Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boron tribromide as a reagent for anti-Markovnikov addition of HBr to cyclopropanes†
Matthew H.
Gieuw
 a,
Shuming
Chen
a,
Shuming
Chen
 b,
Zhihai
Ke
b,
Zhihai
Ke
 a,
K. N.
Houk
a,
K. N.
Houk
 *b and
Ying-Yeung
Yeung
*b and
Ying-Yeung
Yeung
 *a
*a
aDepartment of Chemistry, State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, NT Hong Kong, China. E-mail: yyyeung@cuhk.edu.hk
bDepartment of Chemistry and Biochemistry, University of California, Los Angeles, California 90095, USA. E-mail: houk@chem.ucla.edu
First published on 4th August 2020
Abstract
Although radical formation from a trialkylborane is well documented, the analogous reaction mode is unknown for trihaloboranes. We have discovered the generation of bromine radicals from boron tribromide and simple proton sources, such as water or tert-butanol, under open-flask conditions. Cyclopropanes bearing a variety of substituents were hydro- and deuterio-brominated to furnish anti-Markovnikov products in a highly regioselective fashion. NMR mechanistic studies and DFT calculations point to a radical pathway instead of the conventional ionic mechanism expected for BBr3.
The Lewis acidic nature of organoboranes is well understood, but the participation of BR3 in free-radical processes was largely overlooked until 1966.1 Since the discovery of the potential of organoborane species to undergo radical reactions, many novel and synthetically useful transformations were developed.2 Trialkylboranes (BR3) can easily undergo bimolecular homolytic substitution (SH2) at the boron atom to generate alkyl radicals (Scheme 1A). It was found that alkoxyl, dialkylaminyl, alkylthiyl and carbon-centered radicals, triplet ketones, and triplet oxygen can all initiate the radical reaction by substituting one of the alkyl groups of trialkylboranes to liberate alkyl radicals.3 BEt3/O2 is arguably the most studied organoborane radical-initiating system, with the peroxyl radical being the key to propagate the reaction. Apart from being a radical initiator, BEt3, along with trace amount of O2, can also undergo conjugate addition to unsaturated ketones and aldehydes; addition to ethenyl- and ethynyloxiranes, azidoalkenes, and imines; and addition–elimination to nitroalkenes and nitroarenes, styryl sulfones, sulfoxides and sulfinimides.3 However, apart from changing BEt3 to other trialkylboranes or catecholborane to carry out similar radical reactions, the radical-reaction potential of other organoboranes remains underexplored, given the ease and mild conditions under which they initiate radical chains, often with trace amount of O2 in air at low temperature. The application of such a mild radical-initiation system to stereoselective radical reactions would drastically change the reaction outcome especially when intermediates and products are thermally unstable.4
 | ||
| Scheme 1 Classical radical reactions with trialkylboranes and our work on radical bromination using BBr3. | ||
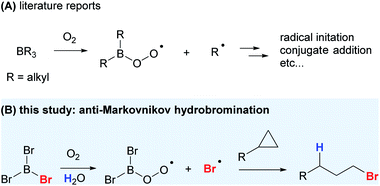
Halogenation is an important class of transformations and the resultant halogenated products can easily be manipulated to give a wide range of functional molecules.5 While trihaloboranes have been employed as halogenating or haloborating agents, their role in reactions is either ambiguous or thought to be exclusively Lewis acidic.6 To date, the use of trihaloboranes as a halogen radical donor has not been reported. With BR3/O2 being a versatile radical-initiator and conjugate-addition system, we envisioned that a suitable halogenated-borane might work similar to that of trialkylboranes in the generation of reactive, yet stable enough halogen radicals for selective halogenation reactions (Scheme 1B).
Trialkylboranes readily undergo SH2 reactions because the formation of stronger B–X (e.g. B–O) bonds via substitution is highly exothermic.3 The BDEs (B–C) of BMe3, BEt3, BnPr3, BiPr3, and BnBu3 range from 344 to 354 kJ mol−1 at 298 K, while their typical autoxidation products, B(OH)3, B(OMe)3, and B(OEt)3, have BDEs (B–O) ranging from 519 to 522 kJ mol−1 at 298 K.7 We hypothesized that organohaloboranes (BXaR3−a, X = halogen) with BDEs (B–X) similar to trialkylboranes would be a halogen radical donor from a thermodynamic viewpoint. As the common trihaloboranes (BX3) BF3, BCl3 and BBr3 have BDEs (B–X) of 644.3, 442.3 and 367.1 kJ mol−1 at 298 K, respectively, BBr3 was the logical option for our purpose.8 Although the BDE (B–I) of BI3 is the lowest among all trihaloboranes and found to be 278.2 kJ mol−1 at 0 K,9 it was not considered suitable as I2 has proven to be a very efficient radical quencher in such reactions,10 and even rigorously purified BI3 invariably contains a trace amount of I2.11
Compared to activated cyclopropanes,12 oxidative functionalization of unactivated cyclopropanes gives a wide range of useful molecules that are otherwise not readily accessible, and protocols for the Markovnikov-selective functionalization of unactivated cyclopropanes have been reported.13–20 Halolyses of cyclopropanes to give 1,3-dihaloalkanes by molecular halogens are also documented although the reactions commonly suffer from the formation of side products via electrophilic aromatic halogenation.21 In contrast, obtaining products with anti-Markovnikov regioselectivity has been considered as one of the top challenges in industry.22–30 Anti-Markovnikov functionalization of unactivated cyclopropanes mostly relies on photo-initiated radical processes with generally poor regioselectivity and limited scope.31–36 To the best of our knowledge, anti-Markovnikov hydrohalogenation of cyclopropanes has not been reported.
