amino-yne click reaction to seed cells on the surfaces with the well-defined patterns. Thanks to their easy fabrication, good biocompatibility, fast native protein immobilization, stable linkage, and no background fluorescence, the activated ethynyl functionalized surfaces together with the proposed technique hold great potential for surface biofunctionalization.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21788102, 21525417, 21490571 and 51620105009), the Natural Science Foundation of Guangdong Province (2019B030301003, 2018A030313337 and 2016A030312002), the National Key Research and Development Program of China (Intergovernmental cooperation project, 2017YFE0132200) and the Innovation and Technology Commission of Hong Kong (ITC-CNERC14S01).Notes and references
- J. S. Seo, S. Lee and C. D. Poulter, J. Am. Chem. Soc., 2013, 135, 8973–8980 CrossRef CAS PubMed.
- P. Nikolaou, A. Vassilakopoulou, D. Papadatos, E. Topoglidis and I. Koutselas, Mater. Chem. Front., 2018, 2, 730–740 RSC.
- K. S. Palla, T. J. Hurlburt, A. M. Buyanin, G. A. Somorjai and M. B. Francis, J. Am. Chem. Soc., 2017, 139, 1967–1974 CrossRef CAS PubMed.
- J. W. Park, Y. J. Park and C. H. Jun, Chem. Commun., 2011, 47, 4860–4871 RSC.
- L. Bai, L. Tan, L. Chen, S. Liu and Y. Wang, J. Mater. Chem. B, 2014, 2, 7785–7794 RSC.
- J. Schartner, J. Güldenhaupt, B. Mei, M. Rögner, M. Muhler, K. Gerwert and C. Kötting, J. Am. Chem. Soc., 2013, 135, 4079–4087 CrossRef CAS PubMed.
- A. C. Lima, C. Cunha, A. Carvalho, H. Ferreira and N. M. Neves, ACS Appl. Mater. Interfaces, 2018, 10, 13839–13850 CrossRef CAS PubMed.
- F. Y. Cao, W. N. Yin, J. X. Fan, L. Tao, S. Y. Qin, R. X. Zhuo and X. Z. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 6698–6705 CrossRef CAS PubMed.
- D. Samanta and A. Sarkar, Chem. Soc. Rev., 2011, 40, 2567–2592 RSC.
- Z. Li, L. Yu, T. Yang and Y. Chen, Sci. China: Chem., 2018, 61, 1243–1260 CrossRef CAS.
- M. O. Çağlayan, F. Sayar, G. Demirel, B. Garipcan, B. Otman, B. Çelen and E. Pişkin, Nanotechnol. Biol. Med., 2009, 5, 152–161 CrossRef PubMed.
- N. Haddour, S. Cosnier and C. Gondran, J. Am. Chem. Soc., 2005, 127, 5752–5753 CrossRef CAS PubMed.
- T. Govindaraju, P. Jonkheijm, L. Gogolin, H. Schroeder, C. F. W. Becker, C. M. Niemeyerd and H. Waldmann, Chem. Commun., 2008, 32, 3723–3725 RSC.
- D. Weinrich, P. C. Lin, P. Jonkheijm, U. T. T. Nguyen, H. Schrder, C. M. Niemeyer, K. Alexandrov, R. Goody and H. Waldmann, Angew. Chem., Int. Ed., 2010, 49, 1252–1257 CrossRef CAS PubMed.
- M. B. Soellner, K. A. Dickson, B. L. Nilsson and R. T. Raines, J. Am. Chem. Soc., 2003, 125, 11790–11791 CrossRef CAS PubMed.
- A. D. de Araújo, J. M. Palomo, J. Cramer, M. Köhn, H. Schröder, R. Wacker, C. Niemeyer, K. Alexandrov and H. Waldmann, Angew. Chem., Int. Ed., 2006, 45, 296–301 CrossRef PubMed.
- S. Sawoo, P. Dutta, A. Chakraborty, R. Mukhopadhyay, O. Bouloussac and A. Sarkar, Chem. Commun., 2008, 45, 5957–5959 RSC.
- N. Lucy and J. Sagiv, J. Am. Chem. Soc., 1983, 105, 674–676 CrossRef.
- S. Herrwerth, T. Rosendahl, C. Feng, J. Fick, W. Eck, M. Himmelhaus, R. Dahint and M. Grunze, Langmuir, 2003, 19, 1880–1887 CrossRef CAS.
