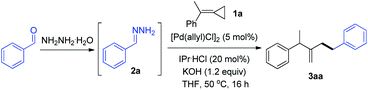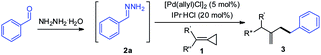Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium-catalyzed hydroalkylation of methylenecyclopropanes with simple hydrazones†
Jinzhong
Yao
ab,
Zhangpei
Chen
a,
Lin
Yu
a,
Leiyang
Lv
 a,
Dawei
Cao
a and
Chao-Jun
Li
a,
Dawei
Cao
a and
Chao-Jun
Li
 *a
*a
aDepartment of Chemistry, FQRNT Centre for Green Chemistry and Catalysis, McGill University, 801 Sherbrooke St. W., Montreal, Quebec H3A 0B8, Canada. E-mail: cj.li@mcgill.ca
bCollege of Biological, Chemical Sciences and Engineering, Jiaxing University, Jiaxing 314001, People's Republic of China
First published on 15th May 2020
Abstract
A palladium-catalyzed hydroalkylation reaction of methylenecyclopropanes via highly selective C–C σ-bond scission was achieved under mild conditions, in which simple hydrazones served as carbanion equivalents. This method featured good functional group compatibility, affording high yields of C-alkylated terminal alkenes.
Introduction
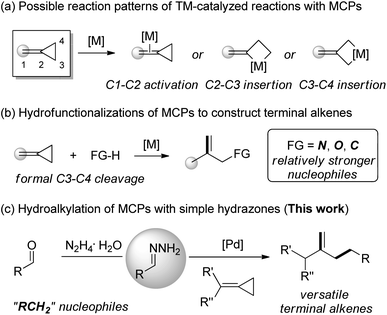
Transition-metal-catalyzed carbon–carbon σ-bond activation towards the reconstruction of new carbon–carbon/hetero bonds is a fundamentally challenging process in organic chemistry.1 Small (three- or four-membered) saturated and unsaturated carbon-rings are ideal candidates for such transformations due to the strain-release driving force.2 Consequently, the reactivity of methylenecyclopropanes (MCPs), a type of three-membered ring tethered by a highly strained double bond, has received much attention in organic synthesis.3 Possible reaction patterns of MCPs with respect to the transition metal catalysis involve the activation of the proximal exo-methylene double bond (C1–C2 activation) and formation of metallacyclobutane species through insertion to the distal single bonds C2–C3 or C3–C4 (Scheme 1a).4 Notably, the hydrofunctionalization of MCPs concerning formal C3–C4 cleavage would provide an efficient way to obtain various terminal alkenes despite confronting with the regioselectivity problem (Scheme 1b).5,6On the other hand, terminal alkenes constitute important intermediates for organic synthesis.7 Particularly, they are not only widely used in the chemical industry for large-scale polymerisations, but also in more special reactions such as metathesis, epoxidations, hydroformylations, hydroaminations, and others.8 Consequently, reliable methods toward the facile generation of versatile terminal alkenes would be very desirable in organic synthesis.9 Toward this target, some efforts have been focused on the hydrofunctionalizations of MCPs through selective C–C distal-bond cleavage, mostly developed by the groups of Yamamoto,10 Shi11 and Mascareñas,12 with several types of pronucleophiles with relatively stronger nucleophilicity, such as nitrogen, oxygen and carbanion nucleophiles. As a result, a variety of functionalized terminal alkenes have been fabricated through these transformations. However, as to the more challenging alkyl substituted terminal alkenes, few successes have been achieved.13 Thus, the development of a modular approach toward hydroalkylation of MCPs with simple alkyl reagents is of great significance.
Recently, our group discovered that hydrazones could serve as alkyl carbanion equivalents in several cross-coupling reactions14 and nucleophilic additions15via polarity reversal. Inspired by our recent work on the palladium-catalyzed Tsuji–Trost reaction, in which hydrazones served as C-nucleophiles instead of traditional alkyl organometallic reagents,16 we wondered if these alkyl pronucleophiles are suitable for the hydroalkylation reaction of MCPs in the presence of the palladium catalyst. To achieve such a transformation would be inherently challenging due to the multiple reactivities of MCPs with the palladium catalyst and the competition between N- and C-nucleophilic attacks of hydrazones (Scheme 1c). Herein, we wish to report a palladium-catalyzed hydroalkylation reaction of MCPs using simple hydrazones as alkyl carbanion equivalents.
