Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Pd/Xiang-Phos-catalyzed enantioselective intermolecular carboheterofunctionalization under mild conditions†
Mengna
Tao
a,
Youshao
Tu
b,
Yu
Liu
 b,
Haihong
Wu
b,
Haihong
Wu
 a,
Lu
Liu
a,
Lu
Liu
 *a and
Junliang
Zhang
*a and
Junliang
Zhang
 *cd
*cd
aShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai 200062, P. R. China. E-mail: lliu@chem.ecnu.edu.cn
bCollege of Chemistry and Life Science, Advanced Institute of Materials Science, Changchun University of Technology, 2055 Yanan Street, Changchun, 130012, P. R. China
cDepartment of Chemistry, Fudan University, 2005 Songhu Road, Shanghai 200438, P. R. China. E-mail: junliangzhang@fudan.edu.cn
dState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, CAS, 345 Lingling Road, Shanghai 200032, P. R. China
First published on 29th May 2020
Abstract
A mild and practical Pd/Xiang-Phos-catalyzed enantioselective intermolecular carboheterofunctionalization reaction of 2,3-dihydrofurans is developed, leading to various optically active fused furoindolines and tetrahydrofurobenzofurans. The key to this transformation is employing two newly modified N-Me-Xiang-Phos ligands ((S, RS)-N-Me-X4/X5) as chiral ligands under mild conditions. Moreover, this synthetic methodology can be efficiently applied to a variety of complex polysubstituted heterocycles with high chemo-, regio-, and enantio-selectivities via introducing diverse substituents on furan rings, which were hard to access by other routes.
Introduction
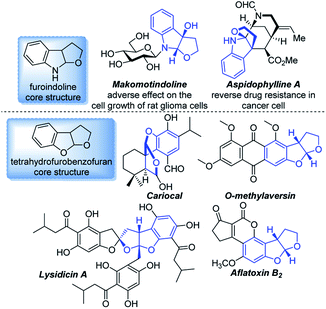
Benzofused heterocycles are ubiquitous moieties in natural products, pharmaceuticals, dyes and herbicides, in which furoindolines and tetrahydrofurobenzofurans are prevalent as key core structures (Fig. 1).1 These derivatives have shown significant anticancer, antimalarial and antimicrobial activities, as well as antioxidant properties, for instance, Makomotindoline and Aspidophylline A have shown a distinct effect on mammalian cells.2,3The first enantioselective total synthesis of Aspidophylline A was described by Garg via a reductive interrupted Fischer indolization.4 You and co-workers developed a copper-catalyzed intermolecular dearomative cascade reaction of indoles, which also provided a powerful synthetic method for the construction of furoindolines.5 E. J. Corey achieved a short, asymmetric total synthesis of Aflatoxin B2via an aromative cascade reaction.6 Despite these seminal reports, hetero-annulation of alkenes developed by Catellani and Larock has become a classic and useful strategy for the construction of various heterocycles from readily available starting materials.7 Although various methods have been developed to construct these two skeletons, it still remains a considerable challenge to extend the substrate scope of asymmetric variants, particularly those that enable access to poly-substituted benzofused heterocycles.
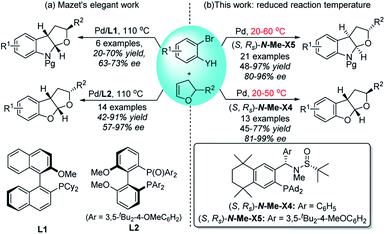
Over the past two decades, palladium-catalyzed carbo-heterofunctionalization of alkenes has been proved to be a reliable and efficient method for the synthesis of a variety of poly-cyclic heterocycles.8 The majority of these reactions proceeded through a crucial hetero-palladation of alkenes with aryl halides along with N- or O-nucleophiles.9,10 However, the development of an enantioselective version, especially under mild conditions, poses a considerable challenge due to the lack of any suitable robust chiral catalyst. Recently, Mazet and co-workers reported the first asymmetric Pd-catalyzed syn-carboetherification and syn-carboamination of 2,3-dihydrofurans (2,3-dhfs) at 110 °C by utilizing two different chiral ligands (Scheme 1a).11 Inspired by the good performance of our chiral sulfinamide phosphine (Sadphos) ligands in the asymmetric construction of C–C and C–X bonds,12 we wondered whether Sadphos could realize the highly enantioselective carboetherification and carboamination of 2,3-dhfs under mild conditions and also address the low enantioselectivity issue of the carboamination reaction. Herein, we report a highly chemo-, regio-, and enantioselective palladium-catalyzed carbohetero-functionalization of 2,3-dhfs employing two newly modified Xiang-Phos ligands as chiral ligands, which can give direct access to enantioenriched poly-substituted functionalized furoindolines and tetrahydrofurobenzofurans in moderate to high yields with high enantio-selectivities at a reduced reaction temperature (Scheme 1b).
Results and discussion
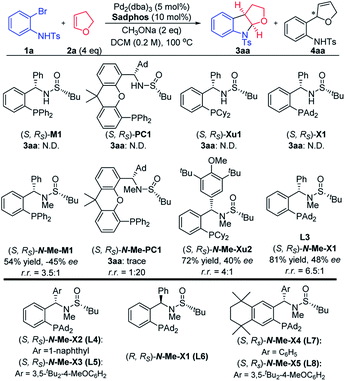
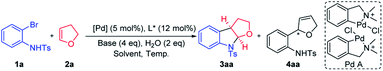
With the use of our developed chiral sulfinamide phosphine ligands as the chiral ligands,13–16 the carboamination reaction of 2-bromoaniline derivative 1a and 2,3-dhf 2a was investigated. It was found that Ming-Phos M1, PC-Phos PC1, Xu-Phos Xu1 and Xiang-Phos X1 did not efficiently deliver the desired product. As observed in our previous work, the N–H bond in ligands could greatly affect the reactivity as well as enantioselectivity in some cases.16 Several representative N-Me sulfinamide phosphine ligands lacking the hydrogen-bonding site were further investigated. We were pleased to find that the desired product 3aa could be obtained in 81% yield with a 48% ee value in the presence of (S, RS)-N-Me-X1 and CH3ONa, albeit with a small amount of the Heck byproduct 4aa. Other chiral ligands such as Ming-Phos N-Me-M1 and Xu-Phos N-Me-Xu2 showed less efficiency comparatively, leading to a lower yield and enantioselectivity along with a poor regioselectivity (Fig. 2).Under the conditions of Xiang-Phos (S, RS)-N-Me-X1 utilized as the chiral ligand, NaOPh appeared to be the optimal base, affording 3aa in 78% ee albeit with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regioselectivity ratio (r.r.) (Table 1, entries 1–5). Solvent screening showed that 1,2-DCE gave a better yield (81%) and r.r. (9
1 regioselectivity ratio (r.r.) (Table 1, entries 1–5). Solvent screening showed that 1,2-DCE gave a better yield (81%) and r.r. (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 87% ee (Table 1, entries 6–9). The result obtained employing other chiral N-Me-Xiang-Phos ligands indicated that the introduction of steric hindrance on the phenyl backbone and enhancement of the electron-donating character were beneficial for the catalytic enantio-selectivity and regioselectivity (Table 1, entries 10–14). Employing the newly modified N-Me-X5 as the chiral ligand, a series of Pd precursors were then screened, showing that a five-membered cyclic palladium precatalyst was competent for the carboamination cyclization (Table 1, entries 15–19). Comparably better outcomes were obtained under mild conditions by lowering the temperature to 20 °C (Table 1, entries 20–23). Inspired by previous findings that the addition of a trace amount of water may help to increase the reactivity and the stereoselectivity,17 the water effect was studied, and indeed, we found that the addition of 2 equivalents of water to the system led to a significantly improved reactivity and reproducible enantioselectivity. In terms of the reactivity and enantio- and regio-selectivity, the reaction conditions illustrated in entry 22 were utilized in the following substrate scope investigations (please see Table S1 in the ESI for details†).
1) with 87% ee (Table 1, entries 6–9). The result obtained employing other chiral N-Me-Xiang-Phos ligands indicated that the introduction of steric hindrance on the phenyl backbone and enhancement of the electron-donating character were beneficial for the catalytic enantio-selectivity and regioselectivity (Table 1, entries 10–14). Employing the newly modified N-Me-X5 as the chiral ligand, a series of Pd precursors were then screened, showing that a five-membered cyclic palladium precatalyst was competent for the carboamination cyclization (Table 1, entries 15–19). Comparably better outcomes were obtained under mild conditions by lowering the temperature to 20 °C (Table 1, entries 20–23). Inspired by previous findings that the addition of a trace amount of water may help to increase the reactivity and the stereoselectivity,17 the water effect was studied, and indeed, we found that the addition of 2 equivalents of water to the system led to a significantly improved reactivity and reproducible enantioselectivity. In terms of the reactivity and enantio- and regio-selectivity, the reaction conditions illustrated in entry 22 were utilized in the following substrate scope investigations (please see Table S1 in the ESI for details†).
| Entry | [Pd]/L | Base | Solvent/T (°C) | 3/4 | Yieldb,c (ee) [%] |
|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out with 1a (0.2 mmol), 2a (0.8 mmol, 4 eq.), a [Pd] source (0.01 mmol, 5 mol%), N-Me-Xiang-phos (0.024 mmol, 12 mol%), base (0.8 mmol, 4 eq.), and H2O (7.2 μL, 2 eq.) in a solvent (1 mL, 0.2 M). b Yield of isolated product 3aa. c Determined by chiral HPLC. d 2 eq. H2O were removed. | |||||
| 1 | Pd2(dba)3/L3 | NaOtBu | DCM/100 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
73(47) |
| 2 | Pd2(dba)3/L3 | LiOtBu | DCM/100 | — | Trace |
| 3 | Pd2(dba)3/L3 | KOtBu | DCM/100 | — | Mix |
| 4 | Pd2(dba)3/L3 | NaOEt | DCM/100 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
52(40) |
| 5 | Pd2(dba)3/L3 | NaOPh | DCM/100 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
63(78) |
| 6 | Pd2(dba)3/L3 | NaOPh | MTBE/100 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
44(60) |
| 7 | Pd2(dba)3/L3 | NaOPh | 1,2-DCE/100 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81(76) |
| 8 | Pd2(dba)3/L3 | NaOPh | Toluene/100 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42(53) |
| 9 | Pd2(dba)3/L3 | NaOPh | MeOH/100 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
39(59) |
| 10 | Pd2(dba)3/L4 | NaOPh | 1,2-DCE/100 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78(87) |
| 11 | Pd2(dba)3/L5 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81(93) |
| 12 | Pd2(dba)3/L6 | NaOPh | 1,2-DCE/100 | — | Trace |
| 13 | Pd2(dba)3/L7 | NaOPh | 1,2-DCE/100 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
77(77) |
| 14 | Pd2(dba)3/L8 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
83(93) |
| 15 | Pd(dba)2/L8 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79(94) |
| 16 | Pd2(dba)3·CHCl3/L8 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81(94) |
| 17 | Pd(OAc)2/L8 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
74(94) |
| 18 | (η3-C3H5)2Pd2Cl2/L8 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
69(94) |
| 19 | Pd A/L8 | NaOPh | 1,2-DCE/100 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
82(94) |
| 20 | Pd A/L8 | NaOPh | 1,2-DCE/80 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81(93) |
| 21 | Pd A/L8 | NaOPh | 1,2-DCE/50 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
81(95) |
| 22 | Pd A/L8 | NaOPh | 1,2-DCE/20 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84(96) |
| 23d | Pd A/L8 | NaOPh | 1,2-DCE/20 | >30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
79(96) |
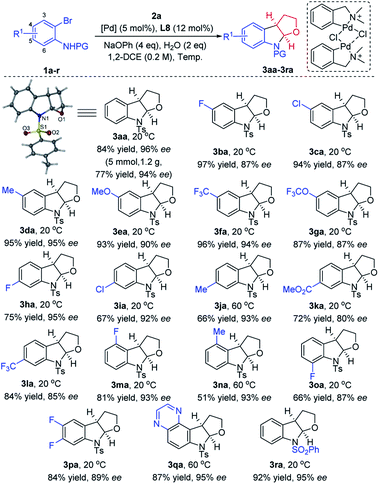
Various substituted N-(2-bromophenyl)-p-tolylsulfonamide derivatives 1a–r were subsequently employed as coupling partners in the enantioselective intermolecular carboamination of 2,3-dhf (Scheme 2). Remarkably, a wide range of 2-Br-anilines bearing electronically diverse substituents at C4 and C5 such as halogens, –Me, –OMe, –CF3, –OCF3, and –CO2Me reacted smoothly and furnished the corresponding furoindolines 3aa–3la in good yields (up to 97%) and ee's (up to 96%). Further substrate scope investigations demonstrated that the electronic properties of substituents at C3 and C6 did affect the yields and enantioselectivities, and produced products 3na and 3oa in lower yields comparatively. Notably, the disubstituents on phenyl rings were also applicable in this cyclization reaction, affording 3pa in 84% yield with 89% ee, as well as 3qa containing a heterocycle in 87% yield and 95% ee. When N-(2-bromophenyl)-benzenesulfonamide was explored as an alternative to 1a, to our delight, the furoindoline product 3ra was formed in a nearly quantitative yield (92%) with high enantioselectivity (95%). We also replaced the protective groups on the nitrogen atom with Ms and Ns, but only trace products could be detected by NMR. Other nitrogen protecting groups on aniline, such as Boc, Cbz and Bz, were not tolerated, and in these cases the desired product was not observed. To our delight, a gram-scale reaction was conducted to further demonstrate the potential synthetic utility of this methodology, delivering 1.2 g of 3aa in 77% yield and 94% ee with 2.5 mol% palladium catalyst at 20 °C for 6 days. The absolute configuration of this series of products was confirmed by the X-ray diffraction analysis of 3aa.18
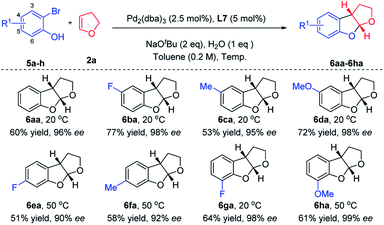
After a quick survey of the construction of tetrahydrofuro-benzofuran (Table 2, please see Table S2 in the ESI for details†), the optimal reaction conditions were identified (Table 2, entry 7). A series of substituted 2-bromophenol derivatives 5a–h were subsequently employed as coupling partners in the enantio-selective intermolecular carboetherification of 2,3-dhf with the use of N-Me-X4 under mild conditions (Scheme 3). Of note, an exciting enantioselective cyclization was realized when substrates containing diverse substituents at C4, C5 and C6 with different electronic properties (–F, –Me, and –OMe) participated smoothly, delivering products in 90% to 99% ee's. It is a pity that only a trace amount of product was observed when substituents were present at C3 of the phenyl ring.
| Entry | [Pd]/L | Base | Solvent | T (°C) | Yieldb,c (ee) [%] |
|---|---|---|---|---|---|
| a Unless otherwise specified, all reactions were carried out with 5a (0.2 mmol), 2a (1 mmol, 5 eq.), a [Pd] source (0.005 mmol, 2.5 mol%), N-Me-Xiang-Phos (0.01 mmol, 5 mol%), base (0.4 mmol, 2 eq.), and H2O (3.6 μL, 1 eq.) in a solvent (1 mL, 0.2 M). b Yield of isolated product. c Determined by chiral HPLC. d Pd2(dba)3 was added to 5 mol%, and L3 was added to 10 mol%. | |||||
| 1 | Pd2(dba)3/L3 | NaOtBu | Toluene | 80 | 40(87) |
| 2 | Pd2(dba)3/L3 | NaOPh | Toluene | 80 | 30(38) |
| 3d | Pd2(dba)3/L3 | NaOtBu | DCM | 80 | 45(33) |
| 4 | Pd2(dba)3/L3 | NaOtBu | Toluene | 20 | 55(95) |
| 5 | Pd A/L3 | NaOtBu | Toluene | 20 | 51(95) |
| 6 | Pd2(dba)3/L5 | NaOtBu | Toluene | 20 | 44(85) |
| 7 | Pd2(dba)3/L7 | NaOtBu | Toluene | 20 | 60(96) |
| 8 | Pd2(dba)3/L8 | NaOtBu | Toluene | 20 | 52(82) |
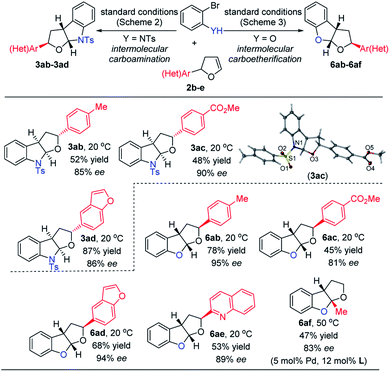
To delve into the construction of poly-substituted fused furo-indolines and tetrahydrofurobenzofurans, a variety of dihydrofuran derivatives 2b–2e, which could be readily prepared by a classic Heck reaction, were subjected to carbofunctionalization. In these two cases, variation of the electronic parameters of the phenyl groups on dhf rings had a slight influence on the yields and enantiocontrol of the carbohetero-functionalizations (Scheme 4, 3ab/6ab, 3ac/6ac). Considering the O- and N-containing heterocycle substituents on dhf rings, the phenyl groups could be swapped for the benzofuran and quinoline substituents (3ad/6ad, 6ae), still maintaining the efficiency of the transformations. 5-Methyl-2,3-dhf was next examined to investigate the formation of an all-carbon quaternary stereocenter. The carboetherification reaction took place smoothly when increasing the loading of the palladium precatalyst and chiral ligand at a higher temperature (6af). However, only the corresponding debromination product was detected in the carboamination reaction system. The absolute configuration of the poly-substituted carboamination products was confirmed by X-ray diffraction analysis of 3ac,18 while the absolute configuration of poly-substituted carbo-etherification products was assigned by comparing the rotational value and 1H,1H-NOESY-NMR spectrum (please see the ESI for details†) of 6ab between our work and Mazet's work.11 The substituted aromatic ring on the tetrahydrofuran ring was in the (S)-configuration, and is a diastereomer of the corresponding product in Mazet's work.
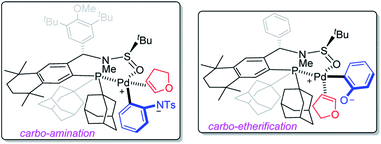
Based on Mazet's studies, as well as our observations on Pd/Sadphos catalytic systems, the chirality-induction models of carbo-amination and -etherification were proposed according to the absolute configuration of products 3aa and 6aa, as shown in Scheme 5. We supposed that the reaction was initiated by a classic oxidative addition, which would be followed by ligand exchange, deprotonation and coordination of 2,3-dhf. The key step of asymmetric hetero-palladation was hypothesized to occur to ultimately construct optically active benzofused heterocycles with high regio- and enantio-selectivities.
Conclusions
In summary, we have demonstrated an efficient Pd-catalyzed enantioselective intermolecular carboheterofunctionalization of 2,3-dihydrofurans for the synthesis of poly-substituted benzofused heterocycles. The new N-Me-Xiang-Phos X4/X5 ligands are responsible for the high reactivity and enantioselectivity. This strategy could be conducted under mild conditions and easily extended to a wide range of chiral fused furoindolines and tetrahydrofurobenzofurans with high chemo-, regio-, and enantio-selectivities, which made the method extremely attractive. In addition, a gram-scale reaction of the representative product 3aa was investigated to further demonstrate the potential synthetic applications of this method. Further applications of Sadphos in other transition-metal-catalyzed reactions are underway in our group and will be reported in due course.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the funding support from the NSFC (21672067), the 973 Program (2015CB856600), and the Program of Eastern Scholar at Shanghai Institutions of Higher Learning and Program for Changjiang Scholars and Innovative Research Team (IRT-16R25).Notes and references
- For books and reviews on benzofused heterocycles, see: (a) K. C. Majumdar and S. K. Chattopadhyay, Heterocycles in Natural Product Synthesis, Wiley-VCH, Weinheim, 2011 CrossRef; (b) A. F. Pozharskii, A. R. Katritzky and A. T. Soldatenkov, Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry and Applications, Wiley, Chichester, 2nd edn, 2011 CrossRef; (c) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed.
- For reviews on indoline motifs, see: (a) D. J. Triggle, J. M. Mitchell and R. Filler, CNS Drug Rev., 1998, 4, 87 CrossRef CAS; (b) J. M. Smith, J. Moreno, B. W. Boal and N. K. Garg, Angew. Chem., 2015, 127, 410 ( Angew. Chem. Int. Ed. , 2015 , 54 , 400 ) CrossRef.
- For reviews on benzofuran motifs, see: (a) S. Bräse, A. Encinas, J. Keck and C. F. Nising, Chem. Rev., 2009, 109, 3903 CrossRef PubMed; (b) F.-P. Miao, X.-D. Li, X.-H. Liu, R. H. Cichewicz and N.-Y. Ji, Mar. Drugs, 2012, 10, 131 CrossRef CAS PubMed; (c) Y. Kwon, S.-H. Kim, Y. Shin, M. Bae, B.-Y. Kim, S. K. Lee, K.-B. Oh, J. Shin and D.-C. Oh, Mar. Drugs, 2014, 12, 2326 CrossRef PubMed.
- For reviews and selected examples of access to indolines, see: (a) Y. Mo, J. Zhao, W. Chen and Q. Wang, Res. Chem. Intermed., 2015, 41, 5869 CrossRef CAS; (b) M. Teng, W. Zi and D. Ma, Angew. Chem., 2014, 126, 1845 ( Angew. Chem., Int. Ed. , 2014 , 53 , 1814 ) CrossRef; (c) J.-C. Zhao, S.-M. Yu, H.-B. Qiu and Z.-J. Yao, Tetrahedron, 2014, 70, 3197 CrossRef CAS; (d) J. M. Smith, J. Moreno, B. W. Boal and N. K. Garg, J. Am. Chem. Soc., 2014, 136, 4504 CrossRef CAS PubMed; (e) Y. Li, S. Zhu, J. Li and A. Li, J. Am. Chem. Soc., 2016, 138, 3982 CrossRef CAS PubMed; (f) J. Moreno, E. Picazo, L. A. Morrill, J. M. Smith and N. K. Garg, J. Am. Chem. Soc., 2016, 138, 1162 CrossRef CAS PubMed; (g) M. J. James, P. O'Brien, R. J. K. Taylor and W. P. Unsworth, Chem.–Eur. J., 2016, 22, 2856 CrossRef CAS PubMed.
- For reviews and selected examples of dearomative cascade reactions, see: (a) C.-X. Zhuo, W. Zhang and S.-L. You, Angew. Chem., 2012, 51, 12834 ( Angew. Chem., Int. Ed. , 2012 , 51 , 12662 ) CrossRef; (b) E. Manoni, A. De Nisi and M. Bandini, Pure Appl. Chem., 2016, 88, 207 CAS; (c) C. Liu, W. Zhang, L.-X. Dai and S.-L. You, Org. Lett., 2012, 14, 4525 CrossRef CAS PubMed; (d) H. Yin, T. Wang and N. Jiao, Org. Lett., 2014, 16, 2302 CrossRef CAS PubMed; (e) H. Liu, G. Jiang, X. Pan, X. Wan, Y. Lai, D. Ma and W. Xie, Org. Lett., 2014, 16, 1908 CrossRef CAS PubMed; (f) R. Beaud, R. Guillot, C. Kouklovsky and G. Vincent, Chem.–Eur. J., 2014, 20, 7492 CrossRef CAS PubMed; (g) W. Yang, L. Huang, Y. Yu, D. Pflästerer, F. Rominger and A. S. K. Hashmi, Chem.–Eur. J., 2014, 20, 3927 CrossRef CAS PubMed; (h) X. Zhang, W. B. Liu, H.-F. Tu and S.-L. You, Chem. Sci., 2015, 6, 4525 RSC; (i) W. Shao, H. Li, C. Liu, C.-J. Liu and S.-L. You, Angew. Chem., 2015, 127, 7794 ( Angew. Chem. Int. Ed. , 2015 , 54 , 7684 ) CrossRef; (j) X. Qin, M. W. Y. Lee and J. Zhou, Angew. Chem., 2017, 129, 12897 ( Angew. Chem. Int. Ed. , 2017 , 56 , 12723 ) CrossRef.
- For selected examples of aromative cascade reactions, see: (a) G. Zhou and E. J. Corey, J. Am. Chem. Soc., 2005, 127, 11958 CrossRef CAS PubMed; (b) H. Zheng, C. Xu, Y. Wang, T. Kang, X. Liu, L. Lina and X. Feng, Chem. Commun., 2017, 53, 6585 RSC.
- For reviews and selected examples of hetero-annulation of alkenes, see: (a) M. Catellani and A. Del Rio, Russ. Chem. Bull., 1998, 47, 928 CrossRef CAS; (b) E. Negishi, C. Copéret, S. Ma, S. Liou and F. Liu, Chem. Rev., 1996, 96, 365 CrossRef CAS PubMed; (c) I. Ojima, M. Tzamarioudaki, Z. Li and R. J. Donovan, Chem. Rev., 1996, 96, 635 CrossRef CAS PubMed; (d) D. E. Emrich and R. C. Larock, J. Organomet. Chem., 2004, 689, 3756 CrossRef CAS; (e) B. A. B. Prasad, A. E. Buechele and S. R. Gilbertson, Org. Lett., 2010, 12, 5422 CrossRef CAS PubMed; (f) H. Zheng, Y. Zhu and Y. Shi, Angew. Chem., 2014, 126, 11462 ( Angew. Chem. Int. Ed. , 2014 , 53 , 11280 ) CrossRef.
- For reviews on Pd-catalyzed carbohetero-functionalization: (a) J. P. Wolfe, Eur. J. Org. Chem., 2007, 2007, 571 CrossRef PubMed; (b) J. P. Wolfe, Synlett, 2008, 2008, 2913 CrossRef; (c) D. M. Schultz and J. P. Wolfe, Synthesis, 2012, 44, 351 CrossRef CAS PubMed; (d) J. P. Wolfe, Top. Heterocycl. Chem., 2013, 32, 1 CAS; (e) Stereoselective Synthesis of Drugs and Natural Products, ed. M. K. Ghorai, S. Halder and S. Samanta, John Wiley & Sons, Hoboken, NJ, 2013 Search PubMed; (f) Z. J. Garlets, D. R. White and J. P. Wolfe, Asian J. Org. Chem., 2017, 6, 636 CrossRef CAS PubMed.
- For selected examples of Pd-catalyzed carboamination, see: (a) B. A. Hopkins and J. P. Wolfe, Chem. Sci., 2014, 5, 4840 RSC; (b) N. R. Babij, G. M. McKenna, R. M. Fornwald and J. P. Wolfe, Org. Lett., 2014, 16, 3412 CrossRef CAS PubMed; (c) A. Faulkner, J. S. Scott and J. F. Bower, J. Am. Chem. Soc., 2015, 137, 7224 CrossRef CAS PubMed; (d) D. R. White, J. T. Hutt and J. P. Wolfe, J. Am. Chem. Soc., 2015, 137, 11246 CrossRef CAS PubMed; (e) S. Kindt, K. Wicht and M. R. Heinrich, Org. Lett., 2015, 17, 6122 CrossRef CAS PubMed; (f) Z. J. Garlets, K. R. Parenti and J. P. Wolfe, Chem.–Eur. J., 2016, 22, 5919 CrossRef CAS PubMed; (g) C. Um and S. R. Chemler, Org. Lett., 2016, 18, 2515 CrossRef CAS PubMed; (h) D. R. White and J. P. Wolfe, Chem.–Eur. J., 2017, 23, 5419 CrossRef CAS PubMed.
- For selected examples of carboetherification, see: (a) J. P. Wolfe and M. A. Rossi, J. Am. Chem. Soc., 2004, 126, 1620 CrossRef CAS PubMed; (b) M. B. Hay and J. P. Wolfe, J. Am. Chem. Soc., 2005, 127, 16468 CrossRef CAS PubMed; (c) J. S. Nakhla, J. W. Kampf and J. P. Wolfe, J. Am. Chem. Soc., 2006, 128, 2893 CrossRef CAS PubMed; (d) S. Hayashi, H. Yorimitsu and K. Oshima, J. Am. Chem. Soc., 2009, 131, 2052 CrossRef CAS PubMed; (e) A. D. Satterfield, A. Kubota and M. S. Sanford, Org. Lett., 2011, 13, 1076 CrossRef CAS PubMed; (f) B. A. Hopkins, Z. J. Garlets and J. P. Wolfe, Angew. Chem., 2015, 127, 13588 ( Angew. Chem. Int. Ed. , 2015 , 54 , 13390 ) CrossRef; (g) J. T. Hutt and J. P. Wolfe, Org. Chem. Front., 2016, 3, 1314 RSC; (h) N. Hu, K. Li, Z. Wang and W. Tang, Angew. Chem., 2016, 128, 5128 ( Angew. Chem. Int. Ed. , 2016 , 55 , 5044 ) CrossRef; (i) D. R. White, M. I. Herman and J. P. Wolfe, Org. Lett., 2017, 19, 4311 CrossRef CAS PubMed; (j) T. Schitter, P. G. Jones and D. B. Werz, Chem.–Eur. J., 2018, 24, 13446 CrossRef CAS PubMed.
- (a) G. M. Borrajo-Calleja, V. Bizet and C. Mazet, J. Am. Chem. Soc., 2016, 138, 4014 CrossRef CAS PubMed; (b) V. Bizet, G. M. Borrajo-Calleja, C. Besnard and C. Mazet, ACS Catal., 2016, 6, 7183 CrossRef CAS; (c) G. M. Borrajo-Calleja, V. Bizet, C. Besnard and C. Mazet, Organometallics, 2017, 36, 3553 CrossRef CAS.