- D. Peelen and L. M. Smith, Langmuir, 2005, 21, 266–271 CrossRef CAS PubMed.
- F. Li, W. Chen and S. Zhang, Biosens. Bioelectron., 2008, 24, 781–786 CrossRef CAS PubMed.
- T. N. Gevrek, I. Kosif and A. Sanyal, ACS Appl. Mater. Interfaces, 2017, 9, 27946–27954 CrossRef CAS PubMed.
- K. M. El Muslemany, A. A. Twite, A. M. ElSohly, A. C. Obermeyer, R. A. Mathies and M. B. Francis, J. Am. Chem. Soc., 2014, 136, 12600–12606 CrossRef CAS PubMed.
- R. D. Rohde, H. D. Agnew, W. S. Yeo, R. C. Bailey and J. R. Heath, J. Am. Chem. Soc., 2006, 128, 9518–9525 CrossRef CAS PubMed.
- S. Prakash, T. M. Long, J. C. Selby, J. S. Moore and M. A. Shannon, Anal. Chem., 2007, 79, 1661–1667 CrossRef CAS PubMed.
- J. S. Seo, S. Lee and D. C. Poulter, J. Am. Chem. Soc., 2013, 135, 8973–8980 CrossRef CAS PubMed.
- M. A. Wijdeven, C. Nicosia, A. Borrmann, J. Huskensb and F. L. van Delft, RSC Adv., 2014, 4, 10549–10552 RSC.
- L. Huang, M. Arndt, K. Gooßen, H. Heydt and L. J. Gooßen, Chem. Rev., 2015, 115, 2596–2697 CrossRef CAS PubMed.
- R. Severin and S. Doye, Chem. Soc. Rev., 2007, 36, 1407–1420 RSC.
- B. He, H. Su, T. Bai, Y. Wu, S. Li, M. Gao, R. Hu, Z. Zhao, A. Qin, J. Ling and B. Z. Tang, J. Am. Chem. Soc., 2017, 139, 5437–5443 CrossRef CAS PubMed.
- J. Huang and X. Jiang, ACS Appl. Mater. Interfaces, 2018, 10, 361–370 CrossRef CAS PubMed.
- H. Li, J. Wang, J. Z. Sun, R. Hu, A. Qin and B. Z. Tang, Polym. Chem., 2012, 3, 1075–1083 RSC.
- X. Hu, X. Zhao, B. He, Z. Zhao, Z. Zheng, P. Zhang, X. Shi, R. T. K. Kwok, J. W. Y. Lam, A. Qin and B. Z. Tang, Research, 2018, 1–12 CrossRef PubMed.
- R. Hu, X. Chen, T. Zhou, H. Si, B. He, R. T. K. Kwok, A. Qin and B. Z. Tang, Sci. China: Chem., 2019, 62, 1198–1203 CrossRef CAS.
- J. A. Camarero, Y. Kwon and M. A. Coleman, J. Am. Chem. Soc., 2004, 126, 14730–14731 CrossRef CAS PubMed.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef CAS PubMed.
- O. Boutureira and G. J. L. Bernardes, Chem. Rev., 2015, 115, 2174–2195 CrossRef CAS PubMed.
- G. MacBeath and S. L. Schreiber, Science, 2000, 289, 1760–1763 CAS.
- D. I. Rozkiewicz, Y. Kraan, M. W. T. Werten, F. A. de Wolf, V. Subramaniam, B. J. Ravoo and D. N. Reinhoudt, Chem.–Eur. J., 2006, 12, 6290–6297 CrossRef CAS PubMed.
- A. Baszkin, M. M. Boissonnade, A. Kamyshny and S. Magdassi, J. Colloid Interface Sci., 2001, 244, 18–23 CrossRef CAS.
- R. Dimitriou, E. Tsiridis and P. V. Giannoudis, Injury, 2005, 36, 1392–1404 CrossRef PubMed.
- A. Shukla, J. H. Slater, J. C. Culver, M. E. Dickinson and J. L. West, ACS Appl. Mater. Interfaces, 2016, 8, 21883–21892 CrossRef CAS PubMed.
- S. Schlie, M. Gruene, H. Dittmar and B. N. Chichkov, Tissue Eng., Part C, 2012, 18, 688–696 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, NMR spectra, photophysical characterization data, UV-vis and photoluminescence spectra, cell imaging, and pros and cons of different techniques for surface immobilization. See DOI: 10.1039/d0sc00062k |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2020 |