Open Access Article
Open Access ArticleTransformations of the cyclo-P4 ligand in [Cp′′′Co(η4-P4)]†
Martin
Piesch
,
Michael
Seidl
and
Manfred
Scheer
 *
*
Institut für Anorganische Chemie, Universität Regensburg, 93040 Regensburg, Germany. E-mail: Manfred.Scheer@ur.de; Web: https://www.uni-regensburg.de/chemie-pharmazie/anorganische-chemie-scheer/
First published on 25th May 2020
Abstract
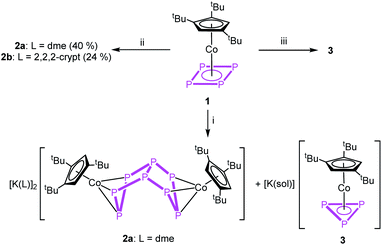
The reactivity of the cyclo-P4 ligand complex [Cp′′′Co(η4-P4)] (1) (Cp′′′ = 1,2,4-tri-tert-butyl-cyclopentadienyl) towards reduction and main group nucleophiles was investigated. By using K[CpFe(CO)2], a selective reduction to the dianionic complex [(Cp′′′Co)2(μ,η3:η3-P8)]2− (2) was achieved. The reaction of 1 with tBuLi and LiCH2SiMe3 as carbon-based nucleophiles yielded [Cp′′′Co(η3-P4R)]− (R = tBu (4), CH2SiMe3 (7)), which, depending on the reaction conditions, undergo subsequent reactions with another equivalent of 1 to form [(Cp′′′Co)2(μ,η3:η3-P8R)]− (R = tBu (5), CH2SiMe3 (8)). In the case of 4, a different pathway was observed, namely a dimerisation followed by a fragmentation into [Cp′′′Co(η3-P5tBu2)]− (6) and [Cp′′′Co(η3-P3)]− (3). With OH− as an oxygen-based nucleophile, the synthesis of [Cp′′′Co(η3-P4(O)H)]− (9) was achieved. All compounds were characterized by X-ray crystal structure analysis, NMR spectroscopy and mass spectrometry. Their electronic structures and reaction behavior were elucidated by DFT calculations.
Introduction
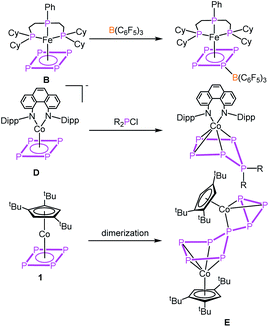
The parent organic carbon aromates CnHn have been widely used as ligands in organometallic chemistry in sandwich, half-sandwich or multi-decker sandwich complexes. While the majority of compounds include Cp (C5H5) and benzene (C6H6) as ligands, examples for complexes with C3H3 and C4H4 ligands are rather limited.1 Due to the isolobal analogy of the CH unit and P, a large variety of ligands have been described where CH units were replaced partially by P atoms as in e.g. phospholes (C4H4P, C2H2P3)2 or phosphinines (C5H5P)3 or completely resulting in aromatic cyclo-Pn ligands. While complexes with cyclo-P3 (e.g. [Cp*Ni(η3-P3)],4 [(Cp′′′Ni)2(μ,η3:η3-P3)]5), cyclo-P5 (e.g. [Cp*M(η5-P5)] (M = Fe,6 Ru7) [(CpRM)2(μ,η5:η5-P5)] (M = Cr,8 Mn9)) and cyclo-P6 [(CpRM)2(μ,η6:η6-P6)] (M = Ti,10 V,11 Nb,12 Mo,13 W11) ligands have been known for decades, the cyclo-P4 Co complex in this row was unknown for a long time. Recently, we succeeded in the synthesis of [Cp′′′Co(η4-P4)] (1).14 This inspired the synthesis of other complexes containing cyclo-P4 ligands a few of which were recently reported as e.g. being based on rare earth metals like [{(DippForm)2Sm}2(μ,η4:η4-P4)],15 group five metals, [CpRM(CO)2(η4-P4)] (M = V, Nb, Ta (A)),16 on chromium, [L2Cr2(η2:η2:η1:η1-P4)] (L = (2,6-diisopropylphenyl)-{6-(2,6-dimethylphenyl)-pyridin-2-yl}-amide)),17 on iron, [PhPP2CyFe(η4-P4)] (B),18 on molybdenum, [(ArDippCN)2Mo(CO)2(η4-P4)] (C)19 and on the anionic iron complex [CpArFe(η4-P4)]− (ref. 20) as well as on the anionic cobalt complex [(PHDI)Co(η4-P4)]− (D).21 The reactivity of these complexes was only little investigated by the coordination chemistry of A and B towards coinage metal salts18,22 and the reactivity of B towards Lewis acids as for instance B(C6F5)3 (Scheme 1).18 The anionic compound D was quenched with phosphorus-based electrophiles (R2PCl) leading to neutral complexes with substituted cyclo-P5R2 ligands (Scheme 1).21 Ring expansion reactions were also reported for 1 using the pnictogenidene complexes [Cp*E{W(CO)5}2] (E = P, As), leading to neutral cobalt complexes with organo-substituted cyclo-P5 and cyclo-P4As ligands,23a with the latter showing that reactions with electrophiles were investigated (other examples cf.ref. 23b and c), though not yet the reactivity towards nucleophiles or reduction reactions in general. As for the former chemistry, however, it was studied for [Cp*Fe(η5-P5)] and [Cp*Ni(η3-P3)],5,24 for which organo-substituted anionic complexes of the types [Cp*Fe(η4-P5R)]− and [Cp′′′Ni(η2-P3R)]− were obtained.With this state of the art in mind, questions arose as to how 1 would respond to redox reactions or to a nucleophilic attack. Would a dimerisation by a P–P bond formation or a simple substitution occur, as known from the reactions of other cyclo-Pn ligand complexes? Would a structural rearrangement be possible, including a reorganisation process? Moreover, controlling the reactivity of 1 is a challenging task since it has a high intrinsic tendency to dimerising irreversibly in solution to give a connected five- and three-membered P8 ligand in [(Cp′′′Co)2(μ,η4:η3-P8)] (E, Scheme 1).14 Furthermore, there are only few examples known of four-membered heterocycles to undergo ring expansion reactions, e.g. by nucleophiles being added to phosphetanes25 or by oxidizing the diphosphete complex [Cp′′′Co(η4-P2(CtBu)2)] to give the five- and the three-membered ring products, [Cp′′′Co(η5-P3(CtBu)2)]+ and [Cp′′′Co(η3-P(CtBu)2)]+, respectively.26
Herein we report the unprecedented transformations of the cyclo-P4 ligand complex 1 upon reduction and the reactions with main-group nucleophiles to give novel P-rich ligand complexes.