Very recently, an anti-Markovnikov hydroboration for unactivated cyclopropanes has been reported using boron tribromide and phenylsilane.37 The reaction was carried out under inert and anhydrous conditions, and mechanistic studies pointed to an ionic mechanism with Lewis acid–base interactions. We show that with a simple twist in the reaction conditions, which is to introduce oxygen, a drastically different reaction outcome and mechanism could be realized. We now report the study and application of BBr3 as a radical Br donor for the anti-Markovnikov addition of HBr to cyclopropanes.
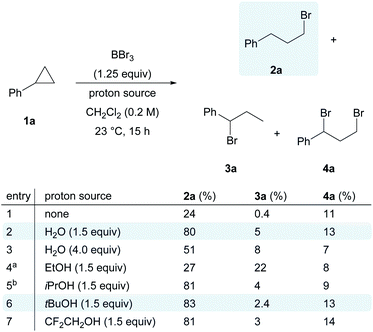
With all these considerations in mind, we initially envisioned that BBr3/O2 as a suitable system to generate bromine radicals, and cyclopropylbenzene (1a) as the model substrate to capture them. The radical reaction might then be terminated by another halogen radical from reagents such as N-chlorosuccinimide or N-iodosuccinimide. Unfortunately, messy mixtures were obtained for all entries (see the ESI, Scheme S1†). On the other hand, a simple proton source, H2O, was found to be effective in terminating the radical species. In the control experiment with only BBr3 and cyclopropylbenzene (1a) (Scheme 2, entry 1), the anti-Markovnikov hydrobrominated product 2a was obtained in 24% yield, together with the formation of Markovnikov product 3a (trace) and dibrominated cyclopropane 4a (11%). We reasoned that the proton source was the trace amount of moisture in commercial BBr3 solution.
Although it is well known that boron-based Lewis acids are moisture sensitive,38 counter-intuitively, the addition of 1.5 equivalents of H2O had a positive impact on the yield of 2a, which was dramatically improved to 80% (Scheme 2, entry 2). Excess water led to a reduction in the yield of 2a and the regioselectivity (Scheme 2, entry 3). Replacing water with ethanol as the proton source resulted in a significant drop in reaction efficiency (Scheme 2, entry 4). In contrast, bulkier alcohols such as i-PrOH or t-BuOH (Scheme 2, entries 5 and 6) and less nucleophilic alcohols such as CF3CH2OH (Scheme 2, entry 7) gave comparable performance to that of water.
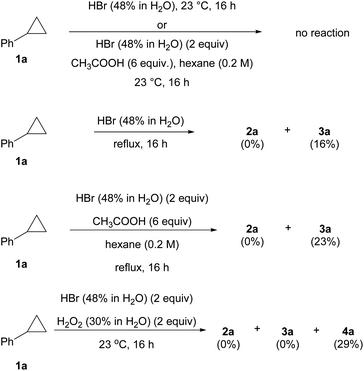
Further study revealed that achieving anti-Markovnikov addition of HBr to cyclopropanes in conventional systems is not a trivial task (Scheme 3). For instance, no reaction was observed when 1a was treated with HBr in either aqueous or water/AcOH co-solvent systems at room temperature.29 Heating both reactions only yielded the Markovnikov product 3a in 16–23% yield, and no anti-Markovnikov product 2a was detected. The classical radical bromination protocol with BBr3/H2O2 only furnished dibrominated product 4a in 29% yield. Similar to the uniqueness of BR3/O2 in several aforementioned radical reactions,4 the incapability of these control experiments in producing 2a as a product contrasted starkly with our BBr3/O2 conditions, which generated a reactive yet selective bromine radical.
Next, we expanded the substrate scope to other unactivated cyclopropanes using either water or t-BuOH as the proton source (Scheme 4). Electron-neutral, deficient and sterically bulky substrates 1a–1g gave the desired anti-Markovnikov products 2a–2g in good yields and regioselectivity. Cyclopropanes with electron-deficient substituents including nitriles (1j–n) and ester (1o) also worked well with excellent regioselectivity. This protocol also exhibits high chemoselectivity towards cyclopropanes. Aryl methyl ether (2i), which is known to be easily cleaved by BBr3 even at low temperature, remained intact under our reaction conditions.39 Due to the tendency of aryl vinyl ketones to polymerize, they are known to be unsuitable for 1,4-conjugate additions mediated by trialkylboranes.40 Nevertheless, aryl cyclopropyl ketones (1h–i) were converted into the corresponding products in high yields, and polymerization was not observed. 1,1-Disubstituted (1p) and simple alkyl (1q) cyclopropanes were also compatible to give products 2p and 2q. When cyclopropyl carboxylic acid (1r) was used as the substrate, the unstable product 2r was detected using HRMS and crude 1H NMR, and γ-butyrolactone was obtained ultimately through cyclization upon a basic work-up procedure. Indene-derived cyclopropyl substrate 1s was also compatible to give 2s. Scaled-up reactions were also performed on selected examples (2a, 2h, 2o, and 2r) and excellent regioselectivities were still obtained.
