Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A boronic acid-functionalized phthalocyanine with an aggregation-enhanced photodynamic effect for combating antibiotic-resistant bacteria†
Eunhye
Lee‡
b,
Xingshu
Li‡
*a,
Juwon
Oh‡
c,
Nahyun
Kwon
b,
Gyoungmi
Kim
b,
Dongho
Kim
 *c and
Juyoung
Yoon
*c and
Juyoung
Yoon
 *b
*b
aCollege of Chemistry, State Key Laboratory of Photocatalysis on Energy and Environment, Fujian Provincial Key Laboratory of Cancer Metastasis Chemoprevention and Chemotherapy, Fuzhou University, Fuzhou 350108, China. E-mail: xingshuli@fzu.edu.cn
bDepartment of Chemistry and Nanoscience, Ewha Womans University, Seoul 120-750, Republic of Korea. E-mail: jyoon@ewha.ac.kr
cSpectroscopy Laboratory for Functional π-Electronic Systems, Department of Chemistry, Yonsei University, Seoul 120-749, Republic of Korea. E-mail: dongho@yonsei.ac.kr
First published on 18th May 2020
Abstract
Most existing photosensitizers (e.g., porphyrins) are often aggregated in aqueous solution because of their large conjugated molecular structures. This aggregation usually results in a lack or low levels of reactive oxygen species (ROS) generation due to aggregation-caused quenching, which severely hampers the application of photosensitizers in photodynamic therapy (PDT). Herein, we make an interesting finding that a boronic acid-functionalized phthalocyanine (PcN4-BA) displays an uncommon phenomenon, an aggregation-enhanced photodynamic effect. The combination of the ability to form uniform nanostructured self-assemblies in water, highly efficient ROS generation and boronic acid-induced targeting give PcN4-BA excellent performances in antimicrobial PDT.
Introduction
Bacterial infection has posed a grave challenge to the lives and health of human beings. In particular, the growing number of antibiotic-resistant bacteria have complicated infection treatments, and some infections (e.g., those caused by superbugs) now fail to respond to traditional first-line treatments.1–4 This promotes a search for new therapeutic strategies. Photodynamic therapy (PDT) is an effective and alternative method for infection treatment. PDT utilizes a photosensitizer (PS) under light exposure to initiate a cascade of photochemical reactions generating reactive oxygen species (ROS) responsible for bacterial inactivation.5–8 A combination of spatiotemporal selectivity, an enhanced immune response and the ability to perform repetitive treatments without resistance endow PDT with great antimicrobial potential.9–11 The full promise of antimicrobial PDT, however, has not yet been realized. This is mainly associated with the lack of ideal PSs and efficient delivery processes. Most PSs (e.g., porphyrins) are large conjugated compounds that often strongly aggregate in aqueous solution, leading to insufficient ROS generation because the aggregation causes quenching. Another problem concerns the low targeting ability that current PSs have for bacterial cells.Zinc(II) phthalocyanines are second-generation PSs for PDT. Compared to porphyrins, zinc(II) phthalocyanines usually have stronger light absorption in the far-red/near-infrared (NIR) region and higher ROS generation efficiencies.12–17 Introduction of quaternary ammonium groups on phthalocyanines is an attractive approach to improve