Results and discussion
In the preliminary investigation, we examined the reaction of MCP 1a with phenyl hydrazone 2a (Table 1). The reaction of 1a with two equivalents of 2a in the presence of catalytic amounts of [Pd(allyl)Cl]2 (5 mol%) and IPr·HCl (20 mol%), and 1.2 equiv. of KOH in THF at 50 °C gave the hydroalkylation product in 80% yield (entry 1). The reaction did not occur in the absence of palladium or base (entries 2 and 3). Other palladium catalysts, such as Pd2(dba)3, showed a relatively lower efficiency (entry 4). The ligand played an important role in this transformation. An N-heterocyclic carbene (NHC) ligand like SIPr·HCl was also suitable for this reaction (entry 5), while other ligands, such as PCy3 and dppp, led to poor results (entries 6 and 7). Among the bases examined, NaOH exhibited a comparable reaction efficiency, while others showed poor reactivity (entries 8–12). 1,4-Dioxane was also effective as a solvent for this reaction, delivering the product with 68% yield (entry 13). The temperature was found to influence the reactivity, as both lower and higher temperatures decreased the yields (entries 14 and 15).| Entry | Variation from “standard conditions” | NMR yield (%) |
|---|---|---|
| a Reaction conditions: phenyl hydrazone 2a (0.2 mmol, 1.0 M generated in situ from benzaldehyde and hydrazine), 1a (0.1 mmol), [Pd(allyl)Cl]2 (5 mol%), IPr·HCl (20 mol%), and KOH (1.2 equiv.) in THF (0.2 M) were stirred under N2 at 50 °C for 16 h. NMR yields were given with mesitylene as the internal standard, and yields were calculated based on 1a. b Isolated yield was given in the parentheses. | ||
| 1 | No change | 80 (75)b |
| 2 | No [Pd(allyl)Cl]2 | 0 |
| 3 | No base | 0 |
| 4 | Pd2(dba)3 instead of [Pd(allyl)Cl]2 | 32 |
| 5 | SIPr·HCl instead of IPr·HCl | 70 |
| 6 | PCy3 instead of IPr·HCl | 40 |
| 7 | dppp instead of IPr·HCl | 0 |
| 8 | t-BuOLi instead of KOH | 54 |
| 9 | t-BuONa instead of KOH | 45 |
| 10 | t-BuOK instead of KOH | 42 |
| 11 | NaOH instead of KOH | 71 |
| 12 | K3PO4 instead of KOH | 30 |
| 13 | 1,4-Dioxane instead of THF | 68 |
| 14 | 35 °C instead of 50 °C | 41 |
| 15 | 65 °C instead of 50 °C | 60 |
|
||
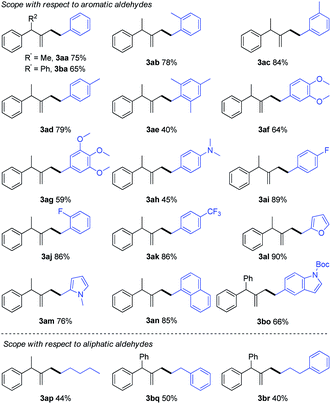
With the optimized reaction conditions in hand, the scope and limitation of hydrazones were examined in their reaction with (1-cyclopropylideneethyl)benzene (1a) or (cyclopropylidenemethylene)dibenzene (1b) as shown in Table 2. A series of hydrazones with functional groups such as methyl, methoxy, fluoro, trifluoromethyl and N,N-dimethylamino participated in the reaction smoothly to give the products in 40–89% yields (3aa–3ak, 3ba). In addition, para-, meta-, ortho-, and multisubstituted aromatic hydrazones were all effective in this reaction. The hydrazone generated from a polycyclic aromatic aldehyde such as 1-naphthaldehyde also led to a smooth reaction to give the desired product (3an) in 85% yield. Moreover, hydrazones prepared from heteroaryl aldehydes containing furan (2l), pyrrole (2m) and indole (2o) were also tolerated in this system (3al, 3am, 3bo). To further expand the utility of this reaction, hydrazones derived from aliphatic aldehydes were examined and found to be also effective, providing the desired products in moderate yields (3ap, 3bq, 3br).
| a Reaction conditions: hydrazone 2 (0.2 mmol, 1.0 M generated in situ from aldehyde and hydrazine), 1a or 1b (0.1 mmol), [Pd(allyl)Cl]2 (5 mol%), IPr·HCl (20 mol%), and KOH (1.2 equiv.) in THF (0.2 M) were stirred under N2 at 50 °C for 16 h; isolated yields were given. |
|---|
|
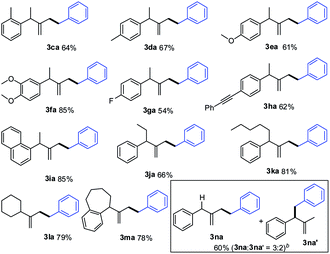
Next, we evaluated the scope of the reaction with regard to the range of methylenecyclopropanes (MCPs) as shown in Table 3. In general, the reaction proceeded smoothly to give the hydroalkylation products in moderate to good yields. A variety of functional groups, including methyl, methoxy, fluoro, on the aryl ring, were compatible under the optimal reaction conditions (3ca–3ga). Notably, the substrate having an alkynyl group was well tolerated, and the corresponding product (3ha) was obtained in 62% yield. Besides the substrates with methyl and phenyl groups at the R′ position, other alkyl-substituted MCPs were all suitable for the reaction (3ja, 3ka), furnishing the desired product with 66% and 81% yields, respectively. Moreover, cyclic MCPs were tolerated to provide products (3la, 3ma) in good yields. However, when R′ was hydrogen, a regioisomeric mixture of 3na and 3na′ was obtained in 60% total yield with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio.
2 ratio.
| a Reaction conditions: phenyl hydrazone 2a (0.2 mmol, 1.0 M generated in situ from benzaldehyde and hydrazine), 1 (0.1 mmol), [Pd(allyl)Cl]2 (5 mol%), IPr·HCl (20 mol%), and KOH (1.2 equiv.) in THF (0.2 M) were stirred under N2 at 50 °C for 16 h; isolated yields were given. b The ratio of 3na and 3na′ was determined by 1H NMR analysis of the crude mixture. |
|---|
|
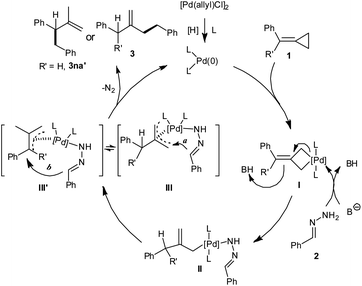
To gain mechanistic insight into this transformation, a preliminary D-labelling experiment was conducted (Scheme 2). When hydrazone (2a-d2) was reacted with 1b, the deuterated product 3ba-d2 was obtained, in which the deuterium isotope is incorporated at the C1 (52% D) and C4 (78% D) positions.
Based on the results and previous studies, a plausible mechanism is proposed as illustrated in Scheme 3. Firstly, palladium(0) is generated from precatalyst [Pd(allyl)Cl]2 upon reduction possibly by the extra hydrazine. Then the direct insertion of palladium(0) species into the distal σ-bond of MCPs 1 gives palladacyclobutane I.10d The base promotes the interaction of hydrazone 2 with palladacyclobutane I to form intermediate II. Then intermediate II leads to π-allyl–Pd species III or III′.6,10a The decomposition of III with N2 extrusion releases product 3, and completes the catalytic cycle (Scheme 3, route a).17 The formation of isomer 3na′ (when R′ = H) could be explained in terms of a possible isomerization of the π-allyl–Pd species of type III to III′ probably due to the lower steric hindrance (Scheme 3, route b).12 The result of the deuterium-labelling experiment supports the proposed mechanism.
Conclusions
In summary, we have developed a novel palladium-catalyzed ring-opening reaction of methylenecyclopropanes with simple hydrazones to produce the corresponding hydroalkylation products in good yields with high regioselectivities. Hydrazones originating from aryl and alkyl aldehydes successfully delivered the C-alkylated products, serving as surrogates of highly reactive organometallic reagents.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Canada Research Chair Foundation (to C.-J. Li), the CFI, FQRNT Center for Green Chemistry and Catalysis, NSERC, the Killam Research Fellowship of the Canada Council of Arts, and McGill University for support of our research. Dr J. Yao thanks the China Scholarship Council for a visiting scholarship.Notes and references
- For selected reviews of C–C activation, see: (a) W. D. Jones, Nature, 1993, 364, 676–677 CrossRef; (b) C.-H. Jun, Chem. Soc. Rev., 2004, 33, 610–618 RSC; (c) T. Seiser and N. Cramer, Org. Biomol. Chem., 2009, 7, 2835–2840 RSC; (d) T. Seiser, T. Saget, D. N. Tran and N. Cramer, Angew. Chem., Int. Ed., 2011, 50, 7740–7752 CrossRef CAS PubMed; (e) A. Dermenci, J. W. Coe and G. Dong, Org. Chem. Front., 2014, 1, 567–581 RSC; (f) M. Murakami and N. Ishida, J. Am. Chem. Soc., 2016, 138, 13759–13769 CrossRef CAS PubMed; (g) P.-h. Chen, B. A. Billett, T. Tsukamoto and G. Dong, ACS Catal., 2017, 7, 1340–1360 CrossRef CAS PubMed; (h) L. Souillart and N. Cramer, Chem. Rev., 2015, 115, 9410–9464 CrossRef CAS PubMed.
- For selected examples of C–C activation, see: (a) S. Komagawa and S. Saito, Angew. Chem., Int. Ed., 2006, 45, 2446–2449 CrossRef CAS PubMed; (b) B. M. Trost and P. J. Morris, Angew. Chem., Int. Ed., 2011, 50, 6167–6170 CrossRef CAS PubMed; (c) B. M. Trost, P. J. Morris and S. J. Sprague, J. Am. Chem. Soc., 2012, 134, 17823–17831 Search PubMed; (d) M. H. Shaw, E. Y. Melikhova, D. P. Kloer, W. G. Whittingham and J. F. Bower, J. Am. Chem. Soc., 2013, 135, 4992–4995 Search PubMed; (e) X. Zhou and G. Dong, J. Am. Chem. Soc., 2015, 137, 13715–13721 Search PubMed; (f) G.-W. Wang, N. G. McCreanor, M. H. Shaw, W. G. Whittingham and J. F. Bower, J. Am. Chem. Soc., 2016, 138, 13501–13504 CrossRef CAS PubMed; (g) J. Yu, H. Yan and C. Zhu, Angew. Chem., Int. Ed., 2016, 55, 1143–1146 CrossRef CAS PubMed; (h) S. Cui, Y. Zhang and Q. Wu, Chem. Sci., 2013, 4, 3421–3426 RSC.
- For selected reviews of reactions on MCPs, see: (a) A. Brandi and A. Goti, Chem. Rev., 1998, 98, 589–636 CrossRef CAS PubMed; (b) A. Brandi, S. Cicchi, F. M. Cordero and A. Goti, Chem. Rev., 2003, 103, 1213–1270 CrossRef CAS PubMed; (c) M. Shi, L.-X. Shao, J.-M. Lu, Y. Wei, K. Mizuno and H. Maeda, Chem. Rev., 2010, 110, 5883–5913 CrossRef CAS PubMed; (d) M. Shi, J.-M. Lu, Y. Wei and L.-X. Shao, Acc. Chem. Res., 2012, 45, 641–652 CrossRef CAS PubMed; (e) L. Yu, M. Liu, F. Chen and Q. Xu, Org. Biomol. Chem., 2015, 13, 8379–8392 Search PubMed; (f) G. Fumagalli, S. Stanton and J. F. Bower, Chem. Rev., 2017, 117, 9404–9432 Search PubMed; (g) L.-Z. Yu, K. Chen, Z.-Z. Zhu and M. Shi, Chem. Commun., 2017, 53, 5935–5945 RSC; (h) M. Rubin, M. Rubina and V. Gevorgyan, Chem. Rev., 2007, 107, 3117–3179 CrossRef CAS PubMed; (i) D.-H. Zhang, X.-Y. Tang and M. Shi, Acc. Chem. Res., 2014, 47, 913–924 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Ma, L. Lu and J. Zhang, J. Am. Chem. Soc., 2004, 126, 9645–9660 CrossRef CAS PubMed; (b) Q. Chen, X. Zhang, S. Su, Z. Xu, N. Li, Y. Li, H. Zhou, M. Bao, Y. Yamamoto and T. Jin, ACS Catal., 2018, 8, 5901–5906 CrossRef CAS; (c) R. Yu and X. Fang, Org. Lett., 2020, 22, 594–597 CrossRef CAS PubMed; (d) S. Simaan and I. Marek, J. Am. Chem. Soc., 2010, 132, 4066–4067 CrossRef CAS PubMed; (e) A. Takahiro, U. Yutaka and I. Katsuhiko, Chem. Lett., 2009, 38, 46–47 CrossRef; (f) T. Pohlmann and A. de Meijere, Org. Lett., 2000, 2, 3877–3879 CrossRef CAS PubMed.
- (a) H. Pellissier, Tetrahedron, 2014, 70, 4991–5031 CrossRef CAS; (b) Y. Yamamoto and U. Radhakrishnan, Chem. Soc. Rev., 1999, 28, 199–207 RSC.
- (a) B. M. Trost and D. M. T. Chan, J. Am. Chem. Soc., 1979, 101, 6432–6433 CrossRef CAS; (b) P. Binger and H. M. Büch, Top. Curr. Chem., 1987, 135, 77–151 CrossRef CAS; (c) E. Nakamura and S. Yamago, Acc. Chem. Res., 2002, 35, 867–877 CrossRef CAS PubMed; (d) S. Yamago and E. Nakamura, J. Org. Chem., 1990, 55, 5553–5555 CrossRef CAS; (e) S. Yamago and E. Nakamura, J. Am. Chem. Soc., 1989, 111, 7285–7286 Search PubMed; (f) P. Binger and B. Schäfer, Tetrahedron Lett., 1988, 29, 529–530 CrossRef CAS; (g) M. Gulías, R. García, A. Delgado, L. Castedo and J. L. Mascareñas, J. Am. Chem. Soc., 2006, 128, 384–385 CrossRef PubMed.
- (a) J. Scheirs and D. B. Priddy, Modern Styrenic Polymers: Polystyrenes and Styrenic Copolymers, John Wiley, Chichester, 2003 Search PubMed; (b) F. Agbossou, J.-F. Carpentier and A. Mortreux, Chem. Rev., 1995, 95, 2485–2506 CrossRef CAS; (c) T. E. Müller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef PubMed.
- (a) M. Beller, J. Seayad, A. Tillack and H. Jiao, Angew. Chem., Int. Ed., 2004, 43, 3368–3398 CrossRef CAS PubMed; (b) D. Kaiser, V. Tona, C. R. Gonçalves, S. Shaaban, A. Oppedisano and N. Maulide, Angew. Chem., Int. Ed., 2019, 58, 14639–14643 CrossRef CAS PubMed; (c) Y. Jiao, M.-F. Chiou, Y. Li and H. Bao, ACS Catal., 2019, 9, 5191–5197 Search PubMed; (d) J. Su, Y. Zhou and X. Xu, Org. Biomol. Chem., 2019, 17, 2013–2019 RSC.
- (a) H. Lebel, D. Guay, V. Paquet and K. Huard, Org. Lett., 2004, 6, 3047–3050 CrossRef CAS PubMed; (b) G. Wienhöfer, F. A. Westerhaus, R. V. Jagadeesh, K. Junge, H. Junge and M. Beller, Chem. Commun., 2012, 48, 4827–4829 RSC; (c) J. Zhou, X. Li, G. Liao and B.-F. Shi, Chin. J. Chem., 2018, 36, 1143–1146 Search PubMed.
- (a) N. Tsukada, A. Shibuya, I. Nakamura and Y. Yamamoto, J. Am. Chem. Soc., 1997, 119, 8123–8124 Search PubMed; (b) D. H. Camacho, I. Nakamura, S. Saito and Y. Yamamoto, Angew. Chem., Int. Ed., 1999, 38, 3365–3367 CrossRef CAS; (c) N. Tsukada, A. Shibuya, I. Nakamura, H. Kitahara and Y. Yamamoto, Tetrahedron, 1999, 55, 8833–8844 CrossRef CAS; (d) I. Nakamura, S. Saito and Y. Yamamoto, J. Am. Chem. Soc., 2000, 122, 2661–2662 CrossRef CAS; (e) D. H. Camacho, I. Nakamura, B. H. Oh, S. Saito and Y. Yamamoto, Tetrahedron Lett., 2002, 43, 2903–2907 CrossRef CAS; (f) I. Nakamura, A. I. Siriwardana, S. Saito and Y. Yamamoto, J. Org. Chem., 2002, 67, 3445–3449 CrossRef CAS PubMed; (g) A. I. Siriwardana, M. Kamada, I. Nakamura and Y. Yamamoto, J. Org. Chem., 2005, 70, 5932–5937 CrossRef CAS PubMed.
- (a) M. Shi, Y. Chen and B. Xu, Org. Lett., 2003, 5, 1225–1228 CrossRef CAS PubMed; (b) M. Jiang and M. Shi, Organometallics, 2009, 28, 5600–5602 CrossRef CAS.
- L. Villarino, F. López, L. Castedo and J. L. Mascareñas, Chem.–Eur. J., 2009, 15, 13308–13312 CrossRef CAS PubMed.
- (a) P. A. Evans and P. A. Inglesby, J. Am. Chem. Soc., 2012, 134, 3635–3638 Search PubMed; (b) P. A. Inglesby, J. Bacsa, D. E. Negru and P. A. Evans, Angew. Chem., Int. Ed., 2014, 53, 3952–3956 CrossRef CAS PubMed; (c) P. A. Evans, P. A. Inglesby and K. Kilbride, Org. Lett., 2013, 15, 1798–1801 CrossRef CAS PubMed.
- (a) L. Lv, D. Zhu, J. Tang, Z. Qiu, C.-C. Li, J. Gao and C.-J. Li, ACS Catal., 2018, 8, 4622–4627 CrossRef CAS; (b) J. Tang, L. Lv, X.-J. Dai, C.-C. Li, L. Li and C.-J. Li, Chem. Commun., 2018, 54, 1750–1753 RSC; (c) C.-J. Li, J. Huang, X.-J. Dai, H. Wang, N. Chen, W. Wei, H. Zeng, J. Tang, C. Li, D. Zhu and L. Lv, Synlett, 2019, 30, 1508–1524 CrossRef CAS; (d) L. Lv, D. Zhu and C.-J. Li, Nat. Commun., 2019, 10, 715–723 CrossRef PubMed.
- (a) H. Wang, X.-J. Dai and C.-J. Li, Nat. Chem., 2016, 9, 374–378 CrossRef PubMed; (b) N. Chen, X.-J. Dai, H. Wang and C.-J. Li, Angew. Chem., Int. Ed., 2017, 56, 6260–6263 CrossRef CAS PubMed; (c) X.-J. Dai, H. Wang and C.-J. Li, Angew. Chem., Int. Ed., 2017, 56, 6302–6306 CrossRef CAS PubMed; (d) W. Wei, X.-J. Dai, H. Wang, C. Li, X. Yang and C.-J. Li, Chem. Sci., 2017, 8, 8193–8197 RSC; (e) L. Lv, D. Zhu, Z. Qiu, J. Li and C.-J. Li, ACS Catal., 2019, 9, 9199–9205 CrossRef CAS; (f) L. Lv, L. Yu, Z. Qiu and C.-J. Li, Angew. Chem., Int. Ed., 2020, 59, 6466–6472 CrossRef CAS PubMed; (g) C.-C. Li, J. Kan, Z. Qiu, J. Li, L. Lv and C.-J. Li, Angew. Chem., Int. Ed., 2020, 59, 4544–4549 CrossRef CAS PubMed.
- (a) D. Zhu, L. Lv, C.-C. Li, S. Ung, J. Gao and C.-J. Li, Angew. Chem., Int. Ed., 2018, 57, 16520–16524 CrossRef CAS PubMed . For a palladium-catalyzed formal hydroalkylation of alkynes with hydrazones, see:; (b) L. Yu, L. Lv, Z. Qiu, Z. Chen, Z. Tan, Y.-F. Liang and C.-J. Li, Angew. Chem., Int. Ed., 2020 DOI:10.1002/anie.202005132.
- (a) J. E. Baldwin, R. M. Adlington, J. C. Bottaro, A. U. Jain, J. N. Kolhe, M. W. D. Perry and I. M. Newington, J. Chem. Soc., Chem. Commun., 1984, 1095–1096 RSC; (b) A. Takemiya and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 14800–14801 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc01221a |
| This journal is © The Royal Society of Chemistry 2020 |