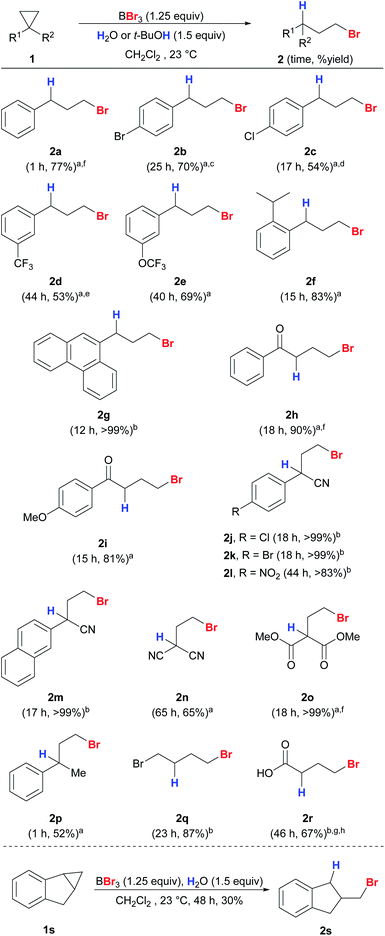 | ||
| Scheme 4 Reaction scope of anti-Markovnikov hydrobromination of cyclopropanes. Conditions: reactions were carried out with 1 (0.2 mmol) unless stated otherwise. Exact reaction conditions for each substrate are stated in the ESI.†at-BuOH was used as the proton source. bH2O was used as the proton source. c4% of 3b was detected. d5% of 3c was detected. e7% of 3b was detected. fThe reaction was conducted on a 1 mmol scale. gThe reaction was conducted on a 2 mmol scale. hThe product cyclized quickly upon work-up and the yield was measured on the basis of the cyclized product γ-butyrolactone. | ||
Cyclopropanes 1t–1y with secondary and tertiary alcohols also gave the corresponding anti-Markovnikov products in excellent yields and with high regioselectivities (Scheme 5). The structure of 2x was confirmed unambiguously by X-ray crystallography.41 The hydroxyl groups in the substrates were converted into bromides simultaneously by the action of BBr3 to give a series of useful dibromides.42 We were interested in whether alcohol-containing substrates can be hydrobrominated in the absence of an external proton source. To our delight, 1t was able to undergo anti-Markovnikov hydrobromination to give 2t with only a slight drop in yield (76%), and 2u was produced in quantitative yield. The hydroxyl groups in 1x and 1y appear to be crucial because a sluggish reaction was observed for 1-phenyl-2-methylcyclopropane that bears no hydroxyl substituent.
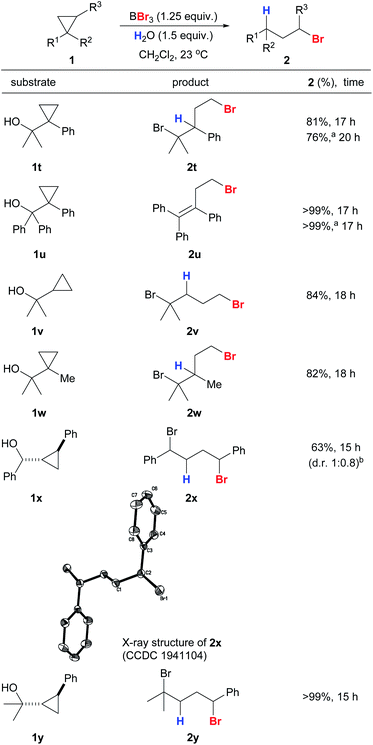 | ||
| Scheme 5 Reaction scope of anti-Markovnikov hydrobromination of cyclopropanes with hydroxyl substituents. Conditions: reactions were carried out with 1 (0.2 mmol). Exact reaction conditions for each substrate are stated in the ESI.†aReaction was conducted in the absence of water. bDiastereoselectivity was determined by a 1H NMR experiment on the crude mixture. | ||
By substituting H2O and t-BuOH with D2O and t-BuOD, deuteriobrominations were also carried out and the corresponding mono-deuterium-labeled compounds were obtained smoothly (Scheme 6). Our protocol offered excellent regio-control in the mono-deuteriation to give 2-D. Unactivated (1a and 1e) and activated cyclopropanes (1j–k, 1m, and 1o) with various substituents worked well and excellent levels of deuterium incorporation were achieved.