hydrophilicity and the affinity for the outer membrane of bacteria.18,19 In addition, recent studies have suggested that boronic acid groups are very useful for bacterial targeting because of their ability to form a pair of covalent bonds with glycans on the surface of bacteria.20–22 Therefore, the 3-{N-(4-boronobenzyl)-N,N-dimethylammonium}phenoxy-substituted zinc(II) phthalocyanine PcN4-BA (Fig. 1a) was herein designed to be a new PS for antimicrobial PDT. The synthesis of PcN4-BA is demonstrated in the ESI.†
Results and discussion
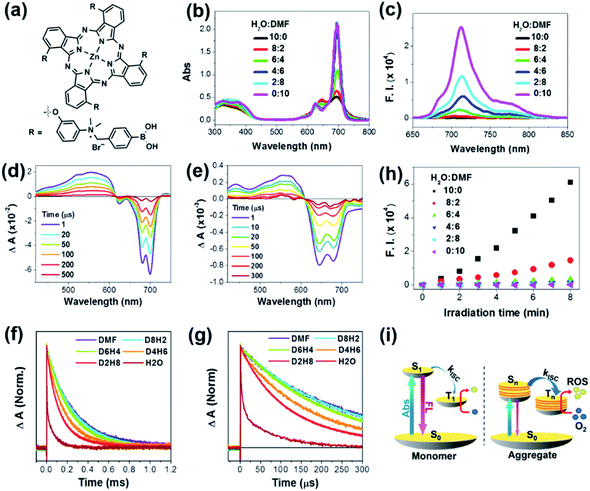
First, we measured the steady-state absorption and emission spectra of PcN4-BA in mixtures of various DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios (Fig. 1b and c). Compared to the sharp and intense absorption features of PcN4-BA in DMF, the absorption spectra were significantly changed with increasing H2O fraction. In H2O, the spectra extend to over 800 nm with a distinct decrease in the extinction coefficient and relatively broad bands. Along with this absorption spectral change, the fluorescence emission gradually attenuated. These distinct absorption and emission spectral changes indicate that PcN4-BA undergoes a significant electronic structural change driven by their aggregation under the polar condition,23,24 which was clearly observed by transmission electron microscopy (vide infra). Here, the broadening of absorption bands with NIR tailing over 800 nm well manifests an increased density of electronic states by intense intermolecular interaction in the PcN4-BA aggregates, where additional collective vibrations promote non-radiative decay processes and result in the lack of fluorescence in H2O.23
H2O ratios (Fig. 1b and c). Compared to the sharp and intense absorption features of PcN4-BA in DMF, the absorption spectra were significantly changed with increasing H2O fraction. In H2O, the spectra extend to over 800 nm with a distinct decrease in the extinction coefficient and relatively broad bands. Along with this absorption spectral change, the fluorescence emission gradually attenuated. These distinct absorption and emission spectral changes indicate that PcN4-BA undergoes a significant electronic structural change driven by their aggregation under the polar condition,23,24 which was clearly observed by transmission electron microscopy (vide infra). Here, the broadening of absorption bands with NIR tailing over 800 nm well manifests an increased density of electronic states by intense intermolecular interaction in the PcN4-BA aggregates, where additional collective vibrations promote non-radiative decay processes and result in the lack of fluorescence in H2O.23
Next, to understand a change of excited state dynamics upon the molecular aggregation, we measured nanosecond transient absorption (ns-TA) spectra of PcN4-BA (Fig. 1d and e). Although the same concentration of PcN4-BA was contained in DMF and H2O solvents, the ns-TA spectra showed significantly different features. Compared to the TA spectra of PcN4-BA in DMF, the corresponding spectra in H2O showed distinct broad negative bleaching with attenuation of signal by a factor of ten, reflecting that the molecular aggregation causes intense intermolecular interaction and a large change in the electronic structure.