Results and discussion
To obtain a first insight into the reactivity of 1, the frontier molecular orbitals (cf. Fig. S32†) were computed by DFT calculations. They reveal that both HOMO and LUMO are mainly located on the cyclo-P4 ligand and, hence, 1 should react with both electrophiles and nucleophiles. The reactivity of 1 towards electrophiles has been shown by reactions with [Cp*E{W(CO)5}2] (E = P, As).23 On the other hand, the cyclic voltammogram (cf. Fig. S18†) of 1 in dme reveals a weak irreversible oxidation process at +275 mV and a strong irreversible reduction process at −2081 mV against [Cp2Fe]/[Cp2Fe]+, indicating the potential ligand-centred redox activity of 1 (for details cf. ESI†).Due to the low reduction potential of 1, we used potassium, potassium graphite and potassium hydride for the chemical reduction. In all cases, the formation of a mixture of [(Cp′′′Co)2(μ,η3:η3-P8)]2− (2) and [Cp′′′Co(η3-P3)]− (3)27 in an approximate ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 2) is observed (cf. Fig. S1†). When the reaction is conducted in the presence of 18-c-6 or 2,2,2-cryptand, only 3 can be detected in the 31P{1H} NMR spectrum. The selective synthesis of 2 can be achieved by using K[CpFe(CO)2] at low temperatures. Interestingly, K[CpFe(CO)2] should not be able to reduce 1 (its reduction potential of −1800 mV in thf against [Cp2Fe]/[Cp2Fe]+ is higher than that of 1 (vide supra)).28 After workup, [K(dme)]2[(Cp′′′Co)2(μ,η3:η3-P8)] (2a) can be obtained as a dark green solid in crystalline yields of 40% (Scheme 2). Although well-diffracting single crystals of 2a could be obtained, a satisfactory refinement of the X-ray data was not possible since the potassium counterions and dme molecules are severely disordered over several positions with side occupancies partially below 8% (cf. ESI†). The addition of 2,2,2-cryptand to a crude reaction mixture of 2a and the recrystallization from a dme/acetonitrile mixture at −30 °C yielded single crystals of [K(2,2,2-cryptand)]2[(Cp′′′Co)2(μ,η3:η3-P8)] (2b) suitable for X-ray diffractions in 24% yield. Both 2a and 2b can be isolated as dark green solids that are extremely air- and moisture-sensitive. The structure in the solid state (Fig. 1) shows a dianionic dinuclear complex with a P8 ligand consisting of a cis-bicyclo[3.3.0]octane core that coordinates to two {Cp′′′Co} fragments. Compound 2 displays the first compound possessing such a ligand. The structural motif is similar to the realgar-like P8 cages (tricyclo[3.3.0.0]octane) as in [(LnM)4(μ4,η1:η1:η1:η1:η1:η1:η1:η1-P8)] (LnM = nacnacGa,29 nacnacFe,30 NNfcSc,31
1 (Scheme 2) is observed (cf. Fig. S1†). When the reaction is conducted in the presence of 18-c-6 or 2,2,2-cryptand, only 3 can be detected in the 31P{1H} NMR spectrum. The selective synthesis of 2 can be achieved by using K[CpFe(CO)2] at low temperatures. Interestingly, K[CpFe(CO)2] should not be able to reduce 1 (its reduction potential of −1800 mV in thf against [Cp2Fe]/[Cp2Fe]+ is higher than that of 1 (vide supra)).28 After workup, [K(dme)]2[(Cp′′′Co)2(μ,η3:η3-P8)] (2a) can be obtained as a dark green solid in crystalline yields of 40% (Scheme 2). Although well-diffracting single crystals of 2a could be obtained, a satisfactory refinement of the X-ray data was not possible since the potassium counterions and dme molecules are severely disordered over several positions with side occupancies partially below 8% (cf. ESI†). The addition of 2,2,2-cryptand to a crude reaction mixture of 2a and the recrystallization from a dme/acetonitrile mixture at −30 °C yielded single crystals of [K(2,2,2-cryptand)]2[(Cp′′′Co)2(μ,η3:η3-P8)] (2b) suitable for X-ray diffractions in 24% yield. Both 2a and 2b can be isolated as dark green solids that are extremely air- and moisture-sensitive. The structure in the solid state (Fig. 1) shows a dianionic dinuclear complex with a P8 ligand consisting of a cis-bicyclo[3.3.0]octane core that coordinates to two {Cp′′′Co} fragments. Compound 2 displays the first compound possessing such a ligand. The structural motif is similar to the realgar-like P8 cages (tricyclo[3.3.0.0]octane) as in [(LnM)4(μ4,η1:η1:η1:η1:η1:η1:η1:η1-P8)] (LnM = nacnacGa,29 nacnacFe,30 NNfcSc,31 32), [(CpMeFe(CO)2)2(CpMeFe(CO))2(μ4,η1:η1:η1:η1:η1:η1-P8)],33 [(Cp*Ir(CO))2(μ5,η1:η1:η1:η1:η1:η1:η1-P8){Cr(CO)5}3]34 or [(Cp′′′Ni)2(μ,η2:η2-P8)]2−,5 with the difference that in 2b one P–P bond is cleaved (P1–P5, Fig. 1). In all mentioned complexes, the P8 ligands coordinate either in an η1:η1 (Ga, Fe, Sc, Sm, Ir) or η2 (Ni) fashion to the metal fragments, while in 2b two allylic P3 subunits (P1–P2 2.1554(6), P1–P8 2.1577(6), P4–P5 2.1519(6), P5–P6 2.1580(6) Å) coordinate to two {Cp′′′Co} fragments in an η3 fashion. These P–P bond lengths are between single35 and double36 bond lengths, in line with the calculated Wiberg Bond Indices (WBIs) of 1.11 and 1.12, respectively.