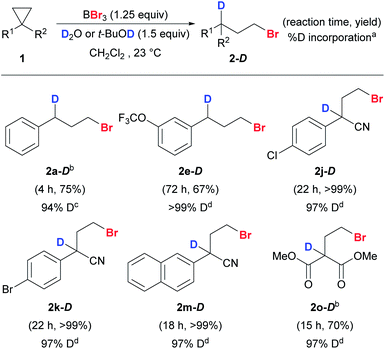 | ||
| Scheme 6 Reaction scope of anti-Markovnikov deuteriobromination of cyclopropanes. Conditions: reactions were carried out with 1 (0.2 mmol) unless stated otherwise. Exact reaction conditions for each substrate are stated in the ESI.†aThe % D incorporation was determined based on the integration of the residual proton signal in 1H NMR. bThe reaction was conducted on a 1 mmol scale. ct-BuOH was used as the deuterium source. dD2O was used as the deuterium source. | ||
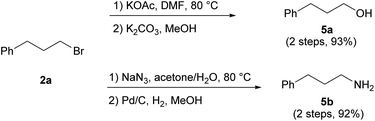
The conversion of products 2 into primary alcohols and amines through nucleophilic substitution proved straightforward. For instance, alcohol 5a and amine 5b were readily prepared from 2a with high conversion (Scheme 7). As the direct synthesis of primary alcohols and amines through anti-Markovnikov hydration and hydroamination has proven to be challenging,22 our protocol provides useful precursors for the synthesis of these highly desired compounds.
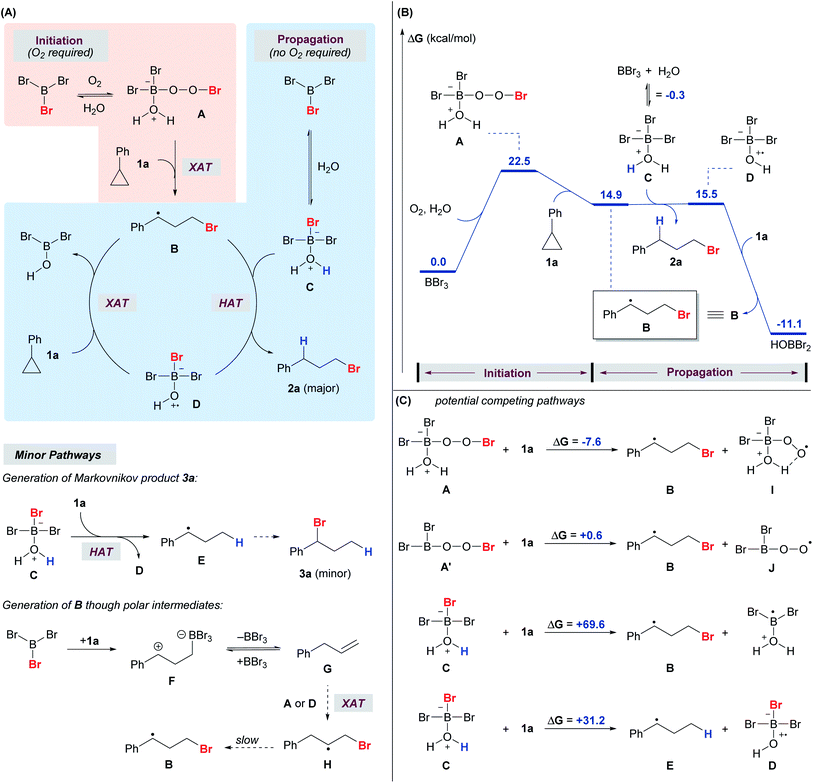
We envision a radical reaction pathway between BBr3 and O2, but given the Lewis acidity of BBr3 and Lewis basicity of H2O and alcohols, an acid-mediated pathway cannot be ruled out.38 However, such a pathway appears highly unlikely, as the treatment of cyclopropanes with aqueous HBr yielded no anti-Markovnikov product 2 (Scheme 3). Several control experiments were performed to further probe the reaction mechanism.
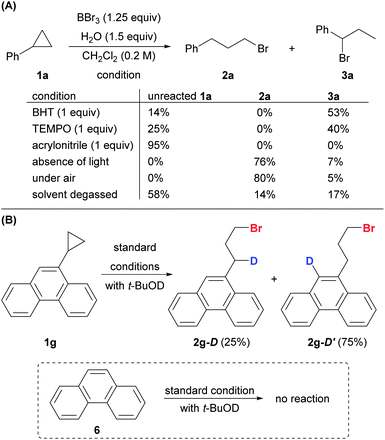
The addition of a radical scavenger, BHT or TEMPO, in slight excess of BBr3 completely shut down the formation of anti-Markovnikov product 2a, and a significant amount of Markovnikov product 3a was detected (Scheme 8A). The addition of the acceptor olefin acrylonitrile completely suppressed the reaction. The absence of light had no impact on the reaction, thereby eliminating the possibility of a photo-triggered pathway. The presence of oxygen was crucial for both the yield and the regioselectivity. The reaction proceeded smoothly to give the desired product 2a (80%) in open air. In contrast, the yield of anti-Markovnikov product 2a dropped to 14% and that of the Markovnikov product 3a increased to 17% when the reaction was conducted with degassed CH2Cl2 and 1a. Deuteriobromination of 1g was also conducted with t-BuOD as the deuterium source (Scheme 8B). Other than the benzylic deuteriation product 2g-D (25%), a substantial amount of 2g-D′ (75%) was obtained. In contrast, no aromatic deuteriation was observed when phenanthrene (6) was used as the substrate under the same conditions. The formation of 2g-D′ could be attributed to the isomerization of benzylic radical species (also see the ESI, Fig. S1†). This preliminary evidence pointed at a radical mechanism, although a carbocation intermediate cannot be ruled out completely.