Furthermore, with increasing H2O fraction, the decay of ns-TA signals accelerated (Fig. 1f and g), indicating a decrease in the triplet-state lifetime. This result indicates that a reduced energy gap and enhancement of intersystem crossing (ISC) between the lowest triplet excited and singlet ground states by the intense intermolecular interaction in the aggregates stimulate their non-radiative decay processes. Here, it is noteworthy that the signal for ROS generation is intensified with increasing H2O fraction (Fig. 1h), which directly describes that more triplet excitons are being produced in the aggregated PcN4-BA than in the monomer by photoexcitation. This is well matched with the ns-TA data manifesting the enhanced ISC by the aggregation. The enhanced ROS generation in PcN4-BA aggregates was also observed in aqueous solutions containing different concentrations of Cremophor EL (Fig. S1†). This enhanced ROS generation can be understood by the aggregation-induced enhanced ISC process (Fig. 1i).24 The large intermolecular interaction in the aggregates increases the density of electronic states which significantly reduces the energy gap between the excited singlet and triplet states. Thus, the enhanced energy overlap between excited singlet and triplet states caused by the aggregation promotes ISC, and the subsequent increase in triplet excitons results in the improvement in ROS generation. Although the lifetimes of triplet excited states are shortened by aggregation, the TA signal is prolonged by more than 200 μs in H2O. This time scale is sufficient for aggregates to collide with other species, such as oxygen, by Brownian motion (ESI†).25
Compared to four other phthalocyanine derivatives (PcN4, PcO4, PcS4, and PcC4) with different substituent groups (Fig. 2a), PcN4-BA promoted ROS generation much more efficiently (Fig. 2b and c). In addition, the ROS generation induced by PcN4-BA was approximately 13 times higher than that induced by the clinically used PS methylene blue (MB). The detection of the superoxide anion (Fig. 2d) and singlet oxygen (Fig. 2e) indicated that PcN4-BA is involved in both type I and type II photochemical reactions.26,27
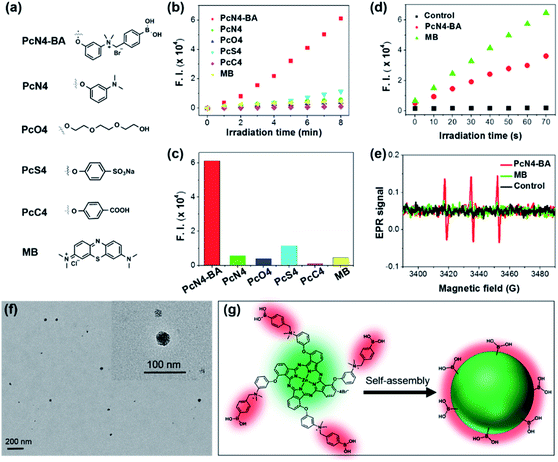 | ||
| Fig. 2 Highly efficient ROS generation and nanostructure self-assembly by PcN4-BA in water. (a) Molecular structures of different PSs. For the phthalocyanine derivatives, only their substituted groups (R, same position as PcN4-BA as shown in Fig. 1a) are displayed. (b) ROS generation plot of different PSs (all at 10 μM) in water detected using 2,7-dichlorofluorescin diacetate (10 μM) as the fluorescent probe. (c) Fluorescence intensity of the ROS probe after incubation with different PSs and irradiation with light for 8 min. (d) Superoxide anion generation of PcN4-BA and MB in water. (e) Singlet oxygen generation of PcN4-BA and MB in water determined by electron paramagnetic resonance (EPR) analysis. The light irradiation time was 10 min. (f) The morphology of PcN4-BA nanoparticles determined by transmission electron microscopy. (g) Schematic illustration of the nanostructured self-assembly of PcN4-BA. | ||
More interestingly, PcN4-BA self-assembled uniform nanostructures in water. From dynamic light scattering tests (Fig. S2†), we found that PcN4-BA in water could form dispersions of stable nanoparticles with average sizes ranging from 40 to 100 nm. In Fig. 2f, transmission electron microscopy images show that nanostructured PcN4-BA assemblies are nearly spherical. We speculate that intermolecular π–π interactions induced by the phthalocyanine macrocyclic structure promotes stacking of the PcN4-BA molecules, and surface hydrophilic interactions (e.g., electrostatic repulsion) between the 3-{N-(4-boronobenzyl)-N,N-dimethylammonium}phenoxy moieties and water sufficiently stabilizes the individual nanoparticles (Fig. 2g).