- A general review: Y. Liu, W. Li and J. Zhang, Natl. Sci. Rev., 2017, 4, 326 CrossRef CAS.
- For Ming-Phos ligands, see: (a) Z.-M. Zhang, P. Chen, W. Li, Y. Niu, X. Zhao and J. Zhang, Angew. Chem., 2014, 126, 4439 ( Angew. Chem., Int. Ed. , 2014 , 53 , 4350 ) CrossRef; (b) M. Chen, Z.-M. Zhang, Z. Yu, H. Qiu, B. Ma, H.-H. Wu and J. Zhang, ACS Catal., 2015, 5, 7488 CrossRef CAS; (c) Z.-M. Zhang, B. Xu, S. Xu, H.-H. Wu and J. Zhang, Angew. Chem., 2016, 128, 6432 ( Angew. Chem., Int. Ed. , 2016 , 55 , 6324 ) CrossRef; (d) B. Xu, Z.-M. Zhang, S. Xu, B. Liu, Y. Xiao and J. Zhang, ACS Catal., 2017, 7, 210 CrossRef CAS.
- For Xiang-Phos ligands, see: (a) H. Hu, Y. Wang, D. Qian, Z.-M. Zhang, L. Liu and J. Zhang, Org. Chem. Front., 2016, 3, 759 RSC; (b) P.-C. Zhang, Y. Wang, Z.-M. Zhang and J. Zhang, Org. Lett., 2018, 20, 7049 CrossRef CAS PubMed.
- For PC-Phos ligands, see: (a) Y. Wang, P. Zhang, X. Di, Q. Dai, Z.-M. Zhang and J. Zhang, Angew. Chem., 2017, 129, 16121 ( Angew. Chem., Int. Ed. , 2017 , 56 , 15905 ) CrossRef; (b) L. Wang, M. Chen, P. Zhang, W. Li and J. Zhang, J. Am. Chem. Soc., 2018, 140, 3467 CrossRef CAS PubMed; (c) P.-C. Zhang, J. Han and J. Zhang, Angew. Chem., 2019, 131, 11566 ( Angew. Chem. Int. Ed. , 2019 , 58 , 11444 ) Search PubMed.
- For Xu-Phos ligands, see: (a) Z.-M. Zhang, B. Xu, Y. Qian, L. Wu, Y. Wu, L. Zhou, Y. Liu and J. Zhang, Angew. Chem., 2018, 130, 10530 ( Angew. Chem. Int. Ed. , 2018 , 57 , 10373 ) CrossRef; (b) Z.-M. Zhang, B. Xu, L. Wu, Y. Wu, Y. Qian, L. Zhou, Y. Liu and J. Zhang, Angew. Chem., 2019, 131, 14795 ( Angew. Chem. Int. Ed. , 2019 , 58 , 14653 ) CrossRef.
- (a) F. Ozawa, A. Kubo and T. Hayashi, Chem. Lett., 1992, 2177 CrossRef CAS; (b) S. Rousseaux, J. García-Fortanet, M. A. D. A. Sanchez and S. L. Buchwald, J. Am. Chem. Soc., 2011, 133, 9282 CrossRef CAS PubMed; (c) B. A. Hopkins and J. P. Wolfe, Angew. Chem., 2012, 124, 10024 ( Angew. Chem. Int. Ed. , 2012 , 51 , 9886 ) CrossRef; (d) Y. Ji, E. Plata, C. S. Regens, M. Hay, M. Schmidt, T. Razler, Y. Qiu, P. Geng, Y. Hsiao, T. Rosner, M. D. Eastgate and D. G. Blackmond, J. Am. Chem. Soc., 2015, 137, 13272 CrossRef CAS PubMed; (e) H. Li, K. M. Belyk, J. Yin, Q. Chen, A. Hyde, Y. Ji, S. Oliver, M. T. Tudge, L.-C. Campeau and K. R. Campos, J. Am. Chem. Soc., 2015, 137, 13728 CrossRef CAS PubMed.
- CCDC 1981382 (3ac) and 1981384 (3aa).
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1981382 (3ac) and 1981384 (3aa). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc01391a |
| This journal is © The Royal Society of Chemistry 2020 |