Consistent with literature reports on BR3,43,44 the reactivity of BBr3 towards homolytic debromination decreases sharply along the series BBr3, BBr2OR, and BBr(OR)2 as a consequence of π-bonding between oxygen and boron. With 0.5 equiv BBr3, only 21% of 2a was obtained even with a prolonged reaction time of 24 h. These data indicated that only the first equivalent of Br from BBr3 is crucial for the reactivity, and contribution from the possible BBra(OR)3−a byproducts should be insignificant.
A series of 1H and 11B NMR experiments were conducted to gain further insight. Upon mixing BBr3 with 1a in the absence of O2 and a proton source, both 1a and BBr3 were mostly consumed, and a new 11B signal at 64 ppm (see the ESI, Fig. S2†) emerged as a singlet, which is characteristic of an alkyldihaloborane species.45,46 From 1H NMR, it is clear that 1a is ring-opened (see the ESI, Fig. S4†), and the species has a similar NMR pattern to a hydroborated cyclopropane, which has been reported as a reaction intermediate in literature examples (see the ESI, Fig. S3†).37 Direct bromoboration of alkynes or allenes with BBr3 is well documented.47,48 While this new species cannot be clearly identified, it is speculated that it could be the direct bromoboration product or hydroxyboration product. Nevertheless, it is clear that the interaction between BBr3 and 1a does not lead to the anti-Markovnikov product 2a in the absence of O2 and a proton source.
When i-PrOH and BBr3 were mixed in CD2Cl2 under air, the 11B signal of BBr3 (39 ppm) disappeared and a new signal at 25.0 ppm emerged. A new proton signal at −2.68 ppm also appeared in the 1H NMR study of the same sample. The two new signals (25.0 ppm in 11B NMR and −2.68 ppm in 1H NMR) diminished gradually upon the addition of 1a and the amount of anti-Markovnikov product 2a increased accordingly (see the ESI, Fig. S5†). On the other hand, a new 11B NMR signal at 18.9 ppm (but no signal at 25.0 ppm) was observed when the same mixture was prepared in the absence of O2 and attributed to the formation of the Lewis adduct between i-PrOH and BBr3 (see the ESI, Fig. S6†). Thus, it is reasonable to propose that the active species, responsible for initiating the anti-Markovnikov hydrobromination of cyclopropanes, was formed only in the presence of O2.
A DFT computational study was also performed to shed light on the mechanism (Fig. 1). While there are no reports on radical reactions triggered by BBr3/O2, we speculate that the reaction mechanism might be analogous to the classical BR3/O2 system in which the putative peroxy-boron species A is generated49 at the initiation stage of the radical process (Fig. 1A) and corresponds to the new NMR signals (25.0 ppm in 11B NMR and −2.68 ppm in 1H NMR).3,50,51 Based on the calculated energy profile, species A is capable of brominating cyclopropane 1a through a radical mechanism to give B (Fig. 1B). It is also calculated that A and A′ could be in equilibrium, but species A (ΔG = −7.6 kcal mol−1) was found to be a more competent Br donor than A′ (ΔG = 0.6 kcal mol−1) in the halogen atom transfer (XAT), potentially due to the intramolecular hydrogen bond that stabilizes the by-product I (Fig. 1C). It was also calculated that BBr3 can reversibly react with water to give adduct C (11B NMR signal = 18.9 ppm). Species C is unable to serve as a Br radical donor to brominate cyclopropane 1a (ΔG = 69.6 kcal mol−1).
However, species C is capable of acting as a hydrogen radical donor to species B, furnishing the desired product 2a. This result is in alignment with the proposal in the literature in which trialkylborane-ROH complexes (R = H, Me) might act as H-donors as a result of the weakened O–H bond.52 Species D, which is formed from species C after the hydrogen atom transfer (HAT), was calculated to be a competent Br radical donor to brominate cyclopropane 1a to give B, thereby propagating the radical chain. Thus, we propose that oxygen is required only in the initiation stage for the generation of species A, while species C and D are responsible for propagation. Indeed, the reaction was sluggish under an inert atmosphere, while the re-introduction of oxygen into a system initially free of oxygen triggered the anti-Markovnikov hydrobromination (see the ESI, Scheme S2†). The HAT from species A to 1a was also explored computationally, but species A could not be optimized as a stable energy minimum. Species C may also serve as a hydrogen radical donor and react with cyclopropane 1a to give species D and E, which would go on to produce the Markovnikov product 3a. However, this hydrogen atom transfer reaction is endergonic by 31.2 kcal mol−1 (Fig. 1C), making it a minor pathway compared to the competing hydrogen atom transfer from C to B that gives D and 2a (Fig. 1A). This result is consistent with the experimental observation that the Markovnikov product 3a became dominant when the reaction was conducted under an inert atmosphere (Scheme 8A).