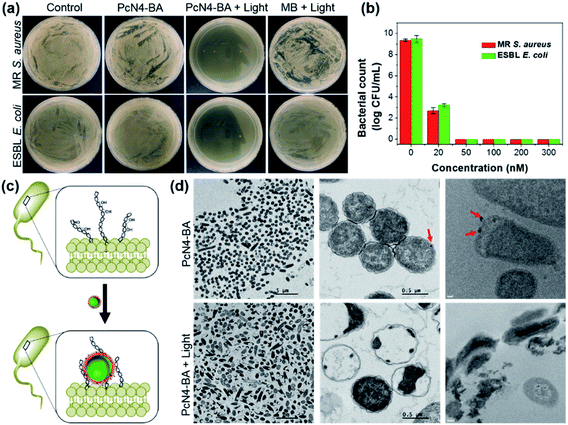
Encouraged by the above uncommon findings, we subsequently evaluated the photodynamic antibacterial efficacy of PcN4-BA. In this investigation, two representative common bacterial strains, Staphylococcus aureus and Escherichia coli, and two representative antibiotic-resistant bacterial strains, methicillin-resistant S. aureus (MR S. aureus) and extended spectrum beta-lactamase E. coli (ESBL E. coli), were selected as model targets. As shown in Fig. 3a, b and S3,† PcN4-BA induced a significant decrease in bacterial colonies upon light irradiation. For example, at a concentration of 50 nM and with 655 nm laser irradiation (0.4 W cm−2) for 10 min, PcN4-BA inhibited the growth of MR S. aureus and ESBL E. coli by almost 100%. However, under the same conditions as PcN4-BA, MB did not show significant photodynamic antibacterial efficiency. We also found that PcN4-BA showed much higher antibacterial efficacy than those of protoporphyrin IX (PPIX) and 4,4′,4′′,4′′′-(porphine-5,10,15,20-tetrayl)tetrakis(benzenesulfonic acid) (TPPS) upon light irradiation (Fig. S4†). More importantly, compared to PcN4-M, which has only quaternary ammonium groups (Fig. S5a†), PcN4-BA showed a much better phototherapeutic index with higher bacterial inactivation under light irradiation (Fig. S5b†) but lower bacterial inactivation in the dark (Fig. S5c†). We speculated that the boronic acid groups of PcN4-BA nanoparticles probably promote the binding of PcN4-BA to the surface of bacteria (Fig. 3c), and then the highly efficient ROS generation induced by light irradiation likely contributes to its high antibacterial effect. Both TEM images and cryo-TEM images confirmed that PcN4-BA nanoparticles bind to the surface of bacteria and that the cell membranes are disrupted after treatment with both PcN4-BA and light irradiation (Fig. 3d).
The cytotoxicity of PcN4-BA against normal mammalian cells was also evaluated for further potential applications. In this study, a human diploid lung fibroblast cell line (WI-38 VA-13 subline 2RA) was employed as the representative cell. Fig. S6† shows that PcN4-BA had no significant cytotoxicity even at a high concentration (5000 nM) that was 100-fold higher than the concentration used for bacterial inactivation. These results suggest that the introduction of boronic acids is an important strategy to enable PcN4-BA to function as an efficient and safe PS for antimicrobial PDT applications.
Conclusions
In conclusion, we have developed an uncommon PS, PcN4-BA, that exhibits an aggregation-enhanced photodynamic effect. In water, this novel PS forms uniform sphere-like nanoparticles via a self-assembling process and displays highly efficient ROS generation through both type I and type II photochemical mechanisms. In addition, PcN4-BA displays excellent photodynamic antimicrobial activity against both common and antibiotic-resistant bacterial strains.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Y. is thankful for financial support from the National Research Foundation of Korea (NRF), which is funded by the Korea government (MSIP) (No. 2012R1A3A2048814). D. K. is thankful for the Strategic Research (NRF-2016R1E1A1A01943379) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science.Notes and references
- E. D. Brown and G. D. Wright, Nature, 2016, 529, 336–343 CrossRef CAS PubMed.
- B. Li and T. J. Webster, J. Orthop. Res., 2018, 36, 22–32 Search PubMed.
- D. Genné, L. Kaiser, T. N. Kinge and D. Lew, Clin. Microbiol. Infect., 2003, 9, 949–954 CrossRef PubMed.
- Z.-H. Yu, X. Li, F. Xu, X.-L. Hu, J. Yan, N. Kwon, G.-R. Chen, T. Tang, X. Dong, Y. Mai, D. Chen, J. Yoon, X.-P. He and H. Tian, Angew. Chem., Int. Ed., 2020, 59, 3658–3664 CrossRef CAS PubMed.
- J. P. Celli, B. Q. Spring, I. Rizvi, C. L. Evans, K. S. Samkoe, S. Verma, B. W. Pogue and T. Hasan, Chem. Rev., 2010, 110, 2795–2838 CrossRef CAS PubMed.
- M. R. Hamblin and T. Hasan, Photochem. Photobiol. Sci., 2004, 3, 436–450 RSC.