32), [(CpMeFe(CO)2)2(CpMeFe(CO))2(μ4,η1:η1:η1:η1:η1:η1-P8)],33 [(Cp*Ir(CO))2(μ5,η1:η1:η1:η1:η1:η1:η1-P8){Cr(CO)5}3]34 or [(Cp′′′Ni)2(μ,η2:η2-P8)]2−,5 with the difference that in 2b one P–P bond is cleaved (P1–P5, Fig. 1). In all mentioned complexes, the P8 ligands coordinate either in an η1:η1 (Ga, Fe, Sc, Sm, Ir) or η2 (Ni) fashion to the metal fragments, while in 2b two allylic P3 subunits (P1–P2 2.1554(6), P1–P8 2.1577(6), P4–P5 2.1519(6), P5–P6 2.1580(6) Å) coordinate to two {Cp′′′Co} fragments in an η3 fashion. These P–P bond lengths are between single35 and double36 bond lengths, in line with the calculated Wiberg Bond Indices (WBIs) of 1.11 and 1.12, respectively.
 | ||
| Scheme 2 Reaction of 1 with (i) K, KH or KC8 in thf at r.t., (ii) K[CpFe(CO)2] in thf at −80 °C and (iii) with K, KH or KC8 in thf at r.t. in the presence of 18-c-6 or 2,2,2-cryptand. | ||
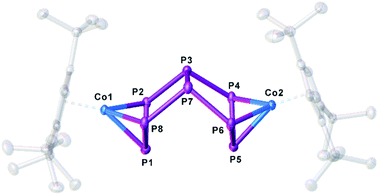 | ||
| Fig. 1 Structure of the dianion in 2b in the solid state. Thermal ellipsoids are shown at 50% probability level. Hydrogen atoms, cations and solvent molecules are omitted for clarity. | ||
All other P–P distances are in the range of single bonds (2.1947(6)–2.2247(6) Å), which is underlined by WBIs between 0.93 and 0.96. The 1H NMR spectrum of 2a in thf-d8 shows three singlets centred at δ = 3.96, 1.31 and 1.22 ppm with an integral ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36
36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 indicating two equivalent freely rotating Cp′′′ ligands. The 31P{1H} NMR spectrum in thf-d8 shows three multiplets centred at δ = 96.2, 83.1 and −124.2 ppm with an integral ratio of 2
18 indicating two equivalent freely rotating Cp′′′ ligands. The 31P{1H} NMR spectrum in thf-d8 shows three multiplets centred at δ = 96.2, 83.1 and −124.2 ppm with an integral ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 of an AA′MM′M′′M′′′XX′ spin system.
2 of an AA′MM′M′′M′′′XX′ spin system.
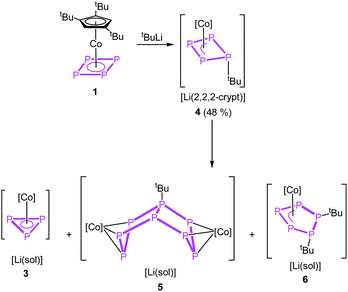
Instead of adding electrons to the system by reduction, nucleophiles can also serve as electron donors. We selected tBuLi as a strong carbon-based nucleophile with a higher steric demand to avoid side reactions. The reaction with 1 in thf at −80 °C proceeds instantly during the addition of tBuLi indicated by an immediate colour change from red to brown. After warming to room temperature, the 1H and 31P{1H} NMR spectra reveal the selective formation of [Cp′′′Co(η3-P4tBu)]− (4) (Scheme 3). Pure 4 can be precipitated from the reaction mixture in a yield of 48% after addition of 2,2,2-cryptand.
Regardless of numerous attempts to obtain single crystals of 4 suitable for X-ray diffractions, we were only able to receive poorly diffracting ones (resolution < 1.20 Å). The atom connectivity can be determined unambiguously, a discussion of the bond lengths and angles, however, would not be appropriate. The solid-state structure (cf. Fig. S22†) reveals a folded cyclo-P4 ligand with one P atom deviating from the former planar P4 unit, to which the tBu substituent is bonded in an axial position (isomer 4a). Theoretically, a second isomer with the tBu substituent in an equatorial position (4b) would be possible, it could, however, not be detected by NMR spectroscopy (vide infra; cf. Fig. S4†). According to DFT calculations, the energy difference between the axial and equatorial isomers of 4 (i.e.4a and 4b) is 11.91 kJ mol−1, with 4a being favoured. During the optimization of the crystallization conditions, we noticed that 4 is not stable in solution over time (especially in the absence of 2,2,2-cryptand) and is converted to [(Cp′′′Co)2(μ,η3:η3-P8tBu)]− (5) or [Cp′′′Co(η3-P3)]− (3) and [Cp′′′Co(η3-P5tBu2)]− (6) (Scheme 3). This process was monitored by 31P{1H} NMR spectroscopy and the products 5 and 6 could be isolated and characterized spectroscopically and by single crystal X-ray diffractions.
For compound 5, few single crystals suitable for X-ray diffractions could be obtained, while the quality of the crystals of 6 gave only very poor data indicating, however, the atom connectivity in 6 (cf. ESI†). The molecular structure of 5 (Fig. 2) shows a binuclear complex with a substituted P8tBu ligand coordinating to two {Cp′′′Co} fragments, each in an η3 fashion similar to 2. All P–P distances are comparable to those in 2. The identity of 3, 5 and 6 was further proven by simulation of the related 31P{1H} NMR spectra. Performing the reaction of 1 with tBuLi at room temperature, the 31P{1H} NMR spectrum of the reaction mixture in thf-d8 reveals a mixture of all four compounds 3, 4, 5 and 6 beside traces of unknown compounds (Fig. 3). The 31P{1H} NMR spectra of 4, 5 and 6 were simulated independent of this spectrum, since 3, 5 and 6 could not be separated preparatively from each other.
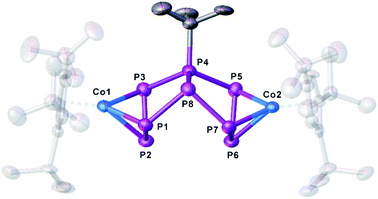 | ||
| Fig. 2 Molecular structure of the anion of 5 in the solid state. Thermal ellipsoids are shown at 50% probability level. Hydrogen atoms, cations and solvent molecules are omitted for clarity. | ||
 | ||
| Fig. 3 31P{1H} NMR spectrum of the reaction of 1 with tBuLi at r.t. in thf-d8 and the simulated NMR spectra of 4, 5 and 6 ([Co] = Cp′′′Co). | ||
The formation of 5 can be explained by the reaction of 4 with another equivalent of 1, which is energetically favoured by 52.48 kJ mol−1 (Fig. 4) and probably driven by the decrease of the ring strain. An alternative pathway to the release of ring strain would be the dimerisation of two equivalents of 4 followed by a subsequent fragmentation to form 6 (containing an unprecedented, disubstituted cyclo-P5 ligand) and 3 (containing a cyclo-P3 ligand). This reaction is exothermic by 161.09 kJ mol−1. Interestingly, these subsequent reactions can be avoided or at least slowed down when the lithium counter ion is completely separated from the anion in 4 by i.e. the addition of 2,2,2-cryptand.
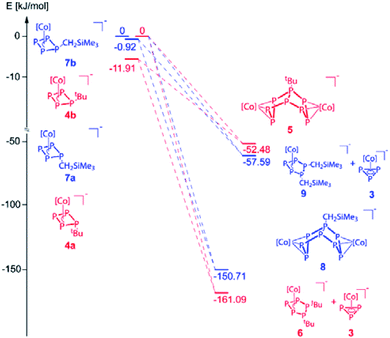 | ||
| Fig. 4 Energetic diagram of the isomers 4a,b and 7a,b and their subsequent reactions. 4b and 7b were each used as reference for the related calculations (0 kJ mol−1, [Co] = Cp′′′Co). | ||
Since the nucleophile tBuLi seems to be too strong and induces subsequent reactions, we employed LiCH2SiMe3 as a weaker and sterically less demanding carbon-based nucleophile in the reaction with 1.
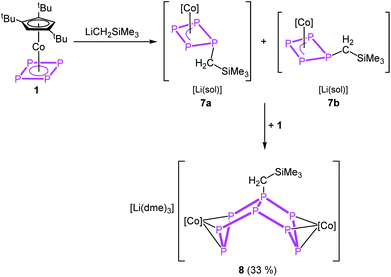
The reaction of 1 with LiCH2SiMe3 in thf at −80 °C leads to the formation of [Cp′′′Co(η3-P3CH2SiMe3)]− (7) and, after warming to room temperature, of [Li(dme)3][(Cp′′′Co)2(μ,η3:η3-P8CH2SiMe3)] (8), which can be isolated in crystalline yields of 33% after workup (Scheme 4). The formation of 7 and 8 becomes manifest by color changes from red (1) to brown (7) to green (8). The outcome of the reaction is independent of whether 0.5 or 1 equivalents of LiCH2SiMe3 are used.
The reaction can be stopped partially and the formation of 8 be reduced when 12-c-4 or 2,2,2-cryptand is added directly after the addition of LiCH2SiMe3 at −80 °C. The crude 31P{1H} NMR spectra of these reactions reveal two isomers of 7 in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 (2,2,2-crypt) and 1
0.25 (2,2,2-crypt) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.33 (12-c-4), respectively, which can be assigned to complexes where the CH2SiMe3 substituent is located in an axial position (7a) or in an equatorial position (7b). The 31P{1H} NMR spectra reveal also the formation of 8 and 3 beside minor amounts of unknown side products, while the reaction in the presence of 2,2,2-cryptand seems to be much more selective to giving 3, 7a, 7b and 8 (cf. Fig. S10 and S11†). According to DFT calculations, the energy of the isomers 7a and 7b differs only by 0.92 kJ mol−1, with 7a being favoured (Fig. 4). Few crystals of the isomer 7a were obtained from a concentrated solution of the mixture of isomers in thf layered with n-hexane at −30 °C. The molecular structure (Fig. 5, left) shows a folded cyclo-P4 ligand with one P atom bent out of the plane bearing the CH2SiMe3 substituent. The P1–P2 (2.2283(16) Å) and P1–P4 (2.2229(19) Å) distances are longer than the P2–P3 (2.1877(17) Å) and P3–P4 (2.1895(16) Å) bonds. All P–P bond lengths are elongated compared to 1.14 The 31P{1H} NMR spectrum of 7 in thf-d8 shows three multiplets of an AMXX′ spin system (7a) centred at δ = 52.6, 46.0 and 18.8 ppm with an integral ratio of 1
1.33 (12-c-4), respectively, which can be assigned to complexes where the CH2SiMe3 substituent is located in an axial position (7a) or in an equatorial position (7b). The 31P{1H} NMR spectra reveal also the formation of 8 and 3 beside minor amounts of unknown side products, while the reaction in the presence of 2,2,2-cryptand seems to be much more selective to giving 3, 7a, 7b and 8 (cf. Fig. S10 and S11†). According to DFT calculations, the energy of the isomers 7a and 7b differs only by 0.92 kJ mol−1, with 7a being favoured (Fig. 4). Few crystals of the isomer 7a were obtained from a concentrated solution of the mixture of isomers in thf layered with n-hexane at −30 °C. The molecular structure (Fig. 5, left) shows a folded cyclo-P4 ligand with one P atom bent out of the plane bearing the CH2SiMe3 substituent. The P1–P2 (2.2283(16) Å) and P1–P4 (2.2229(19) Å) distances are longer than the P2–P3 (2.1877(17) Å) and P3–P4 (2.1895(16) Å) bonds. All P–P bond lengths are elongated compared to 1.14 The 31P{1H} NMR spectrum of 7 in thf-d8 shows three multiplets of an AMXX′ spin system (7a) centred at δ = 52.6, 46.0 and 18.8 ppm with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and three multiplets of an AMM′X spin system (7b) centred at δ = 39.5, 3.94 and −78.0 ppm with an integral ratio of 1
2 and three multiplets of an AMM′X spin system (7b) centred at δ = 39.5, 3.94 and −78.0 ppm with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The JPP coupling constants obtained from the simulation (cf. ESI†) correlate nicely with the bond distances obtained from the X-ray structure determination.
1. The JPP coupling constants obtained from the simulation (cf. ESI†) correlate nicely with the bond distances obtained from the X-ray structure determination.
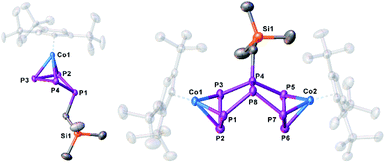 | ||
| Fig. 5 Molecular structure of the anions 7a and 8 in the solid state. Thermal ellipsoids are shown at 50% probability level. Hydrogen atoms, cations and solvent molecules are omitted for clarity. | ||
When the reaction is conducted without the use of 12-c-4 or 2,2,2-cryptand, a colour change from initial red to brown (7) to dark green (8) is observed. Crystals of 8 suitable for single crystal X-ray diffraction were obtained from a concentrated solution in a mixture of n-hexane and dme stored at −30 °C. The structure of 8 (Fig. 5, right) shows a dinuclear anionic complex with a substituted P8 ligand. The P8 structural motif is similar to that observed in the reduced complex 2 and analogous to that found in 5. The P8 ligand consists of two condensed five-membered rings (bicyclo[3.3.0]octane) coordinating to the {Cp′′′Co} fragments via the allylic P3 subunits (P1–P2–P3 and P5–P6–P7). The bond distances (P1–P2 2.1643(11), P2–P3 2.1574(11), P5–P6 2.1494(11), P6–P7 2.1575(11) Å) are in the range between single and double bonds, indicated by the WBIs between 1.09 and 1.11. All other P–P distances are in the range of single bonds. The 31P{1H} NMR spectrum of 8 in thf-d8 shows five multiplets centred at δ = 187.9, 102.8, 74.4, 40.6 and −118.8 ppm with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 indicating an AM2N2OX2 spin system. The simulation of the spectrum at 193 K was conducted to determine the coupling constants and chemical shifts (cf. Fig. S13†). The formation of 8 can be explained by the reaction of 7 with another equivalent of 1, energetically favoured by −150.71 kJ mol−1. Alternatively, as also might be true for the formation of 5, a formal intermolecular nucleophilic attack of 7 at another molecule 7 under release of LiCH2SiMe3 to 8 (SN2 type), which is supported by the VT 31P{1H} NMR of the reaction between 1 and LiCH2SiMe3 (cf. Fig. S14–S16†). At 193 K, the reaction is already complete (absence of signals of 1). The 31P{1H} NMR spectrum shows six broad multiplets that can be assigned to 7a,b. Upon warming, these signals broaden and only two broad triplets remain (at 233 and 253 K). The formation of 8 is detected for the first time at 253 K. At room temperature, 3, 7a,b, 8 and a second set of signals quite similar to those of 8 are observed. The latter might represent an isomer of 8 (starting either from 7a or 7b). After 24 hours at room temperature, the signals of 7a,b disappeared completely. Interestingly, whereas the formation of 6 as an additional product of the reaction between 1 and tBuLi was found, the corresponding product [Cp′′′Co(η3-P5(CH2SiMe3)2)]− (9) for the reaction of 1 with LiCH2SiMe3 could never be detected by NMR, since the dimerisation and subsequent fragmentation of 7 to 9 and 3 would be energetically less favoured (−57.59 kJ mol−1; Fig. 4). Beside the relative thermodynamic stability of the intermediates 4 and 7, the kinetic effects of the transformation processes play an important role, which is indicated by the outcome of these reactions, as well as their conversion rates.
2 indicating an AM2N2OX2 spin system. The simulation of the spectrum at 193 K was conducted to determine the coupling constants and chemical shifts (cf. Fig. S13†). The formation of 8 can be explained by the reaction of 7 with another equivalent of 1, energetically favoured by −150.71 kJ mol−1. Alternatively, as also might be true for the formation of 5, a formal intermolecular nucleophilic attack of 7 at another molecule 7 under release of LiCH2SiMe3 to 8 (SN2 type), which is supported by the VT 31P{1H} NMR of the reaction between 1 and LiCH2SiMe3 (cf. Fig. S14–S16†). At 193 K, the reaction is already complete (absence of signals of 1). The 31P{1H} NMR spectrum shows six broad multiplets that can be assigned to 7a,b. Upon warming, these signals broaden and only two broad triplets remain (at 233 and 253 K). The formation of 8 is detected for the first time at 253 K. At room temperature, 3, 7a,b, 8 and a second set of signals quite similar to those of 8 are observed. The latter might represent an isomer of 8 (starting either from 7a or 7b). After 24 hours at room temperature, the signals of 7a,b disappeared completely. Interestingly, whereas the formation of 6 as an additional product of the reaction between 1 and tBuLi was found, the corresponding product [Cp′′′Co(η3-P5(CH2SiMe3)2)]− (9) for the reaction of 1 with LiCH2SiMe3 could never be detected by NMR, since the dimerisation and subsequent fragmentation of 7 to 9 and 3 would be energetically less favoured (−57.59 kJ mol−1; Fig. 4). Beside the relative thermodynamic stability of the intermediates 4 and 7, the kinetic effects of the transformation processes play an important role, which is indicated by the outcome of these reactions, as well as their conversion rates.
The reaction of the oxygen-based nucleophile NaOH with 1 at room temperature yields [Na(dme)3][Cp′′′Co(η3-P4(O)H)] (10) in crystalline yields of 55% after workup (eqn (1)). In the first step, OH− binds to one phosphorus atom of the cyclo-P4 ligand in 1, followed by a proposed tautomeric rearrangement with the formation of a P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double and P–H single bond.
O double and P–H single bond.
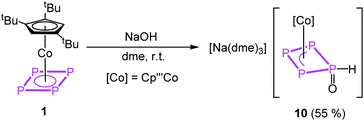 | (1) |
The structure of 10 (Fig. 6) reveals a folded cyclo-P4 ligand in which one P atom is bent out of the plane (P1). The hydrogen atom bonded to phosphorus (H1) was found on the electron density map and was freely refined. The P–P distances are in the range of single or slightly shortened single bonds (P1–P2 2.1659(7), P1–P4 2.1676(7), P2–P3 2.1984(8), P3–P4 2.1938(8) Å; WBIs 0.94–1.07). The P1–O1 bond (1.5200(15) Å) is in the range between a single and a double bond (WBI of 1.08).
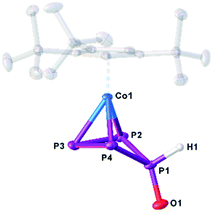 | ||
| Fig. 6 Molecular structure of the anion in 10 in the solid state. Thermal ellipsoids are shown at 50% probability level. Hydrogen atoms, cations and solvent molecules are omitted for clarity. | ||
The 31P{1H} NMR spectrum of 10 in thf-d8 shows three multiplets centred at δ = 74.5, 29.0 and −21.8 ppm with an integral ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 indicating an A2MX spin system. In the 31P NMR spectrum, a coupling between H1 and all P atoms can be observed. In the 1H NMR spectrum, a doublet of triplet of doublets at 7.78 ppm with the coupling constants of 1JPH = 356.8, 2JPH = 20.6 and 3JPH = 5.1 Hz could be detected among the characteristic signals for the Cp′′′ ligand. A similar reaction was reported by Peruzzini and co-workers for the reaction of [Ir(dppm)(Ph2PCH2PPh2PPPP)]+ with water under basic conditions.37
1 indicating an A2MX spin system. In the 31P NMR spectrum, a coupling between H1 and all P atoms can be observed. In the 1H NMR spectrum, a doublet of triplet of doublets at 7.78 ppm with the coupling constants of 1JPH = 356.8, 2JPH = 20.6 and 3JPH = 5.1 Hz could be detected among the characteristic signals for the Cp′′′ ligand. A similar reaction was reported by Peruzzini and co-workers for the reaction of [Ir(dppm)(Ph2PCH2PPh2PPPP)]+ with water under basic conditions.37
Conclusions
In summary, we were able to show that the irreversible dimerisation reaction of 1 can be overcome by carrying out the reactions at room temperature or lower. That way, in reactions with main group nucleophiles and upon reduction, 1 displays a unique tendency to release the ring strain of the cyclo-P4 ligand. Here, unprecedented aggregation and rearrangement processes occur. The reduction of 1 with K[CpFe(CO)2] yields selectively the dinuclear dianionic complex [(Cp′′′Co)2(μ,η3:η3-P8)]2− (2), comprising an unprecedented open realgar-like P8 cage that reveals a double η3-coordinated P8 ligand for the first time. Compound 1 was functionalised with the main group nucleophiles tBuLi and LiCH2SiMe3, respectively, and [Cp′′′Co(η3-P4R)]− (R = tBu (4), CH2SiMe3 (7)) could be isolated as the first reaction products. Both compounds are metastable and show further rearrangement processes such as dimerisation and/or fragmentation. In comparison to other nucleophile-substituted polyphosphorus complexes starting from a four-membered ring in 1, here the first-formed product is highly reactive. Thus, compounds 4 and 7 can act as nucleophiles and react with another equivalent of 1 to the dinuclear complexes [(Cp′′′Co)2(μ,η3:η3-P8R)]− (R = tBu (5), CH2SiMe3 (8)) comprising novel organo-substituted open realgar-like P8R ligands. For 4, an additional reaction pathway was observed. The formal dimerisation of 4 followed by an unprecedented fragmentation gives [Cp′′′Co(η3-P5tBu2)]− (6) with a new diorgano-substituted cyclo-P5 ligand and [Cp′′′Co(η3-P3)]− (3).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft within the project Sche 384/38-1 and Sche 384/33-2. M. P. is grateful to the Fonds der Chemischen Industrie for a PhD fellowship.Notes and references
- Examples for complexes with a C4H4 ligand: (a) P. D. Harvey, W. P. Schaefer, H. B. Gray, D. F. R. Gilson and I. S. Butler, Inorg. Chem., 1988, 27, 57 CrossRef CAS; (b) L. M. Cirjak, J.-S. Huang, Z.-H. Zhu and L. F. Dahl, J. Am. Chem. Soc., 1980, 102, 6623 CrossRef CAS; (c) P. E. Riley and R. E. Davis, J. Organomet. Chem., 1977, 137, 91 CrossRef CAS; (d) R. E. Davis and P. E. Riley, Inorg. Chem., 1980, 19, 674 CrossRef CAS; (e) P. E. Riley and R. E. Davis, J. Organomet. Chem., 1976, 113, 157 CrossRef CAS . For C3H3 no examples were found in the CSD data bank. For more stable substituted derivatives with C3R3 (R = alkyl, aryl, e.g. R. M. Tuggle and D. L. Weaver, Inorg. Chem. 1971, 10, 1504) and C4R4 ligands (R = alkyl, aryl, e.g. A. R. Kudinov, E. V. Mutseneck and D. A. Luginov, Coord. Chem. Rev. 2004, 248, 571) are known and studied.
- (a) R. S. P. Turbervill, A. R. Jupp, P. S. B. McCullough, D. Ergöçmen and J. M. Goicoechea, Organometallics, 2013, 32, 2234 CrossRef CAS; (b) F. Nief and L. Ricard, Organometallics, 2001, 20, 3884 CrossRef CAS; (c) S. Du, Z. Chai, J. Hu, W.-X. Zhang and Z. Xi, Chin. J. Org. Chem., 2019, 39, 2338 CrossRef; (d) S. Du, J. Yin, Y. Chi, L. Xu and W.-X. Zhang, Angew. Chem., Int. Ed., 2017, 56, 15886 CrossRef CAS PubMed; (e) S. Du, J. Yang, J. Hu, Z. Chai, G. Luo, Y. Luo, W.-X. Zhang and Z. Xi, J. Am. Chem. Soc., 2019, 141, 6843 CrossRef CAS PubMed.
- C. Elschenbroich, M. Nowotny, B. Metz, W. Massa, J. Graulich, K. Biehler and W. Sauer, Angew. Chem., Int. Ed. Engl., 1991, 30, 547 CrossRef.
- O. J. Scherer, J. Braun and G. Wolmershäuser, Chem. Ber., 1990, 123, 471 CrossRef CAS.
- E. Mädl, G. Balázs, E. V. Peresypkina and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 7702 CrossRef PubMed.
- O. J. Scherer and T. Brück, Angew. Chem., Int. Ed. Engl., 1987, 26, 59 Search PubMed.
- O. J. Scherer, T. Brück and G. Wolmershäuser, Chem. Ber., 1988, 121, 935 CrossRef CAS.
- (a) L. Y. Goh, R. C. S. Wong, C. K. Chu and T. W. Hambley, J. Chem. Soc., Dalton Trans., 1990, 6, 977 RSC; (b) O. J. Scherer, J. Schwalb, G. Wolmershäuser, W. Kaim and R. Gross, Angew. Chem., Int. Ed. Engl., 1986, 25, 363 CrossRef.
- S. Heinl, G. Balázs, M. Bodensteiner and M. Scheer, Dalton Trans., 2016, 45, 1962 RSC.
- O. J. Scherer, H. Swarowsky, G. Wolmershäuser, W. Kaim and S. Kohlmann, Angew. Chem., Int. Ed. Engl., 1987, 26, 1153 CrossRef.
- O. J. Scherer, J. Schwalb, H. Swarowsky, G. Wolmershäuser, W. Kaim and R. Gross, Chem. Ber., 1988, 121, 443 CrossRef CAS.
- O. J. Scherer, J. Vondung and G. Wolmershäuser, Angew. Chem., Int. Ed. Engl., 1989, 28, 1355 CrossRef.
- (a) M. Fleischmann, C. Heindl, M. Seidl, G. Balázs, A. V. Virovets, E. V. Peresypkina, M. Tsunoda, F. P. Gabbaï and M. Scheer, Angew. Chem., Int. Ed., 2012, 51, 9918 CrossRef CAS PubMed; (b) M. Fleischmann, F. Dielmann, G. Balázs and M. Scheer, Chem.–Eur. J., 2016, 22, 15248 CrossRef PubMed; (c) O. J. Scherer, H. Sitzmann and G. Wolmershäuser, Angew. Chem., Int. Ed. Engl., 1985, 24, 351 CrossRef.
- F. Dielmann, A. Timoshkin, M. Piesch, G. Balázs and M. Scheer, Angew. Chem., Int. Ed., 2017, 56, 1671 CrossRef CAS PubMed.
- (a) C. Schoo, S. Bestgen, M. Schmidt, S. N. Konchenko, M. Scheer and P. W. Roesky, Chem. Commun., 2018, 54, 4770 RSC; (b) L. Qiao, C. Zhang, X.-W. Zhang, Z.-C. Wang, H. Yin and Z.-M. Sun, Chin. J. Chem., 2020, 38, 295 CrossRef CAS.
- (a) O. J. Scherer, R. Winter and G. Wolmershäuser, Z. Anorg. Allg. Chem., 1993, 619, 827 CrossRef CAS; (b) M. Herberhold, G. Frohmader and W. Milius, J. Organomet. Chem., 1996, 522, 185 CrossRef CAS.
- C. Schwarzmaier, A. Noor, G. Glatz, M. Zabel, A. Y. Timoshkin, B. M. Cossairt, C. C. Cummins, R. Kempe and M. Scheer, Angew. Chem., Int. Ed., 2011, 50, 7283 CrossRef CAS PubMed.
- A. Cavaillé, N. Saffon-Merceron, N. Nebra, M. Fustier-Boutignon and N. Mézailles, Angew. Chem., Int. Ed., 2018, 57, 1874 CrossRef PubMed.
- K. A. Mandla, C. E. Moore, A. L. Rheingold and J. S. Figueroa, Angew. Chem., Int. Ed., 2018, 57, 6853 CrossRef CAS PubMed.
- U. Chakraborty, J. Leitl, B. Mühldorf, M. Bodensteiner, S. Pelties and R. Wolf, Dalton Trans., 2018, 47, 3693 RSC.
- C. M. Hoidn, T. M. Maier, K. Trabitsch, J. J. Weigand and R. Wolf, Angew. Chem., Int. Ed., 2019, 58, 18931 CrossRef CAS PubMed.
- F. Dielmann, E. V. Peresypkina, B. Krämer, F. Hastreiter, B. P. Johnson, M. Zabel, C. Heindl and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 14833 CrossRef CAS PubMed.
- (a) M. Piesch, M. Seidl, M. Stubenhofer and M. Scheer, Chem.–Eur. J., 2019, 25, 6311 CrossRef CAS PubMed; (b) G. Capozzi, L. Chiti, M. Di Vaira, M. Peruzzini and P. Stoppioni, J. Chem. Soc., Chem. Commun., 1986, 1799 RSC; (c) P. Barbaro, A. Ienco, C. Mealli, M. Peruzzini, O. Scherer, G. Schmitt, F. Vizza and G. Wolmershäuser, Chem.–Eur. J., 2003, 9, 5195 CrossRef CAS PubMed.
- E. Mädl, M. V. Butovskii, G. Balázs, E. V. Peresypkina, A. V. Virovets, M. Seidl and M. Scheer, Angew. Chem., Int. Ed., 2014, 53, 7643 CrossRef PubMed.
- J. R. Corfield, M. J. P. Harger, J. R. Shutt and S. Trippett, J. Chem. Soc. C, 1970, 1855 RSC.
- E.-M. Rummel, G. Balázs, V. Heinl and M. Scheer, Angew. Chem., Int. Ed., 2017, 56, 9592 CrossRef CAS PubMed.
- [Cp′′′Co(η3-P3)]− was obtained by the reaction of 1 with MeNHC (1,3,4,5-tetramethyl-imidazol-2-ylidene): M. Piesch, S. Reichl, M. Seidl, G. Balázs and M. Scheer, Angew. Chem., Int. Ed., 2019, 58, 16563 CrossRef CAS PubMed.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877 CrossRef CAS PubMed.
- F. Hennersdorf, J. Frötschel and J. J. Weigand, J. Am. Chem. Soc., 2017, 139, 14592 CrossRef CAS PubMed.
- F. Spitzer, C. Graßl, G. Balázs, E. M. Zolnhofer, K. Meyer and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 4340 CrossRef CAS PubMed.
- W. Huang and P. L. Diaconescu, Chem. Commun., 2012, 48, 2216 RSC.
- S. N. Konchenko, N. A. Pushkarevsky, M. T. Gamer, R. Köppe, H. Schnöckel and P. W. Roesky, J. Am. Chem. Soc., 2009, 131, 5740 CrossRef CAS PubMed.
- M. E. Barr, B. R. Adams, R. R. Weller and L. F. Dahl, J. Am. Chem. Soc., 1991, 113, 3052 CrossRef CAS.
- M. Scheer, U. Becker and E. Matern, Chem. Ber., 1996, 129, 721 CrossRef CAS.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 186 CrossRef PubMed.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 12770 CrossRef PubMed.
- V. Mirabello, M. Corpali, L. Gonsalvi, G. Manca, A. Ienco and M. Peruzzini, Chem.–Asian J., 2013, 8, 3177 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1984450–1984455. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc01740j |
| This journal is © The Royal Society of Chemistry 2020 |