In the 1H NMR study of the reaction using 1a, apart from 2a, 3a and 4a (Scheme 2), a trace amount of allylbenzene was detected initially and diminished over time. We speculate that the allylbenzene (Fig. 1A, species G) might be formed through the zwitterionic species F as proposed in the recent studies by Wang and Shi.37,53 The eventual disappearance of allylbenzene could be attributed to the radical bromination to give species H and the subsequent formation of 2a.
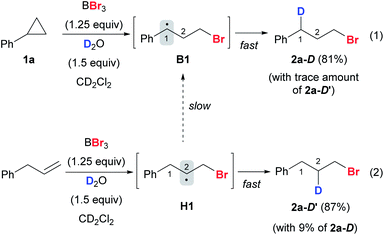
In a deuterium labeling experiment with 1a as the substrate and D2O as the deuterium source, we observed exclusive deuterium incorporation at the benzylic carbon to give product 2a-D, potentially through the C(1) radical species B1 (Scheme 9, eqn (1)). However, the deuterium incorporation pattern is vastly different when using allylbenzene instead of 1a, for which C(2) deuterated product 2a-D′ was obtained predominately (Scheme 9, eqn (2)) (also see the ESI, Fig. S7†). The formation of 2a-D′ from allylbenzene may proceed through the C(2) radical species H1. A small amount of 2a-D (9%) was also detected in the reaction with allylbenzene, attributed to the slow 1,2-hydrogen shift54 converting H1 to the more stable benzylic radical B1. These results suggest that the 1,2-hydrogen shift between the radical species H and B (Fig. 1A) should be much slower than the radical protonation process, implying that allylbenzene is unlikely to be the key intermediate in the reaction.
Conclusions
In summary, we have discovered and exploited the potential of BBr3 to serve as a bromine radical donor in the presence of O2 and a proton source. Through our protocol, cyclopropanes are opened regioselectively to obtain anti-Markovnikov hydro- and deuteriobrominated products in high yields. Mechanistic studies and DFT calculations demonstrate the importance of O2 in the radical initiation process. Further efforts to utilize this reactivity mode of BBr3 on different classes of substrates are currently underway in our laboratory.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Hong Kong Special Administrative Region General Research Funding (grant no. CUHK14304918), The Chinese University of Hong Kong Direct Grant (grant no. 4053394), and Innovation and Technology Commission to the State Key Laboratory of Synthetic Chemistry (GHP/004/16GD). K. N. H thanks the National Science Foundation (Grant CHE-1764328) for financial support. Calculations were performed on the Hoffman2 cluster at the University of California, Los Angeles, and the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by the National Science Foundation (Grant OCI-1053575).Notes and references
- (a) A. G. Davies and B. P. Roberts, Chem. Commun., 1966, 298–299 RSC; (b) A. G. Davies and B. P. Roberts, J. Chem. Soc. B, 1967, 17–22 RSC.
- Radicals in Organic Synthesis, ed, P. Renaud and M. P. Sibi, Wiley-VCH, Weinheim, 2001 Search PubMed.
- C. Ollivier and P. Renaud, Chem. Rev., 2011, 101, 3415–3434 CrossRef PubMed.
- (a) F. R. Mayo and C. Walling, Chem. Rev., 1940, 27, 351–412 CrossRef; (b) M. D. Bachi and E. Bosch, Tetrahedron Lett., 1986, 27, 641–644 CrossRef; (c) K. Nozaki, K. Oshima and K. Utimoto, Tetrahedron Lett., 1988, 29, 6127–6128 CrossRef CAS; (d) K. Nozaki, K. Oshima and K. Utimoto, Bull. Chem. Soc. Jpn., 1990, 63, 2578–2583 CrossRef CAS; (e) P. A. Evans and J. D. Roseman, J. Org. Chem., 1996, 61, 2252–2253 CrossRef CAS.
- (a) M. Hudlicky and T. Hudlicky, Formation of carbon–halogen bonds, in The Chemistry of Functional Groups, Supplement D, ed. S. Patai and Z. Rappoport, John Wiley and Sons Ltd., New Jersey, 1983 Search PubMed; (b) Formation of carbon–halogen bonds (Cl, Br, I), in PATAI's Chemistry of Functional Groups, ed. Y. Sasson, John Wiley and Sons Ltd, 2009 Search PubMed.
- (a) Boron Tribromide in Encyclopedia of Reagents for Organic Synthesis, ed. A. Suzuki, S. Hara and X. Huang, John Wiley & Sons, Ltd., 2006 Search PubMed; (b) Boron Trichloride in Encyclopedia of Reagents for Organic Synthesis, ed. N. Miyaura, Y. Yamamoto and N. Miyaura, John Wiley & Sons, Ltd., 2006 Search PubMed; (c) P. Laszlo and M. Teston, J. Am. Chem. Soc., 1990, 112, 8750–8754 CrossRef CAS.
- J. B. Holbrook, B. C. Smith, C. E. Housecroft and K. Wade, Polyhedron, 1982, 1, 701–706 CrossRef CAS.
- N. N. Greenwood, in Comprehensive Inorganic Chemistry, ed. A. F. Trotman-Dickenson, Pergamon Press, Oxford, 1973 Search PubMed.
- J. D. Grant and D. A. Dixon, J. Phys. Chem. A, 2009, 113, 777–787 CrossRef PubMed.
- (a) M. M. Midland and H. C. Brown, J. Am. Chem. Soc., 1971, 93, 1506–1508 CrossRef; (b) G. W. Kabalka, J. Organomet. Chem., 1971, 33, C25–C28 CrossRef CAS.
- L. V. McCarry and D. R. Carpenter, J. Electrochem. Soc., 1960, 107, 38–42 Search PubMed.
- (a) H.-U. Reissig and R. Zimmer, Chem. Rev., 2003, 103, 1151–1196 CrossRef CAS PubMed; (b) M. Yu and B. L. Pagenkopf, Tetrahedron, 2005, 61, 321–347 CrossRef CAS; (c) F. De Simone and J. Waser, Synthesis, 2009, 20, 3353–3374 Search PubMed; (d) F. de Nanteuil, F. de Simone, R. Frei, F. Benfatti, E. Serrano and J. Waser, Chem. Commun., 2014, 50, 10912–10928 RSC; (e) T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed; (f) M. A. Cavitt, L. H. Phun and S. France, Chem. Soc. Rev., 2014, 43, 804–818 RSC; (g) Y. Xia, X. Liu, H. Zheng, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 227–230 CrossRef CAS PubMed; (h) Y. Xia, L. Lin, F. Chang, X. Fu, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2015, 54, 13748–13752 CrossRef CAS PubMed; (i) Y. Xia, L. Lin, F. Chang, Y. Liao, X. Liu and X. Feng, Angew. Chem., Int. Ed., 2016, 55, 12228–12232 CrossRef CAS PubMed; (j) Y. Xia, F. Chang, L. Lin, Y. Xu, X. Liu and X. Feng, Org. Chem. Front., 2018, 5, 1293–1296 RSC.
- V. Nair, S. B. Panicker and S. Mathai, Res. Chem. Intermed., 2003, 29, 227–231 CrossRef CAS.
- C. Rösner and U. Hennecke, Org. Lett., 2015, 17, 3226–3229 CrossRef PubMed.
- Y.-C. Wong, Z. Ke and Y.-Y. Yeung, Org. Lett., 2015, 17, 4944–4947 CrossRef CAS PubMed.
- Z. Ke, Y.-C. Wong, J.-Y. See and Y.-Y. Yeung, Adv. Synth. Catal., 2016, 358, 1719–1724 CrossRef CAS.
- M. H. Gieuw, V. M.-Y. Leung, Z. Ke and Y.-Y. Yeung, Adv. Synth. Catal., 2018, 360, 4306–4311 CrossRef CAS.
- V. M.-Y. Leung, M. H. Gieuw, Z. Ke and Y.-Y. Yeung, Adv. Synth. Catal., 2020, 362, 2039–2044 CrossRef CAS.
- N. O. Ilchenko, M. Hedberg and K. J. Szabó, Chem. Sci., 2017, 8, 1056–1061 RSC.
- S. M. Banik, K. M. Mennie and E. N. Jacobsen, J. Am. Chem. Soc., 2017, 139, 9152–9155 CrossRef CAS PubMed.
- (a) K. J. Shea and P. S. Skell, J. Am. Chem. Soc., 1973, 95, 6728–6734 CrossRef CAS; (b) D. E. Applequist and L. F. McKenzie, J. Org. Chem., 1976, 41, 2262–2266 CrossRef CAS; (c) J. M. Tanko, R. H. Mas and N. K. Suleman, J. Am. Chem. Soc., 1990, 112, 5557–5562 CrossRef CAS; (d) J. M. Tanko and M. Sadeghipour, Angew. Chem., Int. Ed., 1999, 38, 159–161 CrossRef CAS.
- J. Haggin, Chem. Eng. News Archive, 1993, 71, 23–27 CrossRef.
- J. Takaya and J. F. Hartwig, J. Am. Chem. Soc., 2005, 127, 5756–5757 CrossRef CAS PubMed.
- A. Takemiya and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 6042–6043 CrossRef CAS PubMed.
- G. Dong, P. Teo, Z. K. Wickens and R. H. Grubbs, Science, 2011, 333, 1609–1612 CrossRef CAS PubMed.
- S. Zhu, N. Niljianskul and S. L. Buchwald, J. Am. Chem. Soc., 2013, 135, 15746–15749 CrossRef CAS PubMed.
- A. E. Strom and J. F. Hartwig, J. Org. Chem., 2013, 78, 8909–8914 CrossRef CAS PubMed.
- D. J. Wilger, J.-M. M. Grandjean, T. R. Lammert and D. A. Nicewicz, Nat. Chem., 2014, 6, 720–726 CrossRef CAS PubMed.
- M. Galli, C. J. Flectcher, M. del Pozo and S. M. Goldup, Org. Biomol. Chem., 2016, 14, 5622–5626 RSC.
- Q. Zhu, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 741–747 CrossRef CAS PubMed.
- F. R. Mayo and C. Walling, Chem. Rev., 1940, 27, 351–412 CrossRef CAS.
- C. S. Irving, R. C. Petterson, I. Sarkar, H. Kristinsson, C. S. Aaron, G. W. Griffin and G. J. Boudreaux, J. Am. Chem. Soc., 1966, 88, 5675–5676 CrossRef CAS.
- S. S. Hixson and D. W. Garrett, J. Am. Chem. Soc., 1974, 96, 4872–4879 CrossRef CAS.
- V. R. Rao and S. S. Hixson, J. Am. Chem. Soc., 1979, 101, 6458–6459 CrossRef CAS.
- K. Mizuno, J. Ogawa and Y. Otsuji, Chem. Lett., 1981, 10, 741–744 CrossRef.
- C. R. Pitts, B. Ling, J. A. Synder, A. E. Bragg and T. Leckta, J. Am. Chem. Soc., 2016, 138, 6598–6609 CrossRef CAS PubMed.
- D. Wang, X.-S. Xue, K. N. Houk and Z. Shi, Angew. Chem., Int. Ed., 2018, 57, 16861–16865 CrossRef CAS PubMed.
- H. A. Skinner and N. B. Smith, Trans. Faraday Soc., 1955, 51, 19–22 RSC.
- J. F. W. McOmie, M. L. Watts and D. E. West, Tetrahedron, 1968, 24, 2289–2292 CrossRef CAS.
- A. Suzuki, M. Tabata and M. Ueda, Tetrahedron Lett., 1975, 26, 2195–2198 CrossRef.
- CCDC 1941104 (2w) contains the supplementary crystallographic data for this paper..
- J. D. Pelletier and D. Poirier, Tetrahedron Lett., 1994, 35, 1051–1054 CrossRef CAS.
- A. Suzuki, A. Arase, H. Matsumoto, M. Itoh, H. C. Brown, M. M. Rogic and M. W. Rathke, J. Am. Chem. Soc., 1967, 89, 5708–5709 CrossRef CAS.
- J. A. Baban, N. J. Goodchild and B. P. Roberts, J. Chem. Soc., Perkin Trans. 2, 1986, 157–161 RSC.
- H. C. Brown and J. A. Sikorski, Organometallics, 1982, 1, 28–37 CrossRef CAS.
- R. Soundararajan and D. S. Matteson, Organometallics, 1995, 14, 4157–4166 CrossRef CAS.
- C. Wang, Z. Xu, T. Tobrman and E.-i. Negishi, Adv. Synth. Catal., 2010, 352, 627–631 CrossRef CAS PubMed.
- M. F. Lappert and B. Prokai, J. Organomet. Chem., 1964, 1, 384–400 CrossRef CAS.
- A. M. Kosmas, C. Mpellos, Z. Salta and E. Drougas, Chem. Phys., 2010, 371, 36–42 CrossRef CAS.
- P. Renaud, A. Beauseigneur, A. Brecht-Forster, B. Becattini, V. Darmency, S. Kandhasamy, F. Montermini, C. Olliver, P. Panchaud, D. Pozzi, E. M. Scanlan, A.-P. Schaffner and V. Weber, Pure Appl. Chem., 2007, 79, 223–233 CAS.
- D. P. Curran and T. R. McFadden, J. Am. Chem. Soc., 2016, 138, 7741–7752 CrossRef CAS PubMed.
- (a) D. A. Spiegel, K. B. Wiberg, L. N. Schacherer, M. R. Medeiros and J. L. Wood, J. Am. Chem. Soc., 2005, 127, 12513–12515 CrossRef CAS PubMed; (b) D. Pozzi, E. M. Scanlan and P. Renaud, J. Am. Chem. Soc., 2005, 127, 14204–14205 CrossRef CAS PubMed; (c) M. R. Medeiros, L. N. Schacherer, D. A. Spiegel and J. L. Wood, Org. Lett., 2007, 9, 4427–4429 CrossRef CAS PubMed.
- Z.-Y. Zhang, Z.-Y. Liu, R.-T. Guo, Y.-Q. Zhao, X. Li and X.-C. Wang, Angew. Chem., Int. Ed., 2017, 56, 4028–4032 CrossRef CAS PubMed.
- J. W. Wilt and O. Kolewe, J. Am. Chem. Soc., 1965, 87, 2071–2072 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1941104. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc02567d |
| This journal is © The Royal Society of Chemistry 2020 |