- X. Li, S. Lee and J. Yoon, Chem. Soc. Rev., 2018, 47, 1174–1188 RSC.
- X. Li, H. Bai, Y. Yang, J. Yoon, S. Wang and X. Zhang, Adv. Mater., 2018, 31, e1805092 CrossRef PubMed.
- J. F. Lovell, T. W. B. Liu, J. Chen and G. Zheng, Chem. Rev., 2010, 110, 2839–2857 CrossRef CAS PubMed.
- A. P. Castano, P. Mroz and M. R. Hamblin, Nat. Rev. Cancer, 2006, 6, 535–545 CrossRef CAS PubMed.
- B. C. Wilson and M. S. Patterson, Phys. Med. Biol., 2008, 53, R61–R109 CrossRef CAS PubMed.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19–33 RSC.
- X. Li, B.-D. Zheng, X.-H. Peng, S.-Z. Li, J.-W. Ying, Y. Zhao, J.-D. Huang and J. Yoon, Coord. Chem. Rev., 2019, 379, 147–160 CrossRef CAS.
- X. Li, C.-y. Kim, S. Lee, D. Lee, H.-M. Chung, G. Kim, S.-H. Heo, C. Kim, K.-S. Hong and J. Yoon, J. Am. Chem. Soc., 2017, 139, 10880–10886 CrossRef CAS PubMed.
- X. Li, S. Yu, Y. Lee, T. Guo, N. Kwon, D. Lee, S. C. Yeom, Y. Cho, G. Kim, J.-D. Huang, S. Choi, K. T. Nam and J. Yoon, J. Am. Chem. Soc., 2019, 141, 1366–1372 CrossRef CAS PubMed.
- X.-S. Li, M.-R. Ke, M.-F. Zhang, Q.-Q. Tang, B.-Y. Zheng and J.-D. Huang, Chem. Commun., 2015, 51, 4704–4707 RSC.
- X. Li, K. Jeong, Y. Lee, T. Guo, D. Lee, J. Park, N. Kwon, J.-H. Na, S. K. Hong, S.-S. Cha, J.-D. Huang, S. Choi, S. Kim and J. Yoon, Theranostics, 2019, 9, 6412–6423 CrossRef CAS PubMed.
- I. Roy, D. Shetty, R. Hota, K. Baek, J. Kim, C. Kim, S. Kappert and K. Kim, Angew. Chem., Int. Ed., 2015, 54, 15152–15155 CrossRef CAS PubMed.
- X.-S. Li, J. Guo, J.-J. Zhuang, B.-Y. Zheng, M.-R. Ke and J.-D. Huang, Bioorg. Med. Chem. Lett., 2015, 25, 2386–2389 CrossRef CAS PubMed.
- C. S. Guy, M. I. Gibson and E. Fullam, Chem. Sci., 2019, 10, 5935–5942 RSC.
- A. Galstyan, R. Schiller and U. Dobrindt, Angew. Chem., Int. Ed., 2017, 56, 10362–10366 CrossRef CAS PubMed.
- H.-Y. Know, X. Liu, E. G. Choi, J. Y. Lee, S.-Y. Choi, J.-Y. Kim, L. Wang, S.-J. Park, B. Kim, Y.-A. Lee, J.-J. Kim, N. Y. Kang and Y.-T. Chang, Angew. Chem., Int. Ed., 2019, 58, 8426–8431 CrossRef PubMed.
- J. M. Lim, P. Kim, M.-C. Yoon, J. Sung, V. Dehm, Z. Chen, F. Würthner and D. Kim, Chem. Sci., 2013, 4, 388–397 RSC.
- L. Yang, X. Wang, G. Zhang, X. Chen, G. Zhang and J. Jiang, Nanoscale, 2016, 8, 17422–17426 RSC.
- M. Scholz, R. Dědic, T. Breitenbach and J. Hála, Photochem. Photobiol. Sci., 2013, 12, 1873–1884 RSC.
- X. Li, D. Lee, J.-D. Huang and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 9885–9890 CrossRef CAS PubMed.
- X. Li, N. Kwon, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522–11531 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sc